Abstract
Background
Postoperative intraabdominal abscess (IAA) is the most feared complication after laparoscopic appendectomy (LA). We aimed to evaluate the management of this complication in a large cohort of patients undergoing LA in order to design a treatment algorithm.
Methods
We included a consecutive series of patients undergoing LA for acute appendicitis from January 2008 to December 2018. The cohort of patients with postoperative IAA was divided into three groups based on the implemented treatments: G1: antibiotics only, G2: CT-guided drainage, and G3: laparoscopic lavage. Characteristics of the fluid collections and outcomes were analyzed in the three groups.
Results
A total of 1668 LA were performed; the rate of IAA was 2.2% (36 patients). There were 12 (33%) patients who received antibiotics only (G1), 8 (22%) underwent CT-guided percutaneous drainage (G2), and 16 (45%) underwent laparoscopic lavage (G3). The median size of the abscesses was 2.7 (1.2–4) cm in G1, 6.2 (4.5–8) cm in G2, and 9.6 (8–11.4) cm in G3 (p < 0.04). Patients with two or more fluid collections underwent a laparoscopic lavage in all cases. Treatment failure occurred in 16% (2/12), 12.5% (1/8) and 12.5% (2/16) of the patients in G1, G2, and G3, respectively. None of the patients in the entire cohort required open surgery to resolve the postoperative IAA.
Conclusions
A minimally invasive step-up approach based on the size and number of fluid collections is associated with excellent outcomes. A treatment algorithm for post-appendectomy IAA is proposed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Acute appendicitis is one of the most common abdominal surgical emergencies. The life risk of having this entity range from 7 to 14% in the USA, and every year about 300,000 patients undergo an appendectomy [1]. Since its introduction by Semm in the early 80′s, the laparoscopic approach has gained worldwide acceptance [2, 3].
Laparoscopic appendectomy (LA) is associated with reduction in wound infection rates and ileus, shorter hospital stay, and reduced postoperative intraabdominal adhesions [4]. One of the most feared complications after LA, however, is the intraabdominal abscess (IAA). This entity increases length of hospital stay, costs, morbidity, and mortality rates [5,6,7].
Although several therapeutic options have been proposed, there is lack of information regarding a standardized management of IAA after LA. Therefore, we aimed to evaluate the management of the IAA after LA in a large cohort of patients, and to propose a treatment algorithm.
Methods
After approval by the Institutional Review Board of our Institution, data were collected from all patients who underwent LA for acute appendicitis from January 2008 to December 2018. Patients who required conversion to open surgery were excluded.
The diagnosis of acute appendicitis was based on three aspects: clinical presentation, laboratory parameters, and imaging. Ultrasound was carried out in all patients, and a computed tomography (CT) was performed when the clinical exam and ultrasound findings were inconclusive or in those cases with Alvarado’s Score < 6 [8].
All the procedures were performed by general surgery residents with the supervision of an attending surgeon. A unique preoperative dose of antibiotics 30 min before surgical incision was administered in all cases (ciprofloxacin 500 mg and metronidazole 500 mg or ampicillin-sulbactam 1000 mg, adapted to our local infectology guidelines) [9].
LA was performed using three ports that were placed at the umbilicus region (10 mm), suprapubic region (10 mm) and left lower quadrant (5 mm). An exploratory laparoscopy was accomplished to confirm diagnosis of acute appendicitis. Transection of the appendix with scissors was performed over an endoloop and removed inside a protective bag through the suprapubic port after dissection of the mesoappendix with electrocautery, followed by peritoneal irrigation and suction with warm saline water when free fluid was found in the abdominal cavity. A silicone flexible drain was placed when in cases of perforated appendix with generalized purulent peritonitis. Patients with purulent fluid in the abdominal cavity or perforated appendicitis continued antibiotics for 7 days. Patients were discharged when they could tolerate oral intake and have a stable clinical condition. Follow-up was planned at 7 and 30 days after patient’s discharge.
IAA was defined as a patient with fever, abdominal pain or ileus with the confirmation of an abscess by an imaging method (ultrasonography or CT scan). Patient’s characteristics and intraoperative variables related to postoperative IAA were analyzed.
