Abstract
Background
The effectiveness of subcostal transversus abdominis plane block (TAPB) in laparoscopic gastric cancer surgery is unknown. We aimed to investigate its opioid-sparing and pain-relief effects in laparoscopic gastrectomy for gastric cancer.
Method
One hundred and twelve patients undergoing elective laparoscopic gastrectomy were randomised to the TAPB or control group. The TAPB group received ultrasound-guided bilateral subcostal TAPB at the end of surgery, while the control group did not. We investigated fentanyl consumption administered via intravenous patient-controlled analgesia and as a rescue analgesic, the numeric rating scale (NRS) pain scores at rest and during coughing, and the opioid-related side effects at 6, 12, 24, and 48 h postoperatively. The primary outcome was cumulative fentanyl consumption at 24 h postoperatively.
Results
The study included 53 patients in each group. The cumulative fentanyl consumption 24 h postoperatively was significantly lower in the TAPB group than in the control group (median difference -170 mcg, P = 0.03, 95% CI -360 to -15 mcg). Subcostal TAPB also significantly reduced the resting NRS score at 48 h postoperatively (median difference -1, 95% CI -1 to 0, P = 0.01) and coughing NRS score at all time points (all median difference -1, 95% CI -2 to 0, P < 0.01, P = 0.02, 0.01, and 0.01, respectively). However, it did not reduce the occurrence of opioid-related side effects, except the use of antiemetics during the first 6 h postoperatively (TAPB, 1.9% vs. Control, 15.1%, P = 0.03).
Conclusion
Ultrasound-guided bilateral subcostal TAPB provides efficient postoperative analgesia with an opioid-sparing effect after laparoscopic gastrectomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Gastric cancer is the fifth most frequently diagnosed cancer and the third leading cause of cancer death in the world [1]. The only curative treatment for gastric cancer is surgical resection [2], and recently, laparoscopic gastrectomy has become the standard treatment for early and locally advanced gastric cancer [3,4,5].
Opioid-sparing multimodal analgesia is the current standard method for postoperative pain management. Among the various regional techniques, subcostal transversus abdominis plane block (TAPB) provides analgesic coverage to the upper anterior-lateral abdominal wall and is currently a popular analgesic technique for upper abdominal surgery [6]. Considering the area and duration of the cutaneous sensory blockade of subcostal TAPB [7], subcostal TAPB could be a useful opioid-sparing analgesia in laparoscopic gastrectomy for gastric cancer.
However, to the best of our knowledge, there are no reports on the effects of subcostal TAPB in laparoscopic gastrectomy for gastric cancer. Since TAPB is strongly recommended for laparoscopic colorectal surgery in the recently updated Enhanced Recovery After Surgery (ERAS) guidelines [8], subcostal TAPB may play an important role in ERAS protocol-guided laparoscopic gastrectomy. We hypothesised that subcostal TAPB would reduce opioid requirement and postoperative pain in patients undergoing laparoscopic gastrectomy for gastric cancer. Therefore, we conducted a randomised, controlled, double-blinded study to elucidate the postoperative opioid-sparing and pain relief effects of subcostal TAPB in laparoscopic gastric cancer surgery patients before establishing an in-hospital ERAS protocol.
Materials and methods
This prospective randomised study was approved by the Institutional Review Board of the Seoul National University Hospital (No. H-1909-076-1065) and registered with ClinicalTrials.gov (NCT04138901, 24 October 2019). All participants provided written informed consent before the study. We designed and reported the study findings following the Consolidated Standards of Reporting Trials recommendations [9].
All patients aged between 18 and 80 years who were scheduled for elective laparoscopic gastrectomy for gastric cancer were screened for eligibility. Exclusion criteria were: 1) American Society of Anesthesiologists (ASA) physical status greater than III, 2) chronic opioid use, 3) history of abdominal surgery, 4) allergic to local anaesthetics, fentanyl, and nefopam, 5) wound infiltration analgesia, 6) infection or anatomical abnormalities at the injection site, 7) poorly controlled psychiatric disorders that could interfere with the interpretation of the outcome assessments, 8) pregnancy or lactation, and 9) inability to understand the provided information regarding the study protocol and grant informed consent. Before surgery, we educated the participating patients on intravenous patient-controlled analgesia (IV-PCA) and the method of pain assessment.
