Abstract
Background
Insufficient weight loss or weight regain has many causes including a large gastric pouch. A large gastric pouch may be due to the surgical technique or can be patient related (dilation). Resizing the gastric pouch may lead to additional weight loss. Currently, there is no gold standard for the revisional surgical technique. Therefore this study was performed to determine which surgical technique for revisional bariatric surgery (BS) has superior outcomes in terms of weight loss: sleeve resection of the gastrojejunostomy and gastric pouch (SGP), or resection of the gastrojejunostomy with resizing of the pouch and creation of a new anastomosis (RGJ).
Methods
All patients who underwent revisional BS for insufficient weight loss or weight regain as a result of an enlarged pouch after LRYGB from April 2014 to June 2018 in our hospitals were included in this observational cohort study. Outcomes were measured in percentage total weight loss (%TWL).
Results
A total of 37 patients who underwent SGP and 21 patients who underwent RGJ as revisional BS were included in this study. The median body mass index before revisional BS was 37.6 kg/m2 versus 35.7 kg/m2 (SGP vs RGJ, respectively, P = 0.115). There was no significant difference in %TWL between the two cohorts 1 and 2 years after revisional BS, respectively; SGP 14.5% vs RGJ 11.0%, P = 0.885 and SGP 12.3% vs RGJ 10.8%, P = 0.604. Comparing %TWL based on weight at LRYGB, there was also no significant difference two years after revisional BS (SGP 22.0% vs RGJ 22.2%, P = 0.885). The average use of surgical disposables for the SGP technique were lower compared to the RGJ technique.
Conclusions
Resizing a large pouch leads to additional weight loss. Both techniques have comparable outcomes in terms of weight loss. However, based on average surgical costs, the SGP technique may be preferable.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Laparoscopic Roux-en-Y gastric bypass (LRYGB) has been proven to be an effective treatment of morbid obesity. It leads to substantial excess weight loss and reduction or even remission of metabolic comorbidities [1,2,3]. Unfortunately, insufficient weight loss or weight regain after LRYGB have been described as well [4]. Insufficient weight loss and weight regain may have multifactorial causes. It can either be patient related (e.g. dietary non-compliance, physical inactivity or hormonal/metabolic factors) or surgery related (e.g. enlarged pouch due to construction of a large pouch at the primary LRYGB, gastrogastric fistulas or dilation of the gastrojejunostomy) [4, 5].
Several studies have shown the importance of the size of the pouch in the primary surgery on weight loss [6,7,8]. In case of an anatomical cause of insufficient weight loss or weight regain, surgical treatment is challenging and controversial. Several techniques have been proposed for revisional bariatric surgery (BS) if insufficient weight loss or weight regain is caused by an enlarged pouch [9]. Laparoscopic resizing of the gastric pouch and gastrojejunostomy show good results on percentage excess weight loss (%EWL) with low reoperation rate and no mortalities [10, 11]. Two techniques for resizing of the gastric pouch and gastrojejunostomy are described in literature: sleeve resection of the gastrojejunostomy and gastric pouch (SGP) [11, 12], and resection of the gastrojejunostomy with creation of a smaller pouch and a new anastomosis (RGJ) [13, 14]. So far only short-term results in small groups have been described.
The aim of this study is to determine whether a sleeve resection of the gastrojejunostomy and gastric pouch or a revision of the gastrojejunostomy is the superior technique for additional weight loss after LRYGB.
Materials and methods
Patient selection
All patients who underwent revisional BS for insufficient weight loss or weight regain after LRYGB from April 2014 to June 2018 in two expert centers for BS were included in this observational cohort study. Patients after primary LRYGB as well as patients who underwent previous BS before LRYGB were included. Previous BS was either laparoscopic adjustable gastric banding (LAGB) or laparoscopic sleeve gastrectomy (LSG). The SGP technique was performed in one of the centers, and the RGJ technique was performed in the other center. The performed revisional technique was based on the preference of the bariatric surgeons. Both techniques are described below.
Before qualifying for revisional BS, all patients with insufficient weight loss or weight regain followed an obligatory and standardized trajectory led by a team of psychologists, dietitians and psychotherapists to optimize the patients motivation and compliance to an adjusted life style. Patients were only eligible for revisional BS after failing this standardized trajectory in terms of additional weight loss combined with no sensation of restriction and diagnostics tests showing a large pouch. Diagnostic tests to evaluate a large pouch were either barium swallow test (BST) in combination with gastroscopy or a three-dimensional gastric computed tomography (3D-GCT) scan. A pouch was defined as dilated if the pouch was > 5 cm on the BST in combination with gastroscopy, based on the difference in length measured from the gastrojejunostomy to the Z-line, or if the pouch volume was > 50 ml on the 3D-GCT. Examples of pouch enlargement signs on these tests are depicted in Fig. 1. Patients were excluded from this study if the insufficient weight loss or weight regain was caused by a gastrogastric fistula or if revision of the gastrojejunostomy was due to a marginal ulcer.