The management of patients with IAA were divided into 3 groups based on the implemented treatments: G1: patients treated with empiric intravenous antibiotics only (ciprofloxacin 500 mg every 12 h and metronidazole 500 mg every 8 h or ampicillin-sulbactam 1000 mg every 12 h), G2: patients treated with CT- guided drainage plus intravenous antibiotics (adjusted according to culture and sensitivity results), and G3: patients treated with laparoscopic lavage of abdominal cavity plus intravenous antibiotics (adjusted according to culture and sensitivity results). Treatment failure was defined as persistence of symptoms (fever, ileus or abdominal pain) for more than 48 h after setting up the treatment and evidence of persistence of the fluid collection in CT scan. Characteristics of the fluid collections and outcomes were analyzed in the three groups.
For statistical analysis, the program IBM SPSS (version 20.0) was used. For all statistical methods, p < 0.05 was considered statistically significant.
Results
In the period of the study a total of 1668 laparoscopic appendectomies were performed. The mean age was 35.1 (14–95) years and 878 patients (52%) were male. Most of the patients had a good performance status, with ASA score of I–II in 98% of them. Obesity (BMI > 30 kg/m2) was found in 80 patients (4.8%) (Table 1).
The rate of IAA was 2.2% (36 patients). When comparing to patients without postoperative fluid collections, IAA was significantly associated to obese patients (22% vs. 4.2%, p < 0.01), leukocytosis greater than 20,000/mm3 (25% vs. 6.3%, p < 0.01), and perforated appendicitis (81% vs. 15.7%, p < 0.01). There was also a significant increase on the mean hospital stay in patients with IAA, as compared to those without postoperative IAA: 6.6 (4–34) days vs. 1.6 (1–13), p < 0.01 (Table 2).
“Among the patients with postoperative IAA, 12 (33%) received antibiotics only (G1), 8 (22%) underwent CT-guided percutaneous drainage (G2), and 16 (45%) underwent laparoscopic lavage of the abdominal cavity (G3). The median size of the abscess was 2.7 (1.2–4) cm in G1, 6.2 (4.5–8) cm in G2, and 9.6 (8-11.4) cm in G3 (p <0.04). Patients with two or more fluid collections underwent a laparoscopic lavage in all cases.”
Treatment failure occurred in 16% (2/12), 12.5% (1/8) and 12.5% (2/16) of the patients in G1, G2, and G3, respectively. Two patients in G1 required percutaneous drainage, one patient in G2 underwent a laparoscopic lavage, and two patients in G3 required re-laparoscopic lavage. No patient in the entire cohort needed an open surgery to resolve the postoperative IAA.
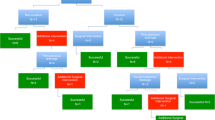
Based on our results, a treatment algorithm for the management of postoperative IAA was proposed (Fig. 1).
Discussion
We aimed to evaluate the management of postoperative IAA after LA in a large cohort of patients in order to design a treatment algorithm. We found that a step-up approach based in the size and number of fluid collections was associated with favorable outcomes.
IAA is a serious complication and has been reported in up to 19% of the patients with complicated appendicitis [10]. A recent meta-analysis conducted by Athanasiou et al. [11] found a rate of IAA of 8% and 6% after laparoscopic and open appendectomies, respectively, with no statistically significant difference between them [OR = 1.11(0.85, 1.45]. In our series, the rate of IAA was 2.2%, which did not vary from a previous report of the same group [5]. There are several established risk factors associated to postoperative IAA such as obesity, leukocytosis, perforated appendicitis, greater operative time, diabetes mellitus, and peritoneal irrigation during the surgery [5, 6]. Most of those factors were indeed found in our cohort. Dhaou et al. [7] reported a hospital stay of 10 (7–14) days after the diagnosis of IAA. We also found that IAA was associated with a significant longer hospital stay.
