Abstract
Background
Although indocyanine green (ICG) fluorescence imaging has been reported to be useful for assessing colorectal perfusion, unstable quantification remains an issue. We performed ICG fluorescence observation from the luminal side and examined the usefulness of the transanal approach.
Methods
A total of 69 patients who underwent left-side colon surgery were enrolled in this cohort study. After the anastomosis had been constructed, ICG 0.2 mg/kg was injected intravenously. The anastomotic site was then observed by a scope inserted transanally. The following items were examined in the areas of the anastomotic site with the highest- and lowest-fluorescence intensity: maximum fluorescence (Fmax), time from ICG injection to Fmax (Tmax), time from start of dyeing to Fmax (ΔT), and the contrast pattern of the mucosa.
Results
Anastomotic leakage (AL) occurred in nine cases. Tmax and ΔT values of the lowest-fluorescence area in the distal intestine showed significant differences in the cases with AL (P = 0.015 and P = 0.040, respectively). Regarding the contrast pattern of the mucosa of the lowest-fluorescence area in the proximal and distal intestine, the patients in whom the vessels were not depicted in the area had a significantly higher incidence of AL than those in whom vessels were depicted in the area (P = 0.031 and P = 0.030, respectively). Some of the areas in which vessels were not depicted by ICG fluorescence observation from the luminal side corresponded to the points of leakage. There were heterogeneous changes that might not be grasped by observation from the serosal side.
Conclusion
Transanal ICG fluorescence imaging can evaluate perfusion over the entire circumference of the anastomosis in detail and aid in assessing the risk of AL. Therefore, the examination of the detailed low-perfusion area enables us to take measures for AL and to search for safer operative managements.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Colorectal cancer is the second most common cause of cancer death in Japan and has been increasing in frequency in recent years [1]. Among colorectal cancer surgeries, rectal cancer surgery is performed in more than 5600 patients each year in Japan [2]. Anastomotic leakage (AL) is a fatal complication of colorectal surgery that can lead to a delay in starting adjuvant chemotherapy, subsequently increasing the risk of recurrence after surgery [3,4,5].
Despite technological advancements in colorectal surgery, AL still occurs in 3–19% of left-side colon and rectal surgery [5,6,7,8,9]. The causes of AL are diverse and include the state of perfusion, tension at the anastomotic site, and the anastomotic procedure [10]. The perfusion of colorectal anastomosis can be assessed using near-infrared (NIR) fluorescence imaging with indocyanine green (ICG), and its efficacy in reducing AL has been reported [11,12,13,14]. Those reports focus on the perfusion of the proximal intestine, and ICG fluorescence observation is performed from the serosal side before anastomosis. However, such studies have the following disadvantages: observation from outside the lumen cannot assess the whole circumference of the anastomotic site; observation before anastomosis cannot evaluate the change of perfusion due to anastomosis; and the distal side of the intestine is not evaluated.
We previously reported our observation of the whole circumference view and contrasted the vessels obtained by transanal ICG fluorescence observation from the luminal side [15]. We also confirmed reproducibly in a basic experiment with swine that the angiogram became obfuscated by arteriovenous clamping but was clarified by declamping. Based on the findings of this basic experiment and focusing on the contrasted vessels and its background, we created the vessel staining pattern classification system (VSPCS) for anastomotic perfusion. In the VSPCS, each of the contrast modes were categorized into four patterns: Category-1 (C-1), the whole region was black, the blood vessels could not be observed on the black background; Category-2 (C-2), thick white blood vessels could not be observed on the white background; Category-3 (C-3), thick white vessels could be observed on a white background; and Category-4 (C-4), white branches of the capillaries could be observed on a black background. Then, we divided these into the C-1,2 group (vessels not depicted pattern) and the C-3,4 group (vessels depicted pattern) (Fig. 1). We examined seven cases of left-side colon or rectal surgery and reported that the C-1,2 group in the proximal intestine tended to have AL more frequently than the C-3,4 group.
