Abstract
Objectives
Transversus abdominis plane (TAP) block is an analgesic technique. Adding dexmedetomidine can enhance regional anesthesia. This study’s aim was to evaluate whether dexmedetomidine prolonged analgesic time of TAP block after gastrectomy.
Methods
Patients scheduled for gastrectomy were randomly assigned to receive a TAP block with saline (group S), ropivacaine (group R), or ropivacaine and dexmedetomidine (group RD). Visual analogue scale (VAS) scores, postoperative nausea and vomiting (PONV) scores, sedation scores, tramadol consumption, ropivacaine concentration, and Quality of Recovery Questionnaire 40 (QoR-40) were recorded.
Results
Patients in group R and group RD had lower VAS scores 2, 4, 12, and 24 h after surgery compared with group S (P < 0.05). PONV scores were lower in group R and group RD compared with group S after 2, 12, 24, and 36 h (P < 0.05). Patients in group R and group RD required less tramadol and had better QoR-40 scores than those in group S (P < 0.05). The aforementioned variables and ropivacaine concentrations did not differ between group R and group RD (P > 0.05). Sedation scores were similar between three groups (P > 0.05).
Conclusions
TAP block can provide analgesia and improve the quality of recovery. Adding dexmedetomidine does not significantly improve the quality or duration of TAP block.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastrectomy can cause severe postoperative pain. Epidural analgesia is often used to relieve pain after gastrectomy. However, epidural analgesia may contribute to hypotension 1. In some cases, epidural anesthesia may be contraindicated, for example because of coagulopathy and sepsis 2. Patient-controlled intravenous analgesia (PCIA) has been widely used as an effective method of relieving pain 3. However, the efficacy of this method is limited by side effects 4, 5. The analgesia and side effects of opioids are dose dependent; therefore, alternative treatments that can reduce the dose of intravenous opioids or avoid central neuraxial blockade are required to manage pain after gastrectomy. In recent years, there has been growing interest in the use of peripheral nerve blocks, such as the transversus abdominis plane (TAP) block 6. The TAP block blocks nerves of the abdominal wall; however, the posterior TAP block can be used for surgery involving the lower abdominal wall 7 and the subcostal TAP block is designed to include analgesia of the upper abdominal wall 8. A dual TAP block which involves separate injection of both the posterior TAP block and the subcostal TAP block can anesthetize the entire abdominal wall, 9 and the effect of the dual TAP block has been demonstrated in healthy volunteers and patients 9, 10. Unfortunately, single-injection TAP block usually provides up to 24 h of effective abdominal wall analgesia, but pain is not covered by this technique. Efforts have been made to identify adjuvants that can prolong the duration and improve the quality of TAP blocks, with fewer side effects. Dexmedetomidine is an agonist of alpha-2 adrenergic receptors, which can inhibit norepinephrine release 11 by blocking hyperpolarization-activated cation current 12 and compound action potential 13. It has previously been shown to prolong the duration of analgesia when combined with local anesthetics in various regional blocks 14–16.
For a TAP block, a large dose of local anesthetic is administered into the plane. Recent evidence suggests that administration of large doses of ropivacaine in a TAP block is potentially toxic 17. Corvetto et al. 18 showed that adding epinephrine to levobupivacaine reduced its peak plasma concentration after a TAP block. However, the effect of dexmedetomidine on the plasma concentration of ropivacaine has not been demonstrated in a TAP block.
We hypothesized that the combined use of ropivacaine and dexmedetomidine in a TAP block would improve visual analogue scale (VAS) scores (primary outcome objective), sedation scores, Quality of Recovery Questionnaire 40 (QoR-40) scores, analgesic consumption, and postoperative nausea and vomiting (PONV) scores. In addition, we identified whether dexmedetomidine affected the concentration of ropivacaine in plasma.
Materials and Methods
This study was a prospective, randomized, double-blind, placebo-controlled trial. Study approval was obtained from the Ethics Committee of Harbin Medical University (HUMURB20140018). Clinical trial registration for this study can be found at www.chictr.org.cn (ChiCTR-TRC-14004930).