Data collection
Baseline characteristics (i.e. age, sex, presence of metabolic comorbidities) and surgical characteristics (i.e. type of primary operation, duration of surgery and early surgical complications) were collected. The initial weight and body mass index (BMI) before the primary BS, the initial weight response to the primary BS, and the weight before revisional BS were recorded. All patients signed a consent form before surgery, in which they agreed that their data could be used for retrospective studies. Therefore, an approval of the international review board was not needed.
During follow-up, outcomes were collected at 3, 6, 12, and 24 months. Additionally, patients with comorbidities were seen by the internal medicine specialist, who evaluated whether metabolic comorbidities persisted, improved or resolved. This evaluation was based on general use of medication. For type 2 diabetes mellitus specifically HbA1c levels, for hypertension specifically blood pressure and screening for concomitant organ damage, for hypercholesterolemia specifically cholesterol levels and lastly for obstructive sleep apnea an evaluation of the pulmonologist was included. The amount of weight loss was described as the percentage of total weight loss (%TWL) and was calculated as ((operative weight − follow-up weight)/operative weight) × 100%. BMI was calculated as weight (kg)/height (m)2.
Weight data were analyzed for patients undergoing primary LRYGB and undergoing LRYGB as conversion from LAGB or LSG separately. In addition, all weight results were presented for the entire group.
Surgical techniques
Laparoscopic sleeve resection of the gastrojejunostomy and gastric pouch (SGP)
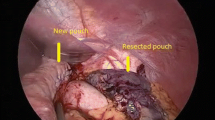
Laparoscopic sleeve resections of the gastrojejunostomy, gastric pouch and blind afferent limb were performed using a 60 mm endoscopic stapler (Fig. 2). A 34-gauge gastric tube was used to calibrate the size of the revised gastric pouch that was transected longitudinally 6 cm from the diaphragm. The resected tissue was removed using an endoscopic bag.
Laparoscopic revision of the gastrojejunostomy (RGJ)
In this technique, the gastric pouch was transected above the level of the anastomosis approximately 6 cm from the diaphragm and reduced by a 60-mm endoscopic stapler, using a 34-gauge gastric tube for calibration (Fig. 3). A new 30-mm linear gastrojejunostomy was created and the remaining defect was laparoscopically sutured. Subsequently, the jejunum was transected below the anastomosis. The resected tissue was then removed using an endoscopic bag.
All operations were performed laparoscopically with the use of one 12-mm vision port for the camera, two 12-mm working ports, one 5-mm working port and a liver retractor. The average use of surgical disposables was calculated and operation time were compared for both techniques.
Statistical analysis
Statistical analysis was performed with IBM SPSS Statistics, version 24 (SPSS, Chicago, IL). Continuous data are presented as median and interquartile range (IQR) and were compared by the Mann–Whitney U-test according to normality. The Chi-square test was used for categorical data analysis. A P value < 0.05 was considered significant. Missing data were addressed with a pair wise deletion in follow-up analysis.
Results
A total of 37 patients who underwent SGP and 21 patients who underwent RGJ as revisional BS were included in our study. The baseline characteristics are presented in Table 1.
Weight loss after resizing the pouch
Weight data are presented in Table 2. Median BMI at primary BS was significantly different but no differences were found in BMI at revisional BS. There were no statistical differences in %TWL between the two techniques during follow-up, as presented in Fig. 3. %TWL was determined based on weight at primary BS (Fig. 4a), at LRYGB (Fig. 4b) and at revisional BS (Fig. 4c). Based on weight at revisional BS, the 24-month %TWL was 12.3% [5.8; 14.5] in the SGP cohort versus 10.8% [3.4; 22.1] in the RGJ cohort (P = 0.604). The total %TWL based on weight at primary BS was %TWL [IQR] was 22.0% [16.4; 30.3] in the SGP cohort and 22.2% [11.7; 32.1] in the RGJ cohort (P = 0.885).