Vessel staining pattern classification system (VSPCS). C-1: There is no inflow of arterial blood, the area remains black. C-2: The whole area is white, and blood vessel shadows are unclear. The whole image has a cloudy appearance. C-3: The background of the whole image is white, which reflects mild venous stasis. C-4: The background of the image is black, and the white branches of the capillaries can be observed. The picture and definition of each category was reprinted courtesy of Dr. Muto (15)
The goal of this study was to verify the clinical utility of transanal ICG-NIR fluorescence imaging for evaluation of colorectal anastomotic perfusion, increasing the numbers of cases. Furthermore, we conducted a more detailed evaluation, including the distal intestine, and examined the cause of AL in the individual cases.
Materials and methods
Study population
A total of 71 patients who underwent left-side colon or rectal surgery with anastomosis at Chiba University Hospital between June 2015 and November 2018 were prospectively enrolled. The study exclusion criteria were allergic hypersensitivity to ICG or iodine. Patients were eligible for enrollment if they were over 20 years old and were scheduled to undergo colorectal surgery. The study protocol was approved by the Clinical Research Ethics Committees of Chiba University and was registered in the University Hospital Medical Information Network (Umin) Clinical Trials Registry as UMIN 000029555 (http://www.umin.ac.jp/ctr/index.htm). The study was conducted according to the Declaration of Helsinki. Written informed consent was obtained from all patients for the use of their clinical data.
Surgical procedure
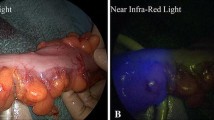
All patients received standard bowel preparation and antibiotic prophylaxis the day before the operation. As antibiotic prophylaxis, kanamycin 2 g and metronidazole 1.5 g were administered. When their anastomosis was completed in the operation, the proximal colon 10 cm or more from the anastomotic site was clamped so as not to clamp the marginal vessels, and a bleeding check and leakage test of the anastomosis were conducted by endoscopy. Anastomotic perfusion was then observed transanally using an NIR scope for laparoscopic use (Olympus Corporation, Tokyo, Japan). Observation was accomplished transanally using a rectoscope-introducer (Karl Storz, Tuttlingen, Germany) for protection of the rectum. To secure the field of view with an appropriate air supply, the rectoscope-introducer was connected to an endoscope using ArgyleTM Universal Bubble Tubing (Covidien, Tokyo, Japan) (Fig. 2). We maintained the expansion of the anastomotic lumen using the air supply of the endoscope. The NIR scope was kept distant enough that the entire anastomotic part was in the field of view. In that state, ICG at 0.2 mg/kg and physiological saline at 5 mL were injected intravenously. The operating light was turned off during observation.
Evaluation methods
All cases were recorded as movie files for the retrospective analysis. Regions of interest (ROI) were set at the following four areas: proximal-high, the highest-fluorescence area in the proximal intestine; proximal-low, the lowest-fluorescence area in the proximal intestine; distal-high, the highest-fluorescence area in the distal intestine; and distal-low, the lowest-fluorescence area in the distal intestine. Fluorescence was measured using the ImageJ software program (ver. 1.39 s; National Institutes of Health, Maryland, USA, available at http://rsb.info.nih.gov/ij), and time intensity curve of fluorescence was created (Fig. 3). ImageJ is a software widely used for fluorescence measurement of ICG [16,17,18,19]. The maximum fluorescence value was defined as Fmax. Fmax was measured as a mean value in ROI to eliminate heterogeneity of the fluorescence in ROI. The time from the intravenous injection of ICG (from the flush by physiological saline) to the maximum fluorescence was defined as Tmax, and the time from the start of dyeing to the maximum fluorescence was defined as ΔT. As a contrast pattern of mucosa by VSPCS, when each area reached Fmax, cases were classified into C-1, 2 (vessels not depicted pattern) and C-3, 4 (vessels depicted pattern). We analyzed the relationship between the Fmax, Tmax, ΔT, VSPCS, and episodes of AL.