Randomisation and blinding
After enrolment, patients were randomly assigned to two groups (subcostal TAPB or control group) according to the stratified block randomisation. Stratification was conducted to reduce the bias of the mini-laparotomy site, the largest incision site, which can compromise the effect of subcostal TAPB in epigastric and lower abdominal incisions (supra- or infra-umbilical, right or left lower quadrant incision) by a ratio of approximately 1:3, based on its recent surgical volume at our institution. Then, randomisation was performed in a 1:1 allocation ratio in mixed-block sizes of 4 and 6 by an anaesthesiologist who was not involved in the study. The results of randomisation were sealed in an envelope and delivered to the researchers on the day of surgery.
The sealed envelopes were opened by the researchers (S.Y. and H-J.L.), who performed subcostal TAPB at the end of surgery. In the TAPB group, a 21-gauge 100 mm needle (Echoplex plus, Vygon, Ecouen, France) was inserted from the bilateral ends of the rectus abdominis muscles using ultrasound guidance (Vivid I, GE Healthcare, Marlborough, MA, USA) with a linear 6–13 MHz transducer. Fifteen millilitres of 0.375% ropivacaine (Ropiva Injection, Hanlim Pharm Co., Ltd, Seoul, Korea) was injected, on each side, from the medial end of the internal oblique abdominis muscle in the outward direction. We did not perform the aforementioned intervention in the control group because TAPB was performed under general anaesthesia. To ensure that the evaluator and patients remained blinded to the group assignment, adhesive foam dressings were attached to include the needle insertion site or the expected insertion site in both groups during the study period.
Anaesthesia and surgical procedure
All procedures, other than the TAPB, were performed equally between the two groups. Anaesthesia was induced using propofol, remifentanil, and rocuronium, and subsequently maintained by desflurane, remifentanil, and rocuronium. During the induction, palonosetron (0.075 mg) was administered to all patients. Additionally, 5 mg of dexamethasone was administered to patients with an Apfel score ≥ 2 to prevent postoperative nausea and vomiting (PONV) [10]. Skin incisions for trocar insertion were performed at five sites (Fig. 1): 12 mm at the periumbilical and both the left and right paraumbilical areas as well as 5 mm at both the left and right costal margins. An additional 5 cm mini-laparotomy was performed according to the surgical methods based on the surgeon's preference: epigastric incision for anastomosis during the laparoscopic-assisted approach and periumbilical or lower quadrant incision for specimen delivery during the totally laparoscopic approach (Fig. 1). After the bowel anastomosis was completed, 20 mg nefopam with 100 mL normal saline was injected intravenously over 30 min. After skin closure, 50 µg fentanyl was administered intravenously as the loading dose of IV-PCA. After reversal of the neuromuscular blockade with 2–4 mg/kg sugammadex, the patients were extubated, and transferred to the post-anaesthesia care unit (PACU) where they remained for at least 30 min.
Postoperative pain management
In the PACU, patients were able to use IV-PCA via an electronic infusion pump with a bolus dose of 1 mL (fentanyl, 20 mcg), a lock-out interval of 10 min, and no continuous infusion. If the patient had a numeric rating scale (NRS) pain score of ≥ 5, 50 µg of IV fentanyl was used as the first-line rescue analgesic. If patients had PONV, 30 mg of IV ketorolac tromethamine was used as an alternative rescue analgesic. Rescue antiemetics were administered upon complaint of moderate to severe PONV, as follows: (1) metoclopramide (10 mg) in the PACU and (2) 0.3 mg ramosetron as an initial rescue drug and 10 mg metoclopramide as a second rescue drug in the ward. The decision to administer the rescue analgesics and antiemetics in the PACU and ward was taken by physicians who were blinded to the groups.