Percentage total weight loss during follow-up. a %TWL based on weight at primary bariatric surgery. b %TWL based on weight at LRYGB. c %TWL based on weight at revisional bariatric surgery. *Significantly different (P < 0.005). SGP sleeve resection of the gastrojejunostomy and gastric pouch, RGJ revision of the gastrojejunostomy, rBS revisional bariatric surgery, %TWL percentage total weight loss, LRYGB laparoscopic Roux-en-Y gastric bypass
Figure 5 shows the trend of overall %TWL based on weight at primary BS for patients who had undergone LYRGB as primary BS (Fig. 5a) and patients who had undergone LRYGB as a conversion from LAGB or SG to LRYGB (Fig. 5b) respectively.
Percentage total weight loss during follow-up. a %TWL based on weight at primary bariatric surgery in patients who underwent LRYGB as primary bariatric procedure. b %TWL based on weight at primary bariatric surgery in patients who underwent LRYGB as a revisional bariatric procedure after LAGB or LSG. *Significantly different (P < 0.005). SGP sleeve resection of the gastrojejunostomy and gastric pouch, RGJ revision of the gastrojejunostomy, rBS revisional bariatric surgery, %TWL percentage total weight loss, LRYGB laparoscopic Roux-en-Y gastric bypass, LAGB laparoscopic adjustable gastric banding, LSG laparoscopic sleeve gastrectomy
Surgical factors, early postoperative complications and surgical costs
Operation time was significantly higher in the RGJ procedure (57.0 min SGP vs 74.0 min RGJ, P = 0.003). At the RGJ group, one patient underwent both revisional surgery, laparoscopic cholecystectomy and closure of the mesenteric defects within one procedure. After exclusion of this patient, operation time was still significantly higher in the RGJ cohort (73.5 min, P = 0.006).
Three early postoperative complications occurred in the SGP group (8.1%) as compared to two complications in the RGJ group (9.5%), as demonstrated in Table 3. According to the Clavien–Dindo classification of surgical complications [15], in the SGP group two grade I and one grade IIIb complications occurred as compared to one grade I complication and one grade IIIB complication in the RGJ group. In both study groups, one patient had an anastomotic leakage (2.7% in the SGP group and 4.8% in the RGJ group). In the SGP group, a jejunal tube was placed endoscopically for enteral feeding to treat the anastomotic leakage. In the RGJ group, a relaparoscopy was performed to close the defect of the anastomotic leakage. Both patients recovered without any negative residual effects. The average surgical costs for the SGP technique were approximately €337 lower than for the SGJ technique (Table 4).
Obesity-related comorbidities
In both groups, all patients achieved either improvement or remission of DM2 after revisional BS (Table 5). Hypertension improved or even resolved in 60% of the patients in the SGP group as compared to 66.7% in the RGJ groups. Two patients (50%) achieved remission of hypercholesterolemia in the SGP groups as compared to one patient (50%) in the RGJ group. There was no remission or improvement of OSAS achieved in both study groups.
Discussion
Resizing a large gastric pouch after LRYGB leads to additional weight loss and has a positive effect on obesity-related comorbidities. The two techniques, SGP and RGJ, were equally effective in terms of weight loss. However, the SGP technique did result in less usage of disposables and a shorter operation time compared to the RGJ technique.
Based on weight prior to resizing the gastric pouch the median %TWL at 24 months after revisional BS was 12.3% [5.8;14.5] in the SGP cohort versus 10.8% [3.4;22.1] in the RGJ cohort (P = 0.604). When we compare the %TWL based on weight prior to the first bariatric procedure, the %TWL is 22.0% in the SGP cohort and 22.2% in the RGJ cohort (P = 0.885), which is comparable to previous studies [16,17,18,19]. Considering these results it should be noted that the additional effect on %TWL of revisional surgery was relatively small. This study, however, shows that the additional effect may be well worth the effort as all patients now achieved %TWL of more than 20% and additional resolution of comorbidities.
Previous studies have shown that the clinical effect on comorbidities in revisional BS is similar to primary BS [20, 21]. In this study, there seems to be an improvement of comorbidities in both groups. As the sample size was small, this study could not demonstrate a statistically significant reduction or improvement of comorbidities between the two techniques. Nevertheless, it can be concluded from this study that revision of a large pouch can exert a positive effect on obesity-related comorbidities. Therefore the continuous presence of obesity-related comorbidities should be considered as an indication for revisional BS.
Although this study did not intend to perform a full cost effectiveness analysis, we did find lower surgical costs of the SGP technique as compared to the RGJ technique. This difference was a result of a shorter operating time and less use of disposables in the SGP technique.