Postoperative data
When AL was suspected based on clinical findings such as fever and abdominal pain, AL was confirmed by the following findings: abdominal drainage included fecal matter; computed tomography showed abscess and gas around the anastomotic site; and contrast enema showed communication between inside and outside the intestinal tract. Patients with AL underwent sigmoidoscopy to estimate the causes of AL, and the point of AL (dehiscence, fistula, or granulation) was determined after the clinical course of the patient was stabilized.
Statistical analyses
Values are expressed as the mean ± standard deviation (SD). The Fisher’s exact test was used for the comparison and analysis of categorical variables. Continuous variables were analyzed using the Wilcoxon’s signed-rank sum test. P values of < 0.05 were considered statistically significant. Statistical analyses were conducted with the JMP Pro software program, version 11.0.0 (SAS Institute Inc., Cary, NC, USA).
Results
Patient population
A total of 71 patients with left-side colon and who underwent rectal surgery were enrolled in this study. Among these, we excluded two patients in whom the distal intestine was not stained at all (C-1) over 5 cm in this ICG study. These patients were excluded because they underwent re-anastomosis during surgery. Therefore, 69 patients were ultimately analyzed in this study.
The patient, tumor, and surgery-related factors are presented in Table 1. Regarding the location of the lesion, those located at the rectum had more AL than those at the sigmoid colon. Regarding other factors, there was no significant difference between cases with AL and without AL. Regarding the anastomotic style, side-to-side anastomosis was performed in 16 cases, side-to-end anastomosis in 40 cases, and end-to-end anastomosis in 13 cases. One case of side-to-end anastomosis and three cases of end-to-end anastomosis were hand-sewn, respectively. Regarding the postoperative course, AL occurred in nine patients (13%), of whom two required reoperation. Patients who did not need reoperation were treated with antibiotics and drainage. Dehiscence parts had not closed for a year in two patients who did not need reoperation due to the existence of diverting ileostomy.
The relation between quantitative value and anastomotic leakage
Fmax had no significant correlation with AL for each of four areas: proximal-high, P = 0.344; proximal-low, P = 0.322; distal-high, P = 0.235; and distal-low, P = 0.380 (Fig. 4).
With respect to Tmax, there was no significant correlation between Tmax and AL in the proximal-high (P = 0.070), proximal-low (P = 0.475), and distal-high (P = 0.892). In contrast, there was a significant correlation between Tmax and AL in the distal-low (74.3 ± 42.3 s vs. 45.4 ± 21.4 s, P = 0.015) (Fig. 5).
With respect to ΔT, there was no significant correlation between ΔT and AL in the proximal-high (P = 0.723), proximal-low (P = 0.420), and distal-high (P = 0.954). However, there was a significant correlation between ΔT and AL in the distal-low (52.8 ± 30.3 s vs. 30.0 ± 15.0 s, P = 0.040) (Fig. 6).
The relation between VSPCS and anastomotic leakage
In the proximal-high, there was no significant difference between C-1,2 and C-3,4 groups (P = 0.355) (Fig. 7). In the proximal-low, AL was observed in 5 of 18 cases of C-1,2 (28%) and 4 of 51 cases of C-3,4 (8%). There was a significant difference in AL between the C-1,2 and C-3,4 groups (P = 0.031).
Relation between VSPCS and anastomotic leakage. The contrast mode of the highest- and the lowest-fluorescence area was divided into the C-1,2 group (vessels not depicted pattern) and the C-3,4 group (vessels depicted pattern). The relationship between VSPCS and AL was then analyzed in the proximal and distal intestine. AL anastomotic leakage
In the distal-high, there was no significant difference between C-1,2 and C-3,4 groups (P = 1.000). In the distal-low, AL was observed in 6 of 29 cases in C-1,2 (21%) and 3 of 40 cases of C-3,4 (8%). There was a significant difference in AL between the C-1,2 and C-3,4 groups (P = 0.030).