Outcome measures
Demographic and intraoperative characteristics were recorded, including age, sex, body mass index, ASA physical status, the Physiological and Operative Severity Score for the enUmeration of Mortality and Morbidity, Apfel score, history of chronic pain, operation type, surgeon, duration of anaesthesia/surgery, and intraoperative remifentanil consumption.
The primary outcome was the cumulative fentanyl consumption for the first 24 h after surgery. Fentanyl consumption was defined as the sum of the dose administered via PCA and that administered as a rescue analgesic after arrival at the PACU. The secondary outcomes were cumulative fentanyl consumption for the first 6, 12, and 48 h postoperatively; interval fentanyl consumption between time points; pain intensity using an 11-point NRS at rest and during coughing at 6, 12, 24, and 48 h postoperatively; and occurrence of opioid-related side effects (nausea, vomiting, dizziness, somnolence, and respiratory depression) at 6, 12, 24, and 48 h, postoperatively. We also investigated the administration of rescue analgesics other than fentanyl and rescue antiemetics, postoperative shoulder pain, and complications related to TAPB (abdominal wall haematoma, visceral wall injury, and local anaesthetic systemic toxicity). All these outcomes were evaluated by a physician who was not involved in this study and was blinded to the group assignment. Data, including the time to first flatus (h), the occurrence of significant surgical complications according to the Clavien–Dindo classification within 7 days postoperatively [11], and the length of hospital stay, were extracted retrospectively from electronic medical records.
Statistical analysis
The sample size calculation was performed before the study with a normal distribution assumption using the G*Power software version 3.1.9 (G*Power, Düsseldorf, Germany). It was based on our acute pain service team’s database, where the mean (standard deviation [SD]) total fentanyl consumption was 800 (450) mcg for the first 24 h postoperatively in laparoscopic gastrectomy patients. Considering that the subcostal TAPB could only cover somatic/incisional pain after the laparoscopic gastrectomy, we hypothesised that the subcostal TAPB could reduce the postoperative opioid consumption by 30% during the first 24 h postoperatively. With the Mann-Whitney U test, a sample size of 45 in each group was required to achieve 80% power to detect a 30% reduction in total fentanyl consumption for the first 24 h after surgery between the two groups, with a two-sided alpha of 0.05. Considering a 20% dropout rate, we aimed to recruit 56 patients per group.
The normal distribution of continuous variables was determined using the Shapiro–Wilk test. The continuous data are reported as medians (interquartile range) and were compared between the two groups using the Mann–Whitney U test. Categorical data are described as frequencies or percentages and were compared between the two groups using the chi-square test or Fisher’s exact test, according to their expected counts. The median differences and 95% confidence intervals (CI), between the two groups, were calculated using the Hodges–Lehmann method. In addition, since it was difficult to convert the administered dose of ketorolac to fentanyl consumption, we performed a subgroup analysis on the difference in the cumulative/interval fentanyl consumption between the two groups, except for patients with postoperative ketorolac use.
Statistical analyses and randomisation were performed using R software, version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria). All the statistical tests of hypotheses were two-sided, and P < 0.05 was considered statistically significant.
Results
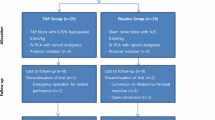
From November 2019 to May 2020, a total of 263 patients were assessed for their eligibility and 112 patients were randomly allocated to the TAPB or control group (Fig. 2). Three patients from each group were excluded because of protocol violations. Two patients in the TAPB group were excluded as they were enrolled in another study, which was identified on the day of surgery. One patient from each group was excluded because of postoperative dexmedetomidine infusion in the intensive care unit. One patient in the control group was excluded because of history of abdominal surgery, which was identified on the day of surgery, and another patient in the control group was excluded because of refusal of IV-PCA on the day of surgery. In total, the data of 106 patients were included in the final analysis. There was no significant difference in the baseline characteristics between the two groups (Table 1).