The effect of pouch size on the achieved weight loss after LRYGB remains controversial. Even though some studies have shown that a small pouch size results in higher achieved weight loss [6,7,8], others could not demonstrate a correlation [22, 23]. In this study, no calibration for pouch size was used at the primary LRYGB. However, revisional BS for a large pouch can lead to additional weight loss. Thus we might suggest the use of a calibration tube for LRYGB to prevent insufficient weight loss or weight regain due to a large pouch.
In this study, there was no consistent diagnostic technique used for pouch volume measurement, as no standardization for pouch volume measurement is defined in literature yet. The barium swallow test (BST) with upper gastrointestinal series has been used to measure pouch volume after LRYGB [6, 14]. However, it is challenging to calculate a three-dimensional pouch volume from two-dimensional radiological imaging. Therefore two other suggested techniques are 3D-GCT and upper endoscopy. The volumes of the gastric pouch and the diameter of the gastrojejunal anastomosis can be measured exactly in these two techniques [24, 25]. Unfortunately, the exact pouch size was not measured according to a standardized protocol preoperatively in this retrospective cohort study. However, all patients had demonstrated a large pouch, either diagnosed by BST, upper endoscopy combined with BST or 3D-GCT. Patients were excluded from this study if the diagnostic technique showed other causes for insufficient weight loss or weight regain, such as gastrogastric fistula.
The revisional BS technique was based upon the preference of the surgeon. Because of this, two bariatric surgeons performed the RGJ and three bariatric surgeons performed the SGP technique. There was no crossover in treatments between the surgeons. As a consequence, there were significant differences at baseline: gender, BMI before LRYGB, BMI before primary BS and best %TWL after primary BS and BMI before primary gastric bypass. In multivariate analysis, only best %TWL after primary BS was a positive predictor of %TWL 12 months postrevisional. However, as standardization of revisional BS is needed, we analyzed the results to assess whether one technique is preferable. Further research in a randomized controlled trial is recommended to prevent selection bias.
In conclusion, both SGP and RGJ techniques are feasible to perform and achieve adequate weight loss after revisional BS for insufficient weight loss or weight regain as a consequence of a large pouch after LRYGB. There was no statistical difference in %TWL between either procedures during follow-up, and both techniques showed improvement of obesity-related comorbidities. However, the average surgical costs of the SGP technique were lower and may therefore be the preferred revisional bariatric technique.
References
Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjostrom CD, Sullivan M, Wedel H, Swedish Obese Subjects Study Scientific G (2004) Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 351:2683–2693
Lager CJ, Esfandiari NH, Luo Y, Subauste AR, Kraftson AT, Brown MB, Varban OA, Meral R, Cassidy RB, Nay CK, Lockwood AL, Bellers D, Buda CM, Oral EA (2018) Metabolic parameters, weight loss, and comorbidities 4 years after Roux-en-Y gastric bypass and sleeve gastrectomy. Obes Surg 28:3415–3423
Dogan K, Betzel B, Homan J, Aarts EO, Ploeger N, de Boer H, Aufenacker TJ, van Laarhoven CJ, Janssen IM, Berends FJ (2014) Long-term effects of laparoscopic Roux-en-Y gastric bypass on diabetes mellitus, hypertension and dyslipidaemia in morbidly obese patients. Obes Surg 24:1835–1842
El Ansari W, Elhag W (2021) Weight regain and insufficient weight loss after bariatric surgery: definitions, prevalence, mechanisms, predictors, prevention and management strategies, and knowledge gaps-a scoping review. Obes Surg 31:1755
Karmali S, Brar B, Shi X, Sharma AM, de Gara C, Birch DW (2013) Weight recidivism post-bariatric surgery: a systematic review. Obes Surg 23:1922–1933
Roberts K, Duffy A, Kaufman J, Burrell M, Dziura J, Bell R (2007) Size matters: gastric pouch size correlates with weight loss after laparoscopic Roux-en-Y gastric bypass. Surg Endosc 21:1397–1402
Reiber BMM, Tenhagen M, Hunfeld M, Cense HA, Demirkiran A (2018) Calibration of the gastric pouch in laparoscopic Roux-en-Y gastric bypass: does it matter? The influence on weight loss. Obes Surg 28:3400–3404