A total of 98 patients scheduled for elective gastrectomy with midline vertical abdominal incision under general anesthesia between July 25, 2014 and February 28, 2015 were enrolled in this study. Exclusion criteria included ≤18 or ≥70 years old, American Society of Anesthesiologists physical status ≥III, body mass index >40 kg/m2 or <50 kg body weight, allergy to any of the medications used in the study, chronic use of analgesics, coagulopathy, chronic hepatic or renal dysfunction, and mental illnesses.
Participants were randomly assigned to three groups. Allocation sequences were generated by a random number table and sealed envelopes were not opened until written informed consent had been obtained. Group S received ultrasound-guided bilateral dual TAP block with 0.9% saline, group R received TAP block with 0.33% ropivacaine, and group RD received TAP block with 0.33% ropivacaine and 1 μg/kg dexmedetomidine. The investigators who performed the block had no involvement in assessing outcome.
During the preoperative visit, a trained investigator explained the study plan and the scales that would be measured in the study to the patients. Pain severity was measured using VAS scores (10 cm line, 0 cm = no pain and 10 cm = worst pain imaginable). PONV scores were measured using a categorical scoring system (none = 0, mild = 1, moderate = 2, and severe = 3). Sedation scores were assigned by the investigator using a sedation scale (awake and alert = 0, quietly awake = 1, asleep but easily roused = 2, and deep sleep = 3). Quality of Recovery Questionnaire 40 (QoR-40) was used to evaluate patient’s recovery after surgery 19. The QoR-40 consists of 40 questions that examine five domains of patient recovery using a five-point Likert scale: none of the time, some of the time, usually, most of the time, and all of the time. The individualized items are presented in Table 1.
Patients received a standardized anesthetic regimen of 2 mg midazolam, 0.3–0.5 μg/kg sufentanil, 1–2 mg/kg lidocaine, 1.5–2 mg/kg propofol, and 0.6 mg/kg rocuronium to induce muscle relaxation. Anesthesia was maintained by a remifentanil infusion and sevoflurane. All patients received 3 mg granisetron for antiemetic prophylaxis and 100 mg flurbiprofen axetil intravenously 30 min before resuscitation from anesthesia. All patients were intravenously administered 100 mg flurbiprofen axetil diluted with 100 mL 0.9% saline every 12 h. A single dose of 100 mg tramadol was administered when patients reported pain equaling or exceeding four VAS points. If required, 10 mg metoclopramide was available.
Ultrasound-guided bilateral dual TAP blocks were performed by experienced anesthesiologists after anesthesia induction. Images were obtained using a Terason 2000+® ultrasound machine (Terason Division Teratach Corporation, Burlington, MA, USA). Under sterile conditions, the investigator placed a high-frequency (5–12 MHz) ultrasound probe (Terason®; Terason Division Teratach Corporation) covered with a sterile sheath, obliquely on the upper abdominal wall, along the subcostal margin near the midline. After identifying the rectus abdominis muscle, the ultrasound probe was gradually moved laterally along the subcostal margin until the transversus abdominis muscle was identified posterior to the rectus muscle (Fig. 1a). A 21G, 100-mm uniplex nanoplex UP 3/100 (Pajunk Corporation, Geisingen, Germany) was introduced through the skin at the medial end of the ultrasound probe and inserted in-plane under real-time ultrasound guidance to lie along the lateral border of the rectus abdominis muscle at the level of the aponeurosis formed by the external and the internal oblique muscles or between the posterior rectus abdominis and transversus abdominis muscle (or posterior rectus sheath). The correct position of the needle was established using direct ultrasound in-plane visualization and distention of the fascial plane by a 1-mL test dose of saline. The correct position was observed as a hypo-echoic lens shaped image (Fig. 1b). Then, 15 mL of the study drug was administered and the ultrasound probe was placed over the anterior abdominal wall immediately inferior and parallel to the costal margin on the anterior-axillary line. The external oblique, internal oblique, and transversus abdominis muscles were identified. The needle was then advanced laterally and posteriorly through the external and internal oblique muscles. The end point was the neurovascular plane between the transversus abdominis and internal oblique muscles (Fig. 1c). Fifteen milliliters of the study drug was injected after a test dose was administered. A contralateral block was performed in the same manner.
