Abstract
Background and aims
Conventional endoscopic submucosal dissection (C-ESD) is a technically demanding procedure with prolonged procedure times and higher risk of adverse events. To overcome the procedural difficulty of ESD, several traction-assisted techniques (T-ESD) have been developed to improve visualization of the submucosa in hopes to facilitate safe and effective dissection. The aim of this study was to conduct a meta-analysis that compares short-term outcomes (30-day) of T-ESD to C-ESD.
Methods
Clinical studies published up to April 2020 comparing the efficacy and safety of T-ESD and C-ESD were identified using electronic bibliographic searches. Both randomized controlled trials and observational studies were included. Outcomes of interests were procedure time, rates of en bloc and R0 resection, and rates of adverse events. Fixed effect and random effect model were used to calculate pooled mean difference for continuous variables and risk differences (RDs) for categorical variables.
Results
Twenty-three studies with 2574 patients were included in this meta-analysis, with a total of 2582 lesions (1292 T-ESD and 1290 C-ESD). Pooled estimates of T-ESD showed shorter procedure times (weighted mean difference = −20.35 min, 95% CI −27.51 to −13.19, p < 0.001), higher R0 resection rates (RD 0.04, 95% CI 0.01–0.06, p = 0.004) and lower perforation rates (RD −0.03, 95% CI −0.04 to −0.01, p = < 0.0001). No significant differences were seen in en bloc rates and bleeding risk between the two groups.
Conclusions
Traction-assisted ESD results in shorter procedure time, improved R0 resection rates and lower risk of perforation as compared to conventional ESD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Endoscopic submucosal dissection (ESD) is an advanced endoscopic technique that allows for en bloc resection of large, superficial luminal gastrointestinal neoplasms [1,2,3,4,5,6]. Prior to ESD, piecemeal endoscopic mucosal resection (EMR) had been the nonsurgical therapeutic option for superficial neoplasms. Major disadvantages of EMR are its high rate of lesion recurrence, and its decreased rate of en bloc resection [7,8,9]. ESD has been shown to be superior to EMR for treatment of superficial gastrointestinal neoplasia by facilitating complete and intact histopathologic evaluation, and lower rates of local recurrence [3, 5]. Despite its advantages and widely adopted practice in East Asia, mainly Japan, ESD has slowly gained popularity worldwide largely due to a steep learning curve, prolonged procedure times and increased risk of complications compared to EMR [10, 11].
Conventional ESD (C-ESD) is a technique first developed in Japan in the 1990s [12]. It involves creating an endoscopic incision through the mucosal layer and into the submucosal space using a small diathermy tipped knife. Careful dissection of the submucosa underneath the lesion is then performed. In order to prevent thermal and mechanical injury to the muscularis propria, submucosal injections, such as saline, are used to raise the lesion and gain more accessibility of the dissection plane [13]. Although many devices have dual dissection and injection functions, the procedure can be long and tedious.
Traction-assisted ESD (T-ESD) is a newer concept that is thought to augment the separation between the muscularis propria and the superficial neoplasm [14, 15]. Established techniques for T-ESD include raising the lesion with external traction (clip-with-thread method, clip-and-snare method, external forceps method, double scope method), or lifting the lesion away from the dissection field using internal devices (rings, clip bands, S–O clips) [15]. A number of clinical studies evaluating efficacy of T-ESD compared to C-ESD have been published, however, the results are variable.
In this study, we conducted a comprehensive systematic review and meta-analysis across multiple study types to assess the safety and efficacy of T-ESD in superficial gastrointestinal neoplasms. We hypothesize that use of traction during ESD will increase efficiency and reduce risk of complications compared to conventional ESD.