Localization of hypoperfusion areas
Among the 18 cases of C-1,2 at the lowest-fluorescence area in the proximal intestine, 11 cases were judged to be C-1,2 throughout the whole proximal intestinal tract, while 7 cases were C-1,2 at only a part of the tract (Fig. 8).
Localization of C-1,2 (non-vessel) areas. Localization of the sites judged to be C-1,2 (vessels non-depicted pattern) in the proximal and distal intestine. Whole: the entire intestinal tract was uniformly distributed to the C-1,2 category, Partial: only a part of the intestinal tract was distributed to the C-1,2 category
Among the 29 cases of C-1,2 at the lowest-fluorescence area in the distal intestine, 13 cases were judged to be C-1,2 throughout the whole distal intestinal tract, while 16 cases were C-1,2 at only a part of the tract. AL occurred not only in cases with C-1,2 throughout the whole intestinal tract but also in cases with C-1,2 at only a part of the tract.
Cases of anastomotic leakage
Figure 9 shows the cases with AL. Case 1 was a patient who underwent low anterior resection with side-to-side anastomosis. In this case, ICG fluorescence imaging showed a partial decline of contrast near the anastomosis in the proximal intestine. Postoperative endoscopy revealed a scar at this point, and it seems that AL occurred at this point.
Anastomotic leakage cases. Case 1: ICG fluorescence imaging showed a partial decline of contrast near the anastomosis in the proximal intestine. Postoperative endoscopy revealed a scar at this point. Case 2: A partial deficit of contrast was observed at the line of the linear stapler on the rectal stump. A granulation was observed. Case 3: The lines of the circular stapler and the linear stapler were very close to each other. A partial decline of contrast and a fistula were also observed at this point
Case 2 was a patient who underwent low anterior resection with side-to-end anastomosis. In this case, a partial deficit of contrast was observed at the line of the linear stapler on the rectal stump. Postoperative endoscopy revealed granulation at this point, and it seems that AL occurred at this point.
Case 3 was a patient who underwent sigmoid colon resection with side-to-end anastomosis. A partial decline of contrast was observed between the anastomotic orifice and the line of the linear stapler in the distal intestine. Postoperative endoscopy revealed a fistula at this point, and it seems that AL occurred at this point.
Discussion
We observed real perfusion after anastomosis in detail and examined the cause of AL. The risk factors for AL consist of preoperative, intraoperative, and postoperative factors. Preoperative factors include radiation, chemotherapy, nutrition, and comorbid conditions, such as diabetes mellitus. Associated intraoperative factors include anastomotic perfusion and the surgical technique, blood loss, operation time, and anastomotic tension. Postoperative factors include the presence of diverting stoma, placement of transanal drainage tube or abdominal drains, and changes of the intestinal microbes [10]. Among these risk factors, perfusion and the surgical technique are considered to play major roles in the development of AL [5, 20,21,22].
One of the methods for assessing perfusion is NIR fluorescence imaging with ICG and many reports have been published regarding the usefulness of ICG fluorescence imaging for assessing perfusion, including multicenter phase II trials [13, 23].
One of the issues with assessing perfusion by ICG fluorescence imaging is the difficulty of quantitative assessment and the lack of unified judgment criteria. At present, there are a few reports of the quantitative assessment of perfusion in the colorectal anastomotic region. To our knowledge, there are only two reports of such a quantitative assessment in colorectal surgery. In one report by Wada et al., they observed a correlation between the Fmax and AL [24]. The other is the report by Son et al., which found a correlation between time from first fluorescence increase to half of maximum (T1/2MAX) and AL, and a correlation between the time ratio (TR = T1/2MAX/ΔT) and AL [25]. In the field of esophagus, Kamiya et al. pointed out the relationship between the time to reach half of the maximum fluorescence and AL [26]. In the present study, we conducted a quantitative assessment. In our study, Fmax, Tmax, and ΔT in the highest-fluorescence area of the anastomosis did not clearly correlate with AL, while Tmax and ΔT of the lowest-fluorescence area of the distal intestine were associated with AL (Table 2).