Ultrasound-guided TAP block. a Sonography of the transversus abdominis muscle laying posterior to the rectus muscle. IO internal oblique muscle, TA transversus abdominis muscle. b Test dose of saline in the TAP block. TA transversus abdominis muscle. c Transversus sonography of abdominal wall with the in-plane technique. SC subcutaneous tissue, EO external oblique muscle, IO internal oblique muscle, TA transversus abdominis muscle, arrows needle shaft
Whole blood was collected at 15, 30, 60, 90, 120, and 240 min after ropivacaine administration. Samples were centrifuged for 15 min at 3000 rpm within 4 h of collection. Plasma samples were stored at −80 °C until assay. Ropivacaine hydrochloride and its injectable product were purchased from AstraZeneca AB (Sodertalje, Sweden). The concentration of ropivacaine in the plasma was measured using high-performance liquid chromatography (Shimadzu Corporation, Kyoto, Japan). Chromatographic analyses were carried out on an LC solution system, which consisted of a quaternary LC-20AT pump connected to an SIL-20A autoinjector and an SPD-20AV ultraviolet detector. A Hypersil ODS-C18 column (4.6 × 200 mm, 5 μm) was utilized at ambient temperature. The gradient elution was used at a flow rate of 0.7 mL/min. The mobile phase was composed of acetonitrile and 0.025 mol/L potassium dihydrogen phosphate (pH was adjusted to 3.0 with phosphoric acid) (25:75, v/v). The detection wavelength was set at 220 nm. To measure the plasma ropivacaine concentration, 200 μL of 2 mol/L sodium hydroxide solution was added to 500 μL plasma samples in a 10-mL tube and vortexed for 1 min. The mixture was extracted with 3 mL of distilled ethyl acetate. After vortexing for 5 min, the samples were centrifuged for 15 min at 4000 rpm. Subsequently, the organic layer was transferred to another tube and evaporated completely under a gentle stream of nitrogen in a water bath at 44–46 °C. The residue was reconstituted with 500 μL acetonitrile and vortexed for 1 min. Finally, 100 μL of supernatant was injected into the HPLC system. A stock solution of 200 μg/mL ropivacaine was made and a standard solution of ropivacaine hydrochloride was prepared by diluting the stock solution with human plasma to concentrations of 0.1, 0.2, 0.5, 1, 2, and 4 μg/mL and stored at 4 °C until use.
The primary outcome measure in this study was the pain score 24 h after surgery. Patient characteristics [age, weight, height, body mass index (BMI)], and the duration of TAP block and surgery were also recorded. After surgery, patients were assessed at 2, 4, 12, 24, 36, and 48 h postoperatively. The following observations were recorded: VAS scores at rest and on movement (coughing or turning the body), sedation scores, and PONV scores. Tramadol consumption during the first and second postoperative days, QoR-40 scores, and ropivacaine plasma concentration were also recorded.
Statistical Analysis
An estimated minimum of 26 patients was required in each group to achieve 95% power of VAS scores, considering a standard deviation (SD) of 2.7 at a significance level of 0.05. This number was raised to 29 in each group to allow for a predicted 10% drop-out. Normally distributed interval data were reported as mean ± SD and were evaluated with one-way analysis of variance (age, weight, height, BMI, surgical time, and time for TAP block). The concentration of ropivacaine in plasma was evaluated with an independent-samples t test. Non-normally distributed interval and ordinal data were reported as medians (range or interquartile range) and were compared among groups using the Kruskal-Wallis H test (VAS scores, PONV scores, sedation scores, cumulative 24 h, 24–48 h of tramadol, QoR-40). Statistical significance in this study was set at P <0.05. We adjusted the significance level to 0.0167 for pairwise comparisons. All reported P values were two-sided. Statistical analysis was performed using SPSS21.0.