Figure 3 and Supplemental Table S1 show the comparisons of the cumulative and interval fentanyl consumptions between the two groups. The TAPB group showed significantly lower cumulative fentanyl consumption at 6, 12, and 24 h postoperatively (6 h: median difference -70 mcg, P = 0.01, 95% CI -120 to -14 mcg; 12 h: median difference -135 mcg, P = 0.01, 95% CI -225 to -40 mcg; and 24 h: median difference -170 mcg, P = 0.03, 95% CI -360 to -15 mcg). The interval fentanyl consumption was significantly lower in the TAPB group during the first 6 h (median difference -70 mcg, P = 0.01, 95% CI − 120 to -14 mcg) and 6–12 h postoperatively (median difference -60 mcg, P = 0.02, 95% CI − 120 to 0 mcg). In the subgroup analysis, the cumulative fentanyl consumption at 48 h postoperatively was also significantly lower in the TAPB group than in the control group (P = 0.03, median difference -270 mcg, 95% CI − 570 to − 20 mcg) (Supplemental Table S2). Postoperative shoulder pain occurred in six patients (11.3%) in the TAPB group and in five patients (9.4%) in the control group.
Comparisons of the cumulative (A) and interval (B) fentanyl consumptions between the two groups. Box plot shows median and interquartile range of fentanyl consumptions in transverse abdominis plane block (TAPB) group and control group during postoperative 48 h. Upper and lower whiskers are maximum and minimum values excluding outliers, respectively. Round symbols show the outliers. Scatter plot (diamond symbols) shows the individual data points
Figure 4 and Supplemental Table S3 show the comparisons of the pain scores at rest and during coughing between the two groups. The TAPB group showed a significantly lower pain score at rest only at 48 h postoperatively (median difference − 1, P = 0.01, 95% CI − 1 to 0). The TAPB group showed a significantly lower pain score during coughing at all time points (6 h: median difference − 1, P < 0.01, 95% CI − 2 to 0; 12 h: median difference − 1, P = 0.02, 95% CI − 2 to 0; 24 h: median difference − 1, P = 0.01, 95% CI − 2 to 0; and 48 h: median difference − 1, P = 0.01, 95% CI − 2 to 0). *P < 0.05.
Comparisons of the pain score at rest (A) and coughing (B) between the two groups. Box plot shows median and interquartile range of numeric rating scale (NRS) in transverse abdominis plane block (TAPB) group and control group during postoperative 48 h. Upper and lower whiskers are maximum and minimum values excluding outliers, respectively. Round symbols show the outliers. Scatter plot (diamond symbols) shows the individual data points
Table 2 shows the comparison of the occurrence of opioid-related side effects, antiemetic use, time to first flatus, occurrence of surgical complications, and the length of hospital stay between the two groups. Antiemetic use within 6 h postoperatively was significantly lower in the TAPB group than in the control group (P = 0.03). There were no complications related directly to subcostal TAPB (abdominal wall haematoma, visceral wall injury, or local anaesthetic systemic toxicity) in the TAPB group. *P < 0.05.
Discussion
Our study investigated the analgesic and opioid-sparing effects of bilateral subcostal TAPB in gastric cancer patients undergoing laparoscopic gastrectomy during the first 48 h postoperatively. Bilateral subcostal TABP significantly reduced postoperative fentanyl consumption during the first 24 h in these patients. It significantly reduced postoperative pain intensity, especially during coughing. Although bilateral subcostal TAPB did not significantly decrease the occurrence of opioid-related side effects, it decreased the antiemetic requirement within 6 h postoperatively. Our results provide valuable information regarding the usefulness of subcostal TAPB in laparoscopic gastrectomy for gastric cancer.
In the guidelines for ERAS after gastrectomy, subcostal TAPB was not highly recommended given the lack of evidence at that time [12]. Thereafter, its effects on open gastrectomy were reported (Supplemental Table S4) [13,14,15,16]. However, postoperative pain after laparoscopic surgery accounts for a smaller portion of somatic pain, which can be reduced by subcostal TAPB, than that after open surgery. Therefore, we considered that it would be difficult to apply the previous results from open gastrectomy to laparoscopic gastrectomy. Since there was lack of evidence for subcostal TAPB in laparoscopic gastric cancer surgery, it might have not been widely used in the ERAS protocol of laparoscopic gastric cancer surgery [17,18,19], despite its promising benefits in various abdominal surgeries [6]. In recent ERAS studies, thoracic epidural analgesia (TEA) was adopted for postoperative pain management for laparoscopic gastrectomy [17, 18]. However, with its relatively common complications, such as hypotension and urinary retention, and rare but serious complications, and the expansion of minimally invasive surgical techniques, the role of TEA in postoperative management is now reduced, whereas less invasive regional analgesic techniques such as TAPB have increased [20].