Methods
Search strategy and study selection
The systematic review and meta-analysis adhered to the PRISMA (Preferred Reporting Items for Systematic reviews and meta-Analyses) statement. Institutional Review Board (IRB) approval and patient informed consent were not needed for this meta-analysis of already published data. A comprehensive search of MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials and Database of Systematic Reviews and Web of Science from the earliest available online year of indexing up to April 30, 2020 was systematically conducted. The search strategy was developed and executed by an experienced librarian, in collaboration with the study investigators. The following free-text terms were used for screening title, abstract and subject heading: a combination of “ESD”, or “endoscopic submucosal resection”, and “traction”. Bibliographies of relevant articles were hand-searched. Randomized controlled trials (RCTs) and observational studies that compared the effects and adverse events between T-ESD and C-ESD were included if they met the following inclusion criteria: (i) ESD was performed in esophagus, stomach or colorectal region (ii) The study provided at least one of our primary outcomes including procedure time, en bloc resection rate, R0 resection rate or adverse events. Exclusion criteria were (1) abstract-only publication; (2) insufficient data defined as not providing any of our outcomes of interest; and (3) no comparative data reported in the article.
To avoid bias in selection of studies, two authors independently selected the eligible studies, data extraction and assessment of risk of bias. Any disagreement was discussed, and the consensus was made with a third reviewer.
Data extraction and quality assessment
A data collection sheet was developed in advance. Details were extracted from each study including publication year, country of origin, study design, baseline patient characteristic, location of lesion, lesion characteristic, type of traction device, sample size, and our outcome measurement (procedure time, en bloc resection rate, R0 resection rate, adverse events including immediate and delayed bleeding, and perforation).
For quality assessment of RCTs, we assessed risk of bias of individual study using the modified Cochrane Collaboration tool. The Newcastle–Ottawa quality assessment scale (NOS), recommended by the Cochrane Collaboration, was used to investigate the quality of each nonrandomized controlled study [16,17,18].
Statistical analysis
We conducted meta-analysis using Review Manager (version 5.3, The Cochrane Collaboration, Denmark) and R Software [19]. To assess comparability of main outcomes between groups, the mean difference and 95% confidence intervals (CIs) were recommended for continuous outcomes. When means and/or standard deviations were not reported in studies, Hozo’s methodology was applied to achieve them using median, range and sample size [20]. The RD and 95% CIs were recommended for dichotomous data.
Heterogeneity among the studies was assessed by the chi-square test and inconsistency index (I2) statistic, with values > 50% suggestive a high heterogeneity level. p value < 0.5 was considered to be significantly heterogeneous. Meta-analysis was to be calculated using fixed effect model. If there was any obvious inter-study heterogeneity (I2 > 50%), they were to be explored by subgroup analysis and a random effect model. To find potential source of heterogeneity, subgroup analyses were performed including lesion location and type of study (RCTs, observational, retrospective or prospective).
Publication bias was evaluated by funnel plot visual analysis and Egger’s test, with p value of 0.05, for each comparison with more than ten studies. Sensitivity analysis was performed if publication bias was detected by running Egger’s tests for RCTs and observational studies, as well as per lesion location.
Results
Study inclusion and assessment
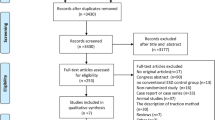
A total of 806 articles were initially identified through database searching (Fig. 1). Of these, 447 were duplicates and 416 did not meet the inclusion criteria outlined above. Of the remaining 31 articles, 23 studies were deemed eligible for inclusion into the meta-analysis. Overall, a total of 2574 patients with 2582 lesions were included, 1292 T-ESD and 1290 C-ESD [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43]. Study characteristics are described in detail in Table 1. Of the 23 published studies, 19, 2, 1 and 1 were from Japan, China, France and Korea, respectively. Nine studies were RCTs and 14 were observational studies which included retrospective (n = 8, 57%) and prospective cohorts (n = 6, 43%). Overall, 1302 (50.4%) lesions were from observational studies and 634 (24.6%) lesions are from retrospective studies. When organized by lesion location, five studies evaluated ESD traction for esophageal lesions, ten for gastric lesions, and eight for colorectal lesions. In term of traction techniques, 18 studies evaluated clip-assisted ESD, 4 studies on double-scope method and 1 study on external grasping forceps method.