Results
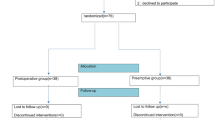
A total of 98 patients were randomly assigned to one of the three groups. Four patients (n = 1 from group S; n = 2 from group R; n = 1 from group RD) were withdrawn from the study because their body weight was <50 kg (n = 2), they suffered from mental illnesses (n = 1), or had an allergy to ropivacaine (n = 1). The VAS scores of 94 patients were assessed and three of these were excluded (n = 2 from group S, n = 1 from group R) because PCIA sulfentanil was required. Therefore, 91 patients completed the study (Fig. 2).
Groups were comparable in terms of age, weight, height, BMI, surgical time, and the time for TAP block (Table 2).
Postoperative VAS scores at rest and VAS scores on movement are reported in Figs. 3 and 4, separately. The VAS scores (rest and movement) were lower in group R and group RD than group S during the first 24 h after surgery (P < 0.0167). In details, the VAS score at rest in group R [median (interquartile range, IQR) = 3 (1–3), P = 0.000] and group RD [median (IQR) = 2 (0.25–3), P = 0.000] was lower than group S [median (IQR) = 4 (4–4)] 2 h postoperatively. The VAS scores on movement in group R [median (IQR) = 4 (3–4), P = 0.009] and group RD [median (IQR) = 3 (2–4), P = 0.000] were lower than group S [median (IQR) = 4 (4–4)] 2 h postoperatively. The median (IQR) VAS score 4 h at rest in group S was 4 (3–4), which was higher than group R [median (IQR) = 2 (1–3), P = 0.003] and group RD [median (IQR) = 2 (1–2), P = 0.000]. The median (IQR) VAS score 4 h on movement in group S was 4 (4–4), which was higher than group R [median (IQR) = 4 (3–4), P = 0.004] and group RD [median (IQR) = 3.5 (3–4), P = 0.002]. Patients in group S had higher VAS scores at rest and on movement 12 and 24 h postoperatively compared with those in group R and group RD. The median (IQR) VAS score at rest 12 h after surgery was 4 (3–4) in group S, 2 (1–3) in group R, and 1.5 (1–3) in group RD (P = 0.000). The median (IQR) VAS score on movement 12 h after surgery was 4 (4–4) in group S, 4 (3–4) in group R, and 3.5 (3–4) in group RD (P = 0.000). The median (IQR) VAS score at rest 24 h after surgery was 3 (1–3.5) in group S, 1 (0–2) in group R, and 1 (1–2) in group RD (P = 0.001). The median (IQR) VAS score on movement 24 h after surgery was 4 (3–4) in group S, 3 (3–4) in group R, and 3 (2–4) in group RD (P = 0.000). However, no significant differences were found between the three groups on VAS scores at rest 36 h (P = 0.069) and 48 h (P = 0.068) after surgery. The VAS scores on movement did not differ 36 h (P = 0.569) and 48 h (P = 0.699) between the three groups. The VAS scores at rest were not significantly different between group R and group RD 2 h (P = 0.684), 4 h (P = 0.708), 12 h (P = 0.582), and 24 h (P = 0.979). Patients in group R and group RD had comparable VAS scores on movement 2 h (P = 0.344), 4 h (P = 0.971), 12 h (P = 0.911), and 24 h (P = 0.911) after surgery.
The median difference in tramadol consumption during the first day after surgery was 100 mg (IQR = 0–200 mg, P = 0.013) in group R, 100 mg (IQR = 0–200 mg, P = 0.002) in group RD, and 200 mg (IQR = 100–300 mg) in group S. Tramadol consumption during the second day was significantly lower in group R [0 mg (range = 0–100 mg)] and group RD [0 mg (range = 0–200 mg)] than group S [100 mg (range = 0–400 mg)] (P < 0.0167). There were no differences between group R and group RD during the first (P = 0.830) and second day (P = 0.988).