Subcostal TAPB demonstrated an opioid-sparing effect after laparoscopic gastrectomy during the first 24 h postoperatively. This effect was noticeable within 12 h postoperatively, which was an expected result considering the half-life of ropivacaine [7]. This opioid-sparing effect of TAPB would have led to a significant decrease in antiemetic administration, representing moderate-to-severe PONV in the early postoperative period. In addition, subcostal TAPB significantly reduced pain severity up to 48 h postoperatively. The analgesic effect of subcostal TAPB could have continued beyond the local anaesthetic’s duration of nerve blockade because of its longer-acting anti-inflammatory effect [21]. The inflammatory response to surgical stress could contribute to acute postoperative pain, which has been reported to be reduced by subcostal TAPB [14, 22]. Further, our finding, in which subcostal TAPB significantly reduced pain during coughing rather than at rest, is consistent with the findings of a recently published meta-analysis regarding the effect of TAPB in laparoscopic colorectal surgery [23]. This may be attributed to the subcostal TAPB effect on somatic pain that is exacerbated by coughing or movement. Movement-evoked pain, such as pain during coughing, was significantly associated with postoperative pulmonary function and showed a stronger association with the postoperative quality of recovery than with pain at rest [24, 25]. Therefore, we expect subcostal TAPB to be useful in improving postoperative recovery.
In this study, we only used systemic opioid analgesia, namely, IV-PCA, in the control group for the following reasons. First, before the study, there was only fentanyl-based IV-PCA in our routine postoperative pain protocol for these patients. Second, we hypothesised that the co-administration of non-opioid analgesics could mask the analgesic effect of subcostal TAPB [26, 27]. However, since it is difficult to control immediate postoperative pain with systemic opioids alone, we administered intravenous nefopam intraoperatively in both groups. Since the opioid-sparing effect of intraoperative nefopam is usually reported within 6 h after surgery [28], the opioid-sparing effect of the subcostal TAPB is also expected to be significant under multimodal analgesia. Further studies on the effects of subcostal TAPB under multimodal analgesia are required.
Our study has several limitations. First, the ultimate purpose of postoperative pain management is to improve the quality of recovery after surgery. Although we investigated the postoperative pain intensity and opioid-related side effects that could affect the overall quality of recovery, these parameters may not be sufficient to assess the multi-dimensional quality of recovery. Second, preoperative dexamethasone was not administered in patients with a low risk of PONV, and we administered it at a fixed dose without considering the patient's weight. Preoperative dexamethasone has been reported to affect postoperative pain and PONV, improving overall postoperative recovery [29]. However, since there was no significant difference in the proportion of dexamethasone use or patient weight between the two groups, its effect on the primary outcome may be insignificant. Third, we similarly conducted subcostal TAPB for all TAPB group patients; however, the effect may vary depending on the mini-laparotomy site. Nonetheless, we performed stratified randomisation according to the mini-laparotomy site to minimise its confounding effect.