Campos GM, Rabl C, Mulligan K, Posselt A, Rogers SJ, Westphalen AC, Lin F, Vittinghoff E (2008) Factors associated with weight loss after gastric bypass. Arch Surg 143:877–883; discussion 884
Tran DD, Nwokeabia ID, Purnell S, Zafar SN, Ortega G, Hughes K, Fullum TM (2016) Revision of Roux-en-Y gastric bypass for weight regain: a systematic review of techniques and outcomes. Obes Surg 26:1627–1634
Al-Bader I, Khoursheed M, Al Sharaf K, Mouzannar DA, Ashraf A, Fingerhut A (2015) Revisional laparoscopic gastric pouch resizing for inadequate weight loss after Roux-en-Y gastric bypass. Obes Surg 25:1103–1108
Iannelli A, Schneck AS, Hebuterne X, Gugenheim J (2013) Gastric pouch resizing for Roux-en-Y gastric bypass failure in patients with a dilated pouch. Surg Obes Relat Dis 9:260–267
Parikh M, Heacock L, Gagner M (2011) Laparoscopic “gastrojejunal sleeve reduction” as a revision procedure for weight loss failure after Roux-en-Y gastric bypass. Obes Surg 21:650–654
Hamdi A, Julien C, Brown P, Woods I, Hamdi A, Ortega G, Fullum T, Tran D (2014) Midterm outcomes of revisional surgery for gastric pouch and gastrojejunal anastomotic enlargement in patients with weight regain after gastric bypass for morbid obesity. Obes Surg 24:1386–1390
Muller MK, Wildi S, Scholz T, Clavien PA, Weber M (2005) Laparoscopic pouch resizing and redo of gastro-jejunal anastomosis for pouch dilatation following gastric bypass. Obes Surg 15:1089–1095
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Delko T, Kostler T, Peev M, Esterman A, Oertli D, Zingg U (2014) Revisional versus primary Roux-en-Y gastric bypass: a case-matched analysis. Surg Endosc 28:552–558
Slegtenhorst BR, van der Harst E, Demirkiran A, de Korte J, Schelfhout LJ, Klaassen RA (2013) Effect of primary versus revisional Roux-en-Y gastric bypass: inferior weight loss of revisional surgery after gastric banding. Surg Obes Relat Dis 9:253–258
Thereaux J, Corigliano N, Poitou C, Oppert JM, Czernichow S, Bouillot JL (2015) Five-year weight loss in primary gastric bypass and revisional gastric bypass for failed adjustable gastric banding: results of a case-matched study. Surg Obes Relat Dis 11:19–25
Wijngaarden LH, Jonker FHW, van den Berg JW, van Rossem CC, van der Harst E, Klaassen RA (2017) Impact of initial response of laparoscopic adjustable gastric banding on outcomes of revisional laparoscopic Roux-en-Y gastric bypass for morbid obesity. Surg Obes Relat Dis 13:594–599
Abdulrazzaq S, Elhag W, El Ansari W, Mohammad AS, Sargsyan D, Bashah M (2020) Is revisional gastric bypass as effective as primary gastric bypass for weight loss and improvement of comorbidities? Obes Surg 30:1219–1229
Mohos E, Jano Z, Richter D, Schmaldienst E, Sandor G, Mohos P, Horzov M, Tornai G, Prager M (2014) Quality of life, weight loss and improvement of co-morbidities after primary and revisional laparoscopic roux Y gastric bypass procedure-comparative match pair study. Obes Surg 24:2048–2054
Cottam DR, Fisher B, Sridhar V, Atkinson J, Dallal R (2009) The effect of stoma size on weight loss after laparoscopic gastric bypass surgery: results of a blinded randomized controlled trial. Obes Surg 19:13–17
Edholm D, Ottosson J, Sundbom M (2016) Importance of pouch size in laparoscopic Roux-en-Y gastric bypass: a cohort study of 14,168 patients. Surg Endosc 30:2011–2015
Heneghan HM, Yimcharoen P, Brethauer SA, Kroh M, Chand B (2012) Influence of pouch and stoma size on weight loss after gastric bypass. Surg Obes Relat Dis 8:408–415
Blanchet MC, Mesmann C, Yanes M, Lepage S, Marion D, Gelas P, Gouillat C (2010) 3D gastric computed tomography as a new imaging in patients with failure or complication after bariatric surgery. Obes Surg 20:1727–1733
Acknowledgements
We would especially like to thank Mara K. K. Veenstra for the illustrations of the two different revisional bariatric surgical techniques.
Funding
This study was not funded.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs Leontine H. Wijngaarden, Drs Beata M.M. Reiber, Drs Heela (F.) Yousufzai, Dr Ahmet Demirkiran and Drs René A. Klaassen have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wijngaarden, L.H., Reiber, B.M.M., Yousufzai, F. et al. Resizing a large pouch after laparoscopic Roux-en-Y gastric bypass: comparing the effect of two techniques on weight loss. Surg Endosc 36, 3495–3503 (2022). https://doi.org/10.1007/s00464-021-08671-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-021-08671-z