Patients experienced nausea and vomiting more frequently in group S 2, 12, 24, and 36 h after surgery (P < 0.0167). No differences were observed in PONV scores between the three groups at 4 and 48 h (P > 0.05). PONV scores were similar between group R and group RD at all time points (P > 0.0167) (Fig. 5).
QoR-40 scores were lower in group S than group R and group RD 24 h [median (IQR) = 157 (152–166) vs. 166.5 (160–173), 167.5 (162.25–177.5), P = 0.000] and 48 h [median (IQR) = 174 (170.5–178.5) vs. 179.5 (178–183), 185.5 (178–187.75), P = 0.000] after surgery. Group R and group RD were balanced for QoR-40 scores at 24 h (P = 0.513) and at 48 h (P = 0.285).
Sedation scores were similar between the three groups at any of the six time points (P > 0.05) (Fig. 6).
Plasma ropivacaine concentrations were not significantly different between group R and group RD at any time point (P > 0.05) (Fig. 7). The mean (±SD) maximal plasma concentration was 1.42 ± 0.28 μg/mL in group R and 1.55 ± 0.41 μg/mL in group RD.
Discussion
The aim of this study was to determine the effect of adding dexmedetomidine to ropivacaine on the quality and duration of TAP block after gastrectomy. Our major finding was the positive effect of TAP block on analgesia and the quality of recovery. However, performing a TAP block with dexmedetomidine did not reduce pain and improve recovery significantly.
TAP block was formally described as a novel approach to abdominal field block by Rafi 20. It is a blind technique and can lead to intramuscular, subcutaneous, or intraperitoneal injection 21 and is associated with injury to the abdominal viscera, including the liver 22. The position of the needle tip and spread of local anesthetic can be monitored in real time by ultrasound guidance. An ultrasound-guided approach was first described by Hebbard et al. 7 as the posterior TAP block. Tran et al. 23 reported that an ultrasound-guided posterior TAP was limited to lower abdominal surgery. Hebbard 8 went on developing an ultrasound-guided subcostal TAP block, which was more suitable for abdominal surgery in the periumbilical region 24. The incisions for gastrectomy were performed through the midline, approximately 5 cm below the xiphoid process to 5 cm below the umbilicus. A single posterior or subcostal injection does not provide sufficient analgesia; therefore, we combined the posterior and oblique subcostal technique in our TAP block to provide wider bilateral analgesic coverage. Børglum et al. 9 showed that an ultrasound-guided dual TAP block provided effective rescue analgesia after major abdominal surgery. Another report demonstrated that a dual TAP block provided dermatomal anesthesia from T6 to T12, while the coverage following a single large volume injection was only reported from T10 to T12. 10 In our study, the VAS scores at rest and on movement improved in patients who received ropivacaine with or without dexmedetomidine. In addition, tramadol consumption and PONV scores significantly decreased and QoR-40 scores improved, indicating better postoperative recovery.
McDonnell et al. 25 demonstrated that the sensory deficit caused by TAP block completely regressed after 24 h. To increase the duration of sensory block, various adjuvants have been described and are used in daily clinical practice. Recently, dexmedetomidine was reported as an adjuvant to local anesthetics to enhance regional anesthesia. Almarakbi and Kaki 26 reported that adding dexmedetomidine to bupivacaine in a TAP block reduced morphine consumption and improved VAS scores after abdominal hysterectomy. At the time of our study, there were no reports on suitable doses of dexmedetomidine for TAP blocks. Zhang et al. 27 showed that 100 μg dexmedetomidine prolonged the sensory and motor duration of axillary brachial block in combination with ropivacaine, while 50 μg dexmedetomidine had no effect on block duration. However, Bharti et al. 28 reported that patients receiving 1.5 μg/kg dexmedetomidine combined with ropivacaine in caudal anesthesia were more sedated compared with the placebo, 0.5 μg/kg, and 1.0 μg/kg groups. In the interest of safety, we administered 1 μg/kg dexmedetomidine to our patients. Unfortunately, the VAS scores and QoR-40 scores were similar between group R and group RD. The optimal dexmedetomidine dosage needs to be further explored after gastrectomy.