Once the diagnosis of IAA is established, several treatment modalities have been proposed [12,13,14]. Image-guided percutaneous drainage and laparoscopic lavage are often chosen to treat this complication [15]. A conservative approach with antibiotics only, however, also seems reasonable in some cases [16]. It has been observed in the pediatric population that antibiotics only (triple scheme) are associated with very good outcomes when compared with surgical management.[17]. In our series, a conservative approach was decided in 12 patients (median size of 2.7 cm) and it was successful with full resolution of the complication in 84% of cases. Only 2 patients (16%) required percutaneous drainage because of abscess persistence.
Image-guided percutaneous drainage is currently available in most institutions, and both ultrasound- and CT-guided drainage have shown very good outcomes with success rates greater than 80% [18,19,20]. To our knowledge, there is a lack of information regarding the radiology´s threshold to perform image-guided drainage of IAA after LA. However, an attempt to establish a radiology cut-off point for percutaneous drainage in abscesses related to other entities has been made [21,22,23]. CT-guided percutaneous drainage election in our cohort was based on abscess size, abscess localization, and patient´s clinical condition, and was performed as initial approach in 8 patients (median size 6.2 cm) with a success rate of about 87.5%. Only 1 patient (12.5%) needed laparoscopic lavage to resolve the complication.
Laparoscopic lavage of the abdominal cavity is often necessary for larger collections. Several publications have supported this approach, mainly when percutaneous drainage is not feasible or it is not available [24, 25]. Considering that a new laparoscopic procedure could be related with other complications such as bowel injury, fistulas or ileus, we believe that a laparoscopic lavage should be reserved for patients with very large or multiple collections in which percutaneous drainage usually fail or is contraindicated. In our cohort, a total of 18 patients (16 as first approach and 2 after percutaneous drainage failure) underwent laparoscopic lavage with a 95% of effectiveness. Only one patient needed a re-laparoscopic lavage (5%) to resolve the complication.
Overall, decision making should be subject to the complexity of the cases with a step-up approach basis. This is in alignment with the EAES consensus which established that IAA can be managed by a conservative method (antibiotics only), or by a percutaneous drainage or a laparoscopic approach in those patients with complicated abscesses, or those who decline in their clinical condition [26]. Our study suggests that unique fluid collections less than 4 cm can be treated conservatively with antibiotics only, collections between 4 and 8 cm with imaging guided percutaneous drainage, and collections greater than 8 cm with laparoscopic lavage. In case of failure, every chosen treatment can be scaled to the next step, with very good overall outcomes (Fig. 1).
The main limitation of our study is its retrospective nature. In addition, a relatively small number of patients developed postoperative IAA and were included in the analysis. However, considering the limited available information in the literature regarding the management of postoperative IAA after LA, we believe our study could help future research collaborations to elucidate the best treatment modalities for this complication.
Conclusion
Obese patients, white blood cell count > 20,000 and perforated appendicitis are associated with a higher risk of postoperative intraabdominal abscess after LA. A minimally invasive step-up approach based on the size and number of fluid collections seems to be safe and feasible. A treatment algorithm is proposed in order to manage this complication to avoid unnecessary procedures.
References
Weiss AJ, Elixhauser A, Andrews RM (2006) Characteristics of operating room procedures in U.S. Hospitals, 2011: statistical brief #170. Agency for healthcare research and quality. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb170-Operating-Room-Procedures-United-States-2011.jsp. Accessed 22 May 2019.