Risk of bias assessment
The risk of bias was evaluated extensively. First, the nine RCTs were addressed using the Cochrane Collaboration Tool (Table 2). Due to nature of the procedure, blinding is not possible. Despite this, most other forms of bias were low risk. Next, the 14 observational studies were analyzed using the NOS. Five studies were considered high quality (score > 7), nine studies were of moderate quality (score 5–7), and no studies were deemed poor quality (score < 5). The details of quality assessment for the observational studies are seen in Table 3.
Procedure times
A total of 2443 participants from 21 studies were included in this primary outcome analysis, of whom 1218 underwent T-ESD and 1225 underwent C-ESD. Two studies had been excluded due to insufficient data [29, 36]. Overall, the T-ESD group had shorter procedure times than did the C-ESD group (weighted mean difference = −20.35 min, 95% CI −27.51 to −13.19, p < 0.001, I2 = 78%). There was substantial heterogeneity amongst the studies, and thus random effect was applied.
To investigate heterogeneity, the study groups were further analyzed via organ subgroup (Fig. 2) and then by study type (RCT versus observational, Fig. 3). The ESD procedural times in the esophagus, stomach and colorectal locations were significantly shorter in the T-ESD group (weighted mean difference: −16, −11, −32 min); however, heterogeneity was highest in the stomach at I2 = 72%, whereas the heterogeneity for the esophagus and colorectal studies was I2 = 38% and 47%, respectively. When analyzed by study type, although both RCTs and observational studies showed shorter procedure times for the T-ESD group, the observational studies had less heterogeneity (I2 = 54%) than that of RCTs (I2 = 88%). After excluding retrospective studies to include only RCT and prospective studies, T-ESD still had shorter procedure times (weighted mean difference −20.5 (CI −29.95 to −11.08, p < 0.001) although heterogeneity was higher (I2 = 84%).
En bloc resection
Nineteen studies with 1139 T-ESD and 1183 C-ESD participants were included in the en bloc resection rate analysis (Fig. 4). The en bloc resection rates for both T-ESD and C-ESD were high at 99.3%, 97.5%, respectively. Overall, there was no significant difference between en bloc resection rate for T-ESD and that of C-ESD even when considering specific organ subgroup. Heterogeneity was high (I2 = 76), but only within the colorectal subgroup [24].
R0 resection
Eleven studies with 874 T-ESD and 839 C-ESD participants were included in the R0 resection rate analysis (Fig. 5). R0 resection rates for both T-ESD and C-ESD were high at 93.2% and 90.5%, respectively. Overall, there was significantly higher R0 resection rate for T-ESD (RD = 0.04, 95% CI 0.01–0.06, p = 0.004). Overall heterogeneity was low (I2 = 31). Subgroups analysis of each lesion location showed borderline but not statistically significant improvement in R0 resection rate.
Adverse events
Perforation
The risk of perforation was reported in all 23 studies (Fig. 6). Overall, the rate of perforation was lower using T-ESD (13/1292, 1%) compared to that of C-ESD (45/1290, 3.5%) (RD −0.03, 95% CI −0.04 to −0.01, p < 0.0001, I2 = 0%). After excluding retrospective studies to include only RCT and prospective studies, perforation rate was also lower in T-ESD (10/950, 1.1%) than in C-ESD (36/998, 3.6%) RD −0.03, 95% CI −0.05 to −0.01, p < 0.001, I2 = 0%). After performing sensitivity analysis to include only RCTs, the rate of perforation was still lower using T-ESD (3/641, 0.5%) compared to C-ESD (16/639, 2.5%) (RD −0.02, 95% −0.03 to −0.00, p = 0.01).