However, these quantitative assessments are too complicated to make quick decisions during the surgery. So, we also examined VSPCS by the visual contrast mode of the mucosa. In the present study, there was a significant difference between the C-1,2 (vessels not depicted pattern) and C-3,4 (vessels depicted pattern) among the AL cases. There were 36 cases with VSPCS 3-4 in both the proximal and distal sides, and it led to AL in only one case. This patient underwent mobilization of splenic flexure because of tension of anastomosis. Although the cause of AL was unclear, the patient recovered in only 3 weeks and abnormal findings were absent when he underwent sigmoidoscopy. We think the presence of the contrasted vessels can be judged simply and in real time during surgery, and VSPCS is a useful, as well as quantitative, assessment.
The localization of the low-perfusion areas in Fig. 8 shows that in some cases, low perfusion occurred throughout the whole intestinal tract, as was generally suspected, while in others, it only occurred in a part of the intestinal tract. In addition, AL can occur in these two patterns as well. Case 1 in Fig. 9 is an example of a case in which only part of the proximal intestinal tract showed low perfusion. It was not clear why a low-perfusion area was observed at this point, but AL occurred at this point. Thus, the observation of a more detailed site is required in assessing the perfusion. Observations from the serosal side, where the visual field is restricted, may be unable to obtain information on these partial low-perfusion areas.
Furthermore, in cases of low anterior resection, it is very difficult to observe the distal intestine from outside of the intestinal lumen in a narrow pelvis. In Case 2, deficiency of the contrast was seen along the staple line of the rectal stump, and fluorescence did not reach the cutting edge. The injury of the straight artery of the mesorectum is a possible reason. In this case, punching of the circular stapler deviated aside. If the circular stapler punched the center of the cutting edge, the low-perfusion area was minimized. There are few reports about perfusion of the distal intestine. However, perfusion at the rectal stump decreased due to intestinal dissection in 76.3% of patients as reported by Vignali et al. [20]. They showed, using laser-Doppler flowmetry, that the perfusion at the rectal stump decreased by an average of 6.2% in cases without AL, and by an average of 16% in cases with AL. In the present study, observation of the whole circumference of anastomosis in the proximal and distal intestines was possible in all 17 cases of lower rectal cancer in a deep pelvis by transanal ICG-NIR imaging. There is only one report, other than ours, about ICG-NIR observation from the luminal side by the transanal approach [27], but we think it is a useful method to assess the perfusion, especially in the lower rectum.
Anastomotic procedures, as well as perfusion, are a cause of AL in left-sided colon cancer surgery. In Case 3 in Fig. 9, the anastomotic orifice made by a circular stapler was shifted to the left, and the lines of the linear stapler and the circular stapler did not cross each other and were very close to each other. In this case, the angle formed by the anastomotic orifice and the stump was too acute to perfuse this area. Investigating the cause of AL by transanal ICG fluorescence imaging is useful in exploring safer anastomotic procedures.
Observation by transanal ICG-NIR fluorescence imaging evaluates not only simple perfusion of the proximal intestine before anastomosis but also the actual perfusion around the anastomosis influenced by factors, such as the anastomotic procedure, tension, and state of the distal intestine.
The limitation of this method is that it is difficult to re-anastomose. We encountered two cases with sigmoid colon cancer which underwent re-anastomosis, but we think it is difficult in the cases with lower rectal cancer. In such a difficult case, construction of a diverting stoma or placement of a transanal drainage tube is considered.
Detailed observation by a transanal approach can contribute to the investigation of how the surgical and patient factors (i.e., the site of vessel ligation, method of anastomosis, age, and complications) influence the anastomotic perfusion. Although there is the limitation of re-anastomosis, it seems to lead to the development of a safer surgical procedure to examine the actual perfusion of the anastomotic site.