A TAP block usually requires a large dose of local anesthetic to provide sufficient analgesia for surgical incisions 24. Even at dilute concentrations, anesthetics may cause systemic toxicity 6. Griffiths et al. 17 confirmed an association between plasma ropivacaine concentration and neurotoxicity after an ultrasound-guided TAP block. In contrast, Kitayama et al. 29 reported that 0.25, 0.5, or 0.75% ropivacaine was safe. This was also well below the threshold of potential toxicity in healthy volunteers 10, 30. In the present study, the mean peak ropivacaine plasma concentrations were not critically toxic in group R and group RD 17. Only one patient in group RD had a peak ropivacaine concentration higher than 2.20 μg/mL without symptoms of local anesthetic toxicity. This might have been ascribed to her low body weight (50 kg). Dexmedetomidine is a highly selective α2-adrenoceptor agonist with little effect on vasoconstriction at low concentrations. There were no differences in ropivacaine plasma concentration between group R and group RD in our study. The effect of dexmedetomidine on ropivacaine plasma concentration was consistent with previous findings. Fristsch et al. 31 showed that dexmedetomidine did not affect ropivacaine plasma levels in interscalene blocks.
There are several limitations to the present study. First, we were unable to evaluate parameters at the onset of anesthesia because patients received general anesthesia. Second, we did not assess clinical signs or symptoms of neurotoxicity. However, there were no significant changes in hemodynamics intraoperatively or postoperatively. Third, the success rate of the TAP block was not properly assessed. Knudsen et al. 32 showed that the peak unbound arterial concentration may represent a more valid predictor of toxicity than the venous level. However, we analyzed venous blood in our analysis. Finally, we did not systemically control the effect of dexmedetomidine.
Conclusion
Ultrasound-guided dual TAP block can provide sufficient analgesia for gastrectomy, decrease the consumption of tramadol, reduce PONV scores, and improve quality of recovery. Adding dexmedetomidine to ropivacaine in TAP block for gastrectomy does not prominently prolong the duration or decrease the consumption of analgesics. In addition, it does not affect the plasma ropivacaine concentration.
Author Contributions
W.L. conceived and designed the study, and reviewed and edited the manuscript. W.D. performed the experiments and reviewed and edited the manuscript. W.L. collected and analyzed the data and wrote the manuscript. X.Z. reviewed and edited the manuscript. J.L. performed the experiments and contributed analysis tools. J.J. and C.G. performed the experiments.
References
Rigg JR, Jamrozik K, Myles PS, Silbert BS, Peyton PJ, Parsons RW, Collins KS, Teoh GS, Mainland P, Lee BB, Ngan Kee WD, Wong E, Liu J, Hg SH, Bee TW, Ong G, Peng NK, Vijayan R, Delilkan A, Lowry C, Prince J, Barratt S, Zammit A, Rodins H, Skirving A, Solomos J, Nicolson M, Pocock J, Fletcher H, Hunt J, Poustie S, Tuck M, Paull J, Sherlock S, Sparrow M, Colson M, Burgin G, Wallace M, Pang J, Blythe C, Martin K, Hobbs M, Storey J, Bassatt J, Paech M, Pavy T, Cokis C, March S, El-Dawlatly AA, Kong CS, Punjasawadwong Y. Epidural anaesthesia and analgesia and outcome of major surgery: a randomised trial. Lancet. 2002;359:1276–1282.