Subgroup analysis showed perforation rate was significantly lower in the T-ESD group in both the stomach and colorectum locations (RD −0.02, 95% CI −0.03 to 0.00, p = 0.02, I2 = 0% and RD −0.06, 95% CI −0.10 to −0.01, p = 0.008, I2 = 10%). No difference was seen between the T-ESD group and the C-ESD group for esophageal lesions (RD −0.02, 95% CI −0.05 to 0.00, p = 0.07, I2 = 0%).
Bleeding
All 23 studies reported rates of bleeding, with most combining intraprocedural or immediate bleeding as one bleeding event for ESD (Fig. 7). Overall, there was no difference in bleeding rate between T-ESD and C-ESD, (RD −0.01, 95% CI −0.03 to 0.01, p = 0.20, I2 = 0%). No difference was seen in the subgroup analysis by lesion location.
Publication bias
Egger test detected publication bias for procedure time (p = 0.009) and perforation (p = 0.04). To explore the source of publication bias, subgroup analyses according to type of study and location of ESD were performed. Subgroup analysis within procedure time showed no significant publication bias in the RCT, control or each organ subgroup. For the perforation subgroup analysis, Egger test detected bias in the observational group (p = 0.002), but not the RCT group or in the specific organ subgroup. Since only eight studies were retrospective, Egger test was not performed for this subgroup. No publication bias was detected for R0 resection rate (p = 0.25), en bloc resection rates (p = 0.06) and bleeding (p = 0.17).
Discussion
In this comprehensive meta-analysis involving 2582 lesions, we found that T-ESD results in shorter procedure time, higher R0 resection rates and lower risk of perforation compared with conventional ESD. Specifically, T-ESD was associated with shorter procedure time in the esophagus, stomach and colorectum, decreased perforation risk in the stomach and colorectum, and higher pooled R0 rates, although this was not significant when analyzed by lesion location. There was no difference between the two groups in rates of en bloc resection or bleeding.
Traction allows clear visualization of the submucosal plane; thus, it facilitates safe dissection and avoids inadvertent injury to the muscle layers. Several traction techniques have been introduced such as clip-and-snare traction method, clip-and-snare methods, external forceps method and double scope method. These techniques are technically simple and use inexpensive accessories that are generally available in endoscopy units. Given that traction is simple and effective in improving efficiency and safety of standard ESD, it is an attractive tool in the armamentarium of endoscopists performing ESD.
Improving efficiency of ESD is critical, as its widespread adoption outside East Asia has been hindered by its lengthy and demanding procedure times [3, 5, 6]. Our study showed that T-ESD decreases procedure time in the esophagus, stomach and colorectum. Although our studies show a reduction in length of procedure, the substantial heterogeneity was noted. By adding observational studies, most with moderate to high quality (NOS score > or = 7), we were able to decrease the heterogeneity modestly (I2 = 89–78%). Reasons for the heterogeneity include variable operator experience (trainees vs experts), type of traction technique and differences in definition of procedure start and end times. Nevertheless, improvement of efficiency in performing ESD is appealing and the traction system can potentially promote widespread acceptance and training of ESD.
The most concerning complication of ESD is perforation. Our study showed that T-ESD was associated with lower rate of perforation (1%) compared to conventional ESD (3.5%). Overall low rates of ESD-related perforation in this meta-analysis are likely because most included studies were primarily based in Japan where endoscopists have extensive experience in performing ESD. Rate of perforation appears to be somewhat higher outside Japan and East Asia [24, 44, 45]. In fact, the risk of complications is highest during the early untutored learning curve, which is more common outside East Asia because of the limited availability of ESD training. In a study from France reporting their early experience in adoption of ESD, rates of perforation was 18% [45]. In this current study, publication bias in the perforation analysis was noted in the observational studies, but not in RCTs. In the subgroup analysis of only RCTs, rate of perforation was still lower in the T-ESD group (0.5%) compared to C-ESD group (2.5%). Thus, our results support T-ESD may potentially decrease risk of ESD-related perforation. Given that traction system can enhance safety of ESD, these techniques should be incorporated in the ESD training.