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Semm K (1983) Endoscopic appendectomy. Endoscopy 15:59–64
Masoomi H, Nguyen NT, Dolich MO et al (2014) Laparoscopic appendectomy trends and outcomes in the united states: data from the nationwide inpatient sample (NIS), 2004–2011. Am Surg 80:1074–1077
Schlottmann F, Sadava EE, Peña ME et al (2017) Laparoscopic appendectomy: risk factors for postoperative intraabdominal abscess. World J Surg 41(5):1254–1258
Reid RI, Dobbs BR, Frizelle FA (1999) Risk factors for post-appendicectomy intra-abdominal abscess. Aust N Z J Surg 69:373–374
Dhaou MB, Ghorbel S, Chouikh T et al (2010) Conservative management of post-appendicectomy intra-abdominal abscesses. Ital J Pediatr 2010(36):68
Alvarado A et al (1986) A practical score for the early diagnosis of acute appendicitis. Ann Emerg Med 15(5):557–564
Clara L et al (2018) Infecciones intraabdominales. puesta al día y recomendaciones de la sociedad argentina de infectología. Medicina (Buenos Aires) 78:417–426
Masoomi H, Nguyen NT, Dolich MO et al (2011) Comparison of laparoscopic versus open appendectomy for acute nonperforated and perforated appendicitis in the obese population. Am J Surg 202:733–739
Athanasiou C, Lockwood S, Markides GA (2017) Systematic review and meta-analysis of laparoscopic versus open appendicectomy in adults with complicated appendicitis: an update of the literature. World J Surg 41(12):3083–3099
Feld R, Eschelman DJ, Sagerman JE et al (1994) Treatment of pelvic abscesses and other fluid collections: efficacy of transvaginal sonographically guided aspiration and drainage. AJR Am J Roentgenol 163:1141–1145
Pereira JK, Chait PG, Miller SF (1996) Deep pelvic abscesses in children: transrectal drainage under radiologic guidance. Radiology 198:393–396
Okoye BO, Rampersad B, Marantos A (1998) Abscess after appendectomy in children: the role of conservative management. Br J Surg 85:1111–1113
Blot S, De Waele JJ, Vogelaers D (2012) Essentials for selecting antimicrobial therapy for intra-abdominal infections. Drugs 72:17–32
Forgues D, Habbig S, Diallo AF et al (2007) Post-appendectomy intra-abdominal abscesses—can they successfully be managed with the sole use of antibiotic therapy? Eur J Pediatr Surg 17(2):104–109
Dobremez E, Lavrand F, Lefevre Y et al (2003) Treatment of post-appendectomy intra-abdominal deep abscesses. Eur J Pediatr Surg 13:393
Betsch A, Wiskirchen J, Trübenbach J et al (2002) CT-guided percutaneous drainage of intra-abdominal abscesses: APACHE III score stratification of 1-year results. Acute physiology, age, chronic health evaluation. Eur Radiol 12:2883–2889
Gronvall J, Gronvall S, Hegedüs V (1977) Ultrasound-guided drainage of fluid-containing masses using angiographic catheterization techniques. Am J Roentgenol 129:997–1002
Gervais DA, Brown SD, Connolly SA et al (2004) Percutaneous imaging guided abdominal and pelvic abscess drainage in children. Radiographics 24:737–754
Gregersen R, Mortensen LQ, Burcharth J, Pommergaard H-C, Rosenberg J (2016) Treatment of patients with acute colonic diverticulitis complicated by abscess formation: a systematic review. Int J Surg 35:201–208
Jaffe TA, Nelson RC, DeLong DM, Paulson EK (2004) Practice patterns in percutaneous image-guided intraabdominal abscess drainage: survey of academic and private practice centers. Radiology 233(3):750–756
Durmishi Y, Gervaz P, Brandt D et al (2006) Results from percutaneous drainage of Hinchey stage II diverticulitis guided by computed tomography scan. Surg Endosc 20:1129–1133
Clark JJ, Johnson SM (2011) Laparoscopic drainage of intraabdominal abscess after appendectomy: an alternative to laparotomy in cases not amenable to percutaneous drainage. J Pediatr Surg 46:1385–1389
Kok K, Yapp S (2000) Laparoscopic drainage of postoperative complicated intra-abdominal abscess. Surg Lap Endo Perc Tech 10:311–313
Gorter RR, Eker HH, Gorter-Stam MAW et al (2016) Diagnosis and management of acute appendicitis. EAES consensus development conference 2015. Surg Endosc 30:4668–4690
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Francisco Laxague MD, Francisco Schlottmann MD, MPH, José M. Piatti MD and Emmanuel E. Sadava MD, have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Laxague, F., Schlottmann, F., Piatti, J.M. et al. Minimally invasive step-up approach for the management of postoperative intraabdominal abscess after laparoscopic appendectomy. Surg Endosc 35, 787–791 (2021). https://doi.org/10.1007/s00464-020-07448-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-020-07448-0