Peyton PJ, Myles PS, Silbert BS, Rigg JA, Jamrozik K, Parsons R. Perioperative epidural analgesia and outcome after major abdominal surgery in high-risk patients. Anesth Analg. 2003;96:548-, table of contents.
Gould TH, Grace K, Thorne G, Thomas M. Effect of thoracic epidural anaesthesia on colonic blood flow. Br J Anaesth. 2002;89:446–451.
Bode RH Jr, Lewis KP, Zarich SW, Pierce ET, Roberts M, Kowalchuk GJ, Satwicz PR, Gibbons GW, Hunter JA, Espanola CC. Cardiac outcome after peripheral vascular surgery. Comparison of general and regional anesthesia. Anesthesiology. 1996;84:3–13.
Werawatganon T, Charuluxanun S. Patient controlled intravenous opioid analgesia versus continuous epidural analgesia for pain after intra-abdominal surgery. Cochrane Database Syst Rev. 2005:CD004088.
Petersen PL, Mathiesen O, Torup H, Dahl JB. The transversus abdominis plane block: a valuable option for postoperative analgesia? A topical review. Acta Anaesthesiol Scand. 2010;54:529–535.
Hebbard P, Fujiwara Y, Shibata Y, Royse C. Ultrasound-guided transversus abdominis plane (TAP) block. Anaesth Intensive Care. 2007;35:616–617.
Hebbard PD. Subcostal transversus abdominis plane block under ultrasound guidance. Anesth Analg 2008; 106: 674–5.
Børglum J, Maschmann C, Belhage B, Jensen K. Ultrasound-guided bilateral dual transversus abdominis plane block: a new four-point approach. Acta Anaesthesiol Scand. 2011;55:658–663.
Børglum J, Jensen K, Christensen AF, Hoegberg LC, Johansen SS, Lönnqvist PA, Jansen T. Distribution patterns, dermatomal anesthesia, and ropivacaine serum concentrations after bilateral dual transversus abdominis plane block. Reg Anesth Pain Med. 2012;37:294–301.
Sato J, Perl ER. Adrenergic excitation of cutaneous pain receptors induced by peripheral nerve injury. Science. 1991; 251: 1608–1610.
Brummett CM, Hong EK, Janda AM, Amodeo FS, Lydic R. Perineural dexmedetomidine added to ropivacaine for sciatic nerve block in rats prolongs the duration of analgesia by blocking the hyperpolarization-activated cation current. Anesthesiology. 2011; 115: 836–843.
Kosugi T, Mizuta K, Fujita T, Nakashima M, Kumamoto E. High concentrations of dexmedetomidine inhibit compound action potentials in frog sciatic nerve without alpha(2) adrenoceptor activation. Br J Pharmacol. 2010; 160: 1662–1676.
Memis D, Turan A, Karamanlioğlu B, Pamukçu Z, Kurt I. Adding dexmedetomidine to lidocaine for intravenous regional anesthesia. Anesth Analg. 2004;98:835–840, table of contents.
Esmaoglu A, Yegenoglu F, Akin A, Turk CY. Dexmedetomidine added to levobupivacaine prolongs axillary brachial plexus block. Anesth Analg. 2010;111(6):1548–1551.
Obayah GM, Refaie A, Aboushanab O, Ibraheem N, Abdelazees M. Addition of dexmedetomidine to bupivacaine for greater palatine nerve block prolongs postoperative analgesia after cleft palate repair. Eur J Anaesthesiol. 2010;27:280–284.
Griffiths JD, Barron FA, Grant S, Bjorksten AR, Hebbard P, Royse CF. Plasma ropivacaine concentrations after ultrasound-guided transversus abdominis plane block. Br J Anaesth. 2010;105(6):853–856.
Corvetto MA, Echevarria GC, De La Fuente N, Mosqueira L, Solari S, Altermatt FR. Comparison of plasma concentrations of levobupivacaine with and without epinephrine for transversus abdominis plane block. Reg Anesth Pain Med. 2012;37:633–637.