In regards to efficacy, ESD has already shown to be superior to EMR for superficial GI neoplasia, as it facilitates complete histopathologic evaluation with en bloc resection, and lowering rates of local recurrence [3, 5, 6]. In the United States and Europe, piecemeal EMR is commonly performed for removing localized, superficial malignancies but recurrence rate is high [46]. Our meta-analysis showed improved R0 resection rate with traction-assisted ESD. Complete R0 resection rate is crucial in the oncologic setting as it is associated with decreased risk of recurrence, particularly for cancerous lesions [47, 48]. Although lesion location did not show a statistically significant improvement in R0 resection, there is a clear trend favoring traction-assisted ESD in the pooled analysis. This could be due to small number of patients in each subgroup location; thus, the analysis was underpowered to detect small differences between the two groups. Further studies are needed to assess efficacy by location and technique.
A previous meta-analysis by Xia et al. explored the benefits of T-ESD compared to C-ESD using only RCTs [49]. Although results were promising for T-ESD, including shorter procedure time and lower perforation rates, we believe they underestimated the benefits of T-ESD by excluding high quality observational studies. By adding well designed retrospective and prospective cohort, comparative and propensity matched control studies, we effectively tripled the number of participants in the pooled analyses without sacrificing heterogeneity and risk of bias. Overall, our results confirmed T-ESD is more efficient, safer, and at least equally effective when considering its improvement in R0 resection.
Our meta-analysis has some limitations. First, approximately half of the lesions were from observational studies, a quarter being from retrospective studies. Retrospective studies inherently have limitations that can confound the data; however, by conducting subgroup analysis to exclude these, the overall conclusions remained the same for all endpoints. Second, most studies included in this meta-analysis were conducted in East Asia, specifically Japan. It is unknown whether the results of this study are applicable globally since only one study was based in France. However, the traction techniques described in these studies are relatively simple and can be performed using readily available accessories in the endoscopy unit. Thus, it is likely that the traction techniques can be easily learned by all endoscopists. Third, we restricted our searches to the English language. Fourth, the studies included in this analysis used various types of traction method, which could partly explain heterogeneity among the studies; however, it should be noted that majority of the included studies evaluating clip-assisted ESD with modified traction techniques. Lastly, we were unable to assess impacts of traction-assisted ESD stratified by the operator experience (trainee vs expert) since only 6 of the 23 studies included trainee status as a variable. Future studies on impact of traction in learning curve of ESD are needed.
In conclusion, this comprehensive meta-analysis showed traction-assisted ESD improved procedure efficacy, efficiency, and safety when compared to conventional ESD. The endoscopists performing ESD should consider and be familiarized with these techniques, particularly when submucosal plane is not well visualized during dissection. Further prospective trials are needed to compare different type of traction methods and evaluate its role on the learning curve of ESD.