Conclusion
ICG-NIR fluorescence observation by transanal approach can be an effective method to evaluate local perfusion over the entire circumference of anastomosis in detail and in real time. We believe this method is useful to search for safer operative management.
References
National Cancer Center. Cancer Registry and Statistics. Tokyo, Japan. Available at: https://ganjoho.jp/en/professional/statistics/table_download.html. Accessed 6 Dec 2018
Ministry of Health, Labour and Welfare. Patient Survey, 2014. Tokyo, Japan. Available at: https://www.e-stat.go.jp/dbview?sid=0003128799. Accessed 6 Dec 2018
Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, Finan P (2011) Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg 253:890–899
Branagan G, Finnis D (2005) Prognosis after anastomotic leakage in colorectal surgery. Dis Colon Rectum 48:1021–1026
Kingham TP, Pachter HL (2009) Colonic anastomotic leak: risk factors, diagnosis, and treatment. J Am Coll Surg 208:269–278
Shiomi A, Ito M, Maeda K, Kinugasa Y, Ota M, Yamaue H, Shiozawa M, Horie H, Kuriu Y, Saito N (2015) Effects of a diverting stoma on symptomatic anastomotic leakage after low anterior resection for rectal cancer: a propensity score matching analysis of 1014 consecutive patients. J Am Coll Surg 220:186–194
Qu H, Liu Y, Bi DS (2015) Clinical risk factors for anastomotic leakage after laparoscopic anterior resection for rectal cancer: a systematic review and meta-analysis. Surg Endosc 29:3608–3617
Kang CY, Halabi WJ, Chaudhry OO, Nguyen V, Pigazzi A, Carmichael JC, Mills S, Stamos MJ (2013) Risk factors for anastomotic leakage after anterior resection for rectal cancer. JAMA Surg 148:65–71
Matsubara N, Miyata H, Gotoh M, Tomita N, Baba H, Kimura W, Nakagoe T, Simada M, Kitagawa Y, Sugihara K, Mori M (2014) Mortality after common rectal surgery in Japan: a study on low anterior resection from a newly established nationwide large-scale clinical database. Dis Colon Rectum 57:1075–1081
Kawada K, Sakai Y (2016) Preoperative, intraoperative and postoperative risk factors for anastomotic leakage after laparoscopic low anterior resection with double stapling technique anastomosis. World J Gastroenterol 22:5718–5727
Boni L, Fingerhut A, Marzorati A, Rausei S, Dionigi G, Cassinotti E (2017) Indocyanine green fluorescence angiography during laparoscopic low anterior resection: results of a case-matched study. Surg Endosc 31:1836–1840
Kim JC, Lee JL, Yoon YS, Alotaibi AM, Kim J (2016) Utility of indocyanine-green fluorescent imaging during robot-assisted sphincter-saving surgery on rectal cancer patients. Int J Med Robot Comput Assist Surg 12:710–717
Jafari MD, Lee KH, Halabi WJ, Mills SD, Carmichael JC, Stamos MJ, Pigazzi A (2013) The use of indocyanine green fluorescence to assess anastomotic perfusion during robotic assisted laparoscopic rectal surgery. Surg Endosc 27:3003–3008
Kudszus S, Roesel C, Schachtrupp A, Höer JJ (2010) Intraoperative laser fluorescence angiography in colorectal surgery: a noninvasive analysis to reduce the rate of anastomotic leakage. Langenbecks Arch Surg 395:1025–1030
Muto Y, Miyauchi H, Ohira G, Hayano K, Narushima K, Suzuki K, Matsubara H (2018) Evaluation of the anastomotic blood flow with near-infrared ICG fluorescence angiography with intraoperative transanal endoscopy to predict anastomotic leakage after colorectal surgery. Chiba Med J 94:25–31
Son YJ, Kim JE, Park SB, Lee SH, Chung YS, Yang HJ (2013) Quantitative analysis of intraoperative indocyanine green video angiography in aneurysm surgery. J Cerebrovasc