Myles PS, Hunt JO, Nightingale CE, Fletcher H, Beh T, Tanil D, Nagy A, Rubinstein A, Ponsford JL. Development and psychometric testing of a quality of recovery score after general anesthesia and surgery in adults. Anesth Analg. 1999;88:83–90.
Rafi AN. Abdominal field block: a new approach via the lumbar triangle. Anaesthesia. 2001;56:1024–1026.
McDermott G, Korba E, Mata U, Jaigirdar M, Narayanan N, Boylan J, Conlon N. Should we stop doing blind transversus abdominis plane blocks? Br J Anaesth. 2012;108:499–502.
Farooq M, Carey M. A case of liver trauma with a blunt regional anesthesia needle while performing transversus abdominis plane block. Reg Anesth Pain Med. 2008;33:274–275.
Tran TM, Ivanusic JJ, Hebbard P, Barrington MJ. Determination of spread of injectate after ultrasound-guided transversus abdominis plane block: a cadaveric study. Br J Anaesth. 2009;102:123–127.
Barrington MJ, Ivanusic JJ, Rozen WM, Hebbard P. Spread of injectate after ultrasound-guided subcostal transversus abdominis plane block: a cadaveric study. Anaesthesia. 2009;64:745–750.
McDonnell JG, O’Donnell BD, Farrell T, Gough N, Tuite D, Power C, Laffey JG. Transversus abdominis plane block: a cadaveric and radiological evaluation. Reg Anesth Pain Med. 2007;32:399–404.
Almarakbi WA, Kaki AM. Addition of dexmedetomidine to bupivacaine in transversus abdominis plane block potentiates post-operative pain relief among abdominal hysterectomy patients: a prospective randomized controlled trial. Saudi J Anaesth. 2014;8:161–166.
Zhang Y, Wang CS, Shi JH, Sun B, Liu SJ, Li P, Li EY. Perineural administration of dexmedetomidine in combination with ropivacaine prolongs axillary brachial plexus block. Int J Clin Exp Med. 2014;7:680–685.
Bharti N, Praveen R, Bala I. A dose-response study of caudal dexmedetomidine with ropivacaine in pediatric day care patients undergoing lower abdominal and perineal surgeries: a randomized controlled trial. Paediatr Anaesth. 2014;24:1158–1163.
Kitayama M, Wada M, Hashimoto H, Kudo T, Yakoshi C, Hirota K. Plasma ropivacaine concentrations after ultrasound-guided transversus abdominis plane block for open retropubic prostatectomy. J Anesth. 2014;28:576–579.
Latzke D, Marhofer P, Kettner SC, Koppatz K, Turnheim K, Lackner E, Sauermann R, Müller M, Zeitlinger M. Pharmacokinetics of the local anesthetic ropivacaine after transversus abdominis plane block in healthy volunteers. Eur J Clin Pharmacol. 2012;68:419–425.
Fritsch G, Danninger T, Allerberger K, Tsodikov A, Felder TK, Kapeller M, Gerner P, Brummett CM. Dexmedetomidine added to ropivacaine extends the duration of interscalene brachial plexus blocks for elective shoulder surgery when compared with ropivacaine alone: a single-center, prospective, triple-blind, randomized controlled trial. Reg Anesth Pain Med. 2014;39:37–47.
Knudsen K, Beckman Suurkula M, Blomberg S, Sjövall J, Edvardsson N. Central nervous and cardiovascular effects of i.v. infusions of ropivacaine, bupivacaine and placebo in volunteers. Br J Anaesth. 1997;78:507–514.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Permissions
There is no previously published material.
Rights and permissions
About this article
Cite this article
Ding, W., Li, W., Zeng, X. et al. Effect of Adding Dexmedetomidine to Ropivacaine on Ultrasound-Guided Dual Transversus Abdominis Plane Block after Gastrectomy. J Gastrointest Surg 21, 936–946 (2017). https://doi.org/10.1007/s11605-017-3402-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-017-3402-5