Abbreviations
- OR:
-
Odds ratio
- ESD:
-
Endoscopic submucosal dissection
- GI:
-
Gastrointestinal
- EMR:
-
Endoscopic mucosal resection
- T-ESD:
-
Traction-assisted endoscopic submucosal dissection
- C-ESD:
-
Conventional endoscopic submucosal dissection
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
- RCTs:
-
Randomized controlled trials
- NOS:
-
Newcastle–Ottawa quality assessment scale
- CIs:
-
Confidence intervals
References
Choi K-S, Jung H-Y, Choi KD et al (2011) EMR versus gastrectomy for intramucosal gastric cancer: comparison of long-term outcomes. Gastrointest Endosc 73(5):942–948. https://doi.org/10.1016/j.gie.2010.12.032
Gotoda T, Yamamoto H, Soetikno RM (2006) Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol 41(10):929–942. https://doi.org/10.1007/s00535-006-1954-3
Isomoto H, Shikuwa S, Yamaguchi N et al (2009) Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut 58(3):331–336. https://doi.org/10.1136/gut.2008.165381
Pyo JH, Lee H, Min BH et al (2016) Long-term outcome of endoscopic resection vs. surgery for early gastric cancer: a non-inferiority-matched cohort study. Am J Gastroenterol 111(2):240–249. https://doi.org/10.1038/ajg.2015.427
Yokoi C, Gotoda T, Hamanaka H, Oda I (2006) Endoscopic submucosal dissection allows curative resection of locally recurrent early gastric cancer after prior endoscopic mucosal resection. Gastrointest Endosc 64(2):212–218. https://doi.org/10.1016/j.gie.2005.10.038
Imagawa A, Okada H, Kawahara Y et al (2006) Endoscopic submucosal dissection for early gastric cancer: results and degrees of technical difficulty as well as success. Endoscopy 38(10):987–990. https://doi.org/10.1055/s-2006-944716
Saito Y, Fukuzawa M, Matsuda T, Fukunaga S, Sakamoto T, Uraoka T, Nakajima T, Ikehara H, Fu K, Itoi T, Fujii T (2010) Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc 24(2):343–352. https://doi.org/10.1007/s00464-009-0562-8
Bhatt A, Abe S, Kumaravel A, Vargo J, Saito J (2015) Indications and techniques for endoscopic submucosal dissection. Am J Gastroenterol 110(6):784–791. https://doi.org/10.1038/ajg.2014.425
De Ceglie A, Hassan C, Mangiavillano B et al (2016) Endoscopic mucosal resection and endoscopic submucosal dissection for colorectal lesions: a systematic review. Crit Rev Oncol Hematol 104:138–155
Schlachterman A, Yang D, Goddard A, Gotoda T, Draganov P (2018) Perspectives on endoscopic submucosal dissection training in the United States: a survey analysis. Endosc Int Open 6(4):E399–E409. https://doi.org/10.1055/s-0044-101452
Ma MX, Bourke MJ (2018) Endoscopic submucosal dissection in the West: current status and future directions. Dig Endosc 30(3):310–320
Hosokawa K, Yoshida S (1998) Recent advances in endoscopic mucosal resection for early gastric cancer. Gan To Kagaku Ryoho 25(4):476–483
Maple JT, Dayyeh BKA, Chauhan SS et al (2015) Endoscopic submucosal dissection. Gastrointest Endosc 81(6):1311–1325
Mizutani H, Ono S, Ohki D et al (2017) Recent development of techniques and devices in colorectal endoscopic submucosal dissection. Clin Endosc 50(6):562–568. https://doi.org/10.5946/ce.2017.108
Tsuji K, Yoshida N, Nakanishi H, Takemura K, Yamada S, Doyama H (2016) Recent traction methods for endoscopic submucosal dissection. World J Gastroenterol 22(26):5917–5926. https://doi.org/10.3748/wjg.v22.i26.5917
Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P (2015) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
Reeves BC, Deeks JJ, Higgins JPT, Wells GA (2008) Including non-randomized studies. In: Higgins JPT, Green S (eds) Cochrane handbook for systematic reviews of interventions. Wiley, Chichester, pp 389–432. https://doi.org/10.1002/9780470712184.ch13
Higgins JPT, Green S (eds) (2011) Cochrane handbook for systematic reviews of interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. www.handbook.cochrane.org.