In conclusion, ultrasound-guided bilateral subcostal TAPB provides efficient postoperative analgesia with an opioid-sparing effect in gastric cancer patients undergoing laparoscopic gastrectomy. Subcostal TAPB is a safe analgesic method, without serious complications, and is a useful option for multimodal analgesia in these patients.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H (2016) Gastric cancer. Lancet 388:2654–2664
Kim W, Kim HH, Han SU, Kim MC, Hyung WJ, Ryu SW, Cho GS, Kim CY, Yang HK, Park DJ, Song KY, Lee SI, Ryu SY, Lee JH, Lee HJ (2016) Decreased morbidity of laparoscopic distal gastrectomy compared with open distal gastrectomy for stage I gastric cancer: Short-term Outcomes From a Multicenter Randomized Controlled Trial (KLASS-01). Ann Surg 263:28–35
Lee HJ, Hyung WJ, Yang HK, Han SU, Park YK, An JY, Kim W, Kim HI, Kim HH, Ryu SW, Hur H, Kong SH, Cho GS, Kim JJ, Park DJ, Ryu KW, Kim YW, Kim JW, Lee JH, Kim MC (2019) Short-term outcomes of a multicenter randomized controlled trial comparing laparoscopic distal gastrectomy with D2 lymphadenectomy to open distal gastrectomy for locally advanced gastric cancer (KLASS-02-RCT). Ann Surg 270:983–991
Kim HH, Han SU, Kim MC, Kim W, Lee HJ, Ryu SW, Cho GS, Kim CY, Yang HK, Park DJ, Song KY, Lee SI, Ryu SY, Lee JH, Hyung WJ (2019) Effect of laparoscopic distal gastrectomy vs open distal gastrectomy on long-term survival among patients with stage I gastric cancer: the KLASS-01 Randomized Clinical Trial. JAMA Oncol 5:506–513
Tran DQ, Bravo D, Leurcharusmee P, Neal JM (2019) Transversus abdominis plane block A narrative review. Anesthesiology 131:1166–1190
Chen Y, Shi K, Xia Y, Zhang X, Papadimos TJ, Xu X, Wang Q (2018) Sensory assessment and regression rate of bilateral oblique subcostal transversus abdominis plane block in volunteers. Reg Anesth Pain Med 43:174–179
Gustafsson UO, Scott MJ, Hubner M, Nygren J, Demartines N, Francis N, Rockal TA, Young-Fadok TM, Soop M, De Boer HD, Urman RD, Chang GJ, Fichera A, Kessler H, Grass F, Whang EE, Fawcett WJ, Carli F, Lobo DN, Rollins KE, Balfour A, Baldini G, Riedel B, Ljungqvist O (2019) Guidelines for perioperative care in elective colorectal surgery: Enhanced Recovery After Surgery (ERAS®) society recommendations: 2018. World J Surg 43:659–959
Schulz KF, Altman DG, Moher D (2010) CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Trials. https://doi.org/10.1186/1745-6215-11-32
Gan TJ, Diemunsch P, Habib AS, Kovac A, Kranke P, Meyer T, Watcha M, Chung F, Angus S, Apfel C, Bergese S, Candiotti K, Chan M, Davis P, Hooper V, Lagoo-Deenadayalan S, Myles P, Nezat G, Philip B, Tramèr M (2014) Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg 118:85–113
Clavien PA, Barkun J, De Oliveira ML, Vauthey JN, Dindo D, Schulick R, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron J, Makuuchi M (2009) The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250:187–196
Mortensen K, Nilsson M, Slim K, Schäfer M, Mariette C, Braga M, Carli F, Demartines N, Griffin SM, Lassen K (2014) Consensus guidelines for enhanced recovery after gastrectomy. Br J Surg 101:1209–1229
Wu Y, Liu F, Tang H, Wang Q, Chen L, Wu H, Zhang X, Miao J, Zhu M, Hu C, Goldsworthy M, You JM, Xu X (2013) The analgesic efficacy of subcostal transversus abdominis plane block compared with thoracic epidural analgesia and intravenous opioid analgesia after radical gastrectomy. Anesth Analg 117:507–513
Liu R, Qin H, Wang M, Li K, Zhao G (2019) Transversus abdominis plane block with general anesthesia blunts the perioperative stress response in patients undergoing radical gastrectomy. BMC Anesthesiol. https://doi.org/10.1186/s12871-019-0861-0,November7
Ding W, Li W, Zeng X, Li J, Jiang J, Guo C, Li W (2017) Effect of Adding Dexmedetomidine to Ropivacaine on Ultrasound-Guided Dual Transversus Abdominis Plane Block after Gastrectomy. J Gastrointest Surg 21:936–946