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5(1):13. https://doi.org/10.1186/1471-2288-5-13
Ahn JY, Choi KD, Lee JH et al (2013) Is transnasal endoscope-assisted endoscopic submucosal dissection for gastric neoplasm useful in training beginners? Prospect randomized trial. Surg Endosc 27(4):1158–1165. https://doi.org/10.1007/s00464-012-2567-y
Hashimoto R, Hirasawa D, Iwaki T et al (2018) Usefulness of the S–O clip for gastric endoscopic submucosal dissection (with video). Surg Endosc 32(2):908–914. https://doi.org/10.1007/s00464-017-5765-9
Higuchi K, Tanabe S, Azuma M et al (2013) Double-endoscope endoscopic submucosal dissection for the treatment of early gastric cancer accompanied by an ulcer scar (with video). Gastrointest Endosc 78(2):266–273. https://doi.org/10.1016/j.gie.2013.01.010
Jacques J, Charissoux A, Bordillon P et al (2019) High proficiency of colonic endoscopic submucosal dissection in Europe thanks to countertraction strategy using a double clip and rubber band. Endosc Int Open 7(9):E1166–E1174. https://doi.org/10.1055/a-0965-8531
Koike Y, Hirasawa D, Fujita N et al (2015) Usefulness of thread-traction-method in esophageal endoscopic submucosal dissection: randomised control trial. Gastroenterol Endosc 57(1):66–74
Mori H, Kobara H, Nishiyama N, Fujihara S, Matsunaga T, Masaki T (2017) Novel effective and repeatedly available ring-thread counter traction for safer colorectal endoscopic submucosal dissection. Surg Endosc 31(7):3040–3047. https://doi.org/10.1007/s00464-016-5326-7
Noda H, Ogasawara N, Koshino A et al (2016) Thread-traction with a sheath of polypectomy snare facilitates endoscopic submucosal dissection of early gastric cancers. Gastroenterol Res Pract 2016:9415497. https://doi.org/10.1155/2016/9415497
Okamoto K, Muguruma N, Kitamura S, Kimura T, Takayama T (2012) Endoscopic submucosal dissection for large colorectal tumors using a cross-counter technique and a novel large-diameter balloon overtube. Dig Endosc 24(Suppl 1):96–99. https://doi.org/10.1111/j.1443-1661.2012.01264.x
Okamoto K, Okamura S, Muguruma N et al (2012) Endoscopic submucosal dissection for early gastric cancer using a cross-counter technique. Surg Endosc 26(12):3676–3681. https://doi.org/10.1007/s00464-012-2364-7
Ota M, Nakamura T, Hayashi K et al (2012) Usefulness of clip traction in the early phase of esophageal endoscopic submucosal dissection. Dig Endosc 24(5):315–318. https://doi.org/10.1111/j.1443-1661.2012.01286.x
Ritsuno H, Sakamoto N, Osada T et al (2014) Prospective clinical trial of traction device-assisted endoscopic submucosal dissection of large superficial colorectal tumors using the S–O clip. Surg Endosc 28(11):3143–3149. https://doi.org/10.1007/s00464-014-3572-0
Sohda M, Kuriyama K, Yoshida T et al (2020) Comparable data between double endoscopic intraluminal operation and conventional endoscopic submucosal dissection for esophageal cancer. J Gastrointest Surg 24(2):307–312. https://doi.org/10.1007/s11605-019-04137-9
Suzuki S, Gotoda T, Kobayashi Y et al (2016) Usefulness of a traction method using dental floss and a hemoclip for gastric endoscopic submucosal dissection: a propensity score matching analysis (with videos). Gastrointest Endosc 83(2):337–346. https://doi.org/10.1016/j.gie.2015.07.014
Uraoka T, Ishikawa S, Kato J et al (2010) Advantages of using thin endoscope-assisted endoscopic submucosal dissection technique for large colorectal tumors. Dig Endosc 22(3):186–191. https://doi.org/10.1111/j.1443-1661.2010.00992.x
Wang F, Leng X, Gao Y et al (2019) Endoscopic submucosal dissection of distal intestinal tumors using grasping forceps for traction. Tech Coloproctol 23(11):1079–1083. https://doi.org/10.1007/s10151-019-02102-x
Xie X, Bai JY, Fan CQ et al (2017) Application of clip traction in endoscopic submucosal dissection to the treatment of early esophageal carcinoma and precancerous lesions. Surg Endosc 31(1):462–468. https://doi.org/10.1007/s00464-016-4939-1
Yamada S, Doyama H, Ota R et al (2016) Impact of the clip and snare method using the prelooping technique for colorectal endoscopic submucosal dissection. Endoscopy 48(3):281–285. https://doi.org/10.1055/s-0034-1393241
Yamasaki Y, Takeuchi Y, Uedo N et al (2018) Efficacy of traction-assisted colorectal endoscopic submucosal dissection using a clip-and-thread technique: a prospective randomized study. Dig Endosc 30(4):467–476. https://doi.org/10.1111/den.13036
Yoshida M, Takizawa K, Nonaka S et al (2020) Conventional versus traction-assisted endoscopic submucosal dissection for large esophageal cancers: a multicenter, randomized controlled trial (with video). Gastrointest Endosc 91(1):55-65.e2. https://doi.org/10.1016/j.gie.2019.08.014
Yoshida M, Takizawa K, Ono H et al (2016) Efficacy of endoscopic submucosal dissection with dental floss clip traction for gastric epithelial neoplasia: a pilot study (with video). Surg Endosc 30(7):3100–3106. https://doi.org/10.1007/s00464-015-4580-4
Yoshida M, Takizawa K, Suzuki S et al (2018) Conventional versus traction-assisted endoscopic submucosal dissection for gastric neoplasms: a multicenter, randomized controlled trial (with video). Gastrointest Endosc 87(5):1231–1240. https://doi.org/10.1016/j.gie.2017.11.031
Yoshida N, Doyama H, Ota R et al (2016) Effectiveness of clip-and-snare method using pre-looping technique for gastric endoscopic submucosal dissection. World J Gastrointest Endosc 8(12):451–457. https://doi.org/10.4253/wjge.v8.i12.451
Ban H, Sugimoto M, Otsuka T et al (2018) Usefulness of the clip-flap method of endoscopic submucosal dissection: a randomized controlled trial. World J Gastroenterol 24(35):4077–4085. https://doi.org/10.3748/wjg.v24.i35.4077
Ngamruengphong S, Ferri L, Aihara H et al (2020) Efficacy of endoscopic submucosal dissection for superficial gastric neoplasia in a large cohort in North America. Clin Gastroenterol Hepatol. https://doi.org/10.1016/j.cgh.2020.06.023
Farhat S, Chaussade S, Ponchon T et al (2011) Endoscopic submucosal dissection in a European setting. A multi-institutional report of a technique in development. Endoscopy 43(08):664–670. https://doi.org/10.1055/s-0030-1256413
Moss A, Williams SJ, Hourigan LF et al (2015) Long-term adenoma recurrence following wide-field endoscopic mucosal resection (WF-EMR) for advanced colonic mucosal neoplasia is infrequent: results and risk factors in 1000 cases from the Australian Colonic EMR (ACE) study. Gut 64(1):57–65
Fuccio L, Bhandari P, Maselli R et al (2018) Ten quality indicators for endoscopic submucosal dissection: what should be monitored and reported to improve quality. Ann Transl Med 6(13):5
Raja S, Rice TW, Goldblum JR et al (2011) Esophageal submucosa: the watershed for esophageal cancer. J Thorac Cardiovasc Surg 142(6):1403-1411.e1
Xia M, Zhou Y, Yu J, Chen W, Huang X, Liao J (2019) Short-term outcomes of traction-assisted versus conventional endoscopic submucosal dissection for superficial gastrointestinal neoplasms: a systematic review and meta-analysis of randomized controlled studies. World J Surg Oncol 17(1):1–10
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Saowanee Ngamruengphong is a consultant for Boston Scientific. Chawin Lopimpisuth, Malorie Simons, Venkata S. Akshintala, Klaorat Prasongdee and Julie Nanavati have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lopimpisuth, C., Simons, M., Akshintala, V.S. et al. Traction-assisted endoscopic submucosal dissection reduces procedure time and risk of serious adverse events: a systematic review and meta-analysis. Surg Endosc 36, 1775–1788 (2022). https://doi.org/10.1007/s00464-021-08452-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-021-08452-8