Li K, Li L, Gao M, Zhu Z, Chen P, Yang L, Zhao G (2015) Application of ultrasound-guided subcostal transversus abdominis plane block in gastric cancer patients undergoing open gastrectomy. Int J Clin Exp Med 8:13976–21382
Kang SH, Lee Y, Min S-H, Park YS, Ahn S-H, Park DJ, Kim H-H (2018) Multimodal enhanced recovery after surgery (ERAS) program is the optimal perioperative care in patients undergoing totally laparoscopic distal gastrectomy for gastric cancer: a prospective, randomized, clinical trial. Ann Surg Oncol 25:3231–3238
Aoyama T, Yoshikawa T, Sato T, Hayashi T, Yamada T, Ogata T, Cho H (2019) Equivalent feasibility and safety of perioperative care by ERAS in open and laparoscopy-assisted distal gastrectomy for gastric cancer: a single-institution ancillary study using the patient cohort enrolled in the JCOG0912 phase III trial. Gastric Cancer 22:617–623
Roh CK, Son SY, Lee SY, Hur H, Han SU (2020) Clinical pathway for enhanced recovery after surgery for gastric cancer: a prospective single-center phase II clinical trial for safety and efficacy. J Surg Oncol 121:662–669
Rawal N (2016) Current issues in postoperative pain management. Eur J Anaesthesiol 33:160–171
Hollmann MW, Durieux ME (2000) Local anesthetics and the inflammatory response: a new therapeutic indication? Anesthesiology 93:858–875
Zhang JM, An J (2007) Cytokines, inflammation, and pain. Int Anesthesiol Clin 45:27–37
Oh TK, Lee SJ, Do SH, Song IA (2018) Transversus abdominis plane block using a short-acting local anesthetic for postoperative pain after laparoscopic colorectal surgery: a systematic review and meta-analysis. Surg Endosc 32:545–552
Gilron I, Tod D, Goldstein DH, Parlow JL, Orr E (2002) The relationship between movement-evoked versus spontaneous pain and peak expiratory flow after abdominal hysterectomy. Anesth Analg 95:1702–1707
Wu CL, Rowlingson AJ, Partin AW, Kalish MA, Courpas GE, Walsh PC, Fleisher LA (2005) Correlation of postoperative pain to quality of recovery in the immediate postoperative period. Reg Anesth Pain Med 30:516–522
Torup H, Hansen EG, Bøgeskov M, Rosenberg J, Mitchell AU, Petersen P, Mathiesen O, Dahl J, Møller AM (2016) Transversus abdominis plane block after laparoscopic colonic resection in cancer patients: a randomised clinical trial. Eur J Anaesthesiol 33:725–730
Oh TK (2017) Should the transversus abdominis plane block be performed for laparoscopic colorectal surgery? Eur J Anaesthesiol 34:631
Na H-S, Oh A-Y, Ryu J-H, Koo B-W, Nam S-W, Jo J, Park J-H (2018) Intraoperative nefopam reduces acute postoperative pain after laparoscopic gastrectomy: a prospective, randomized study. J Gastrointest Surg 22:771–777
Murphy GS, Szokol JW, Greenberg SB, Avram MJ, Vender JS, Nisman M, Vaughn J (2011) Preoperative dexamethasone enhances quality of recovery after laparoscopic cholecystectomyeffect on in-hospital and postdischarge recovery outcomes. Anesthesiology 114:882–890
Acknowledgments
We would like to thank Editage (www.editage.co.kr) for English language editing.
Funding
The authors have no sources of funding to declare for this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Susie Yoon, Gyu Young Song, Jihye Lee, Ho-Jin Lee, Seong-Ho Kong, Won Ho Kim, Do Joong Park, Hyuk-Joon Lee, and Han-Kwang Yang have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yoon, S., Song, G.Y., Lee, J. et al. Ultrasound-guided bilateral subcostal transversus abdominis plane block in gastric cancer patients undergoing laparoscopic gastrectomy: a randomised-controlled double-blinded study. Surg Endosc 36, 1044–1052 (2022). https://doi.org/10.1007/s00464-021-08370-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-021-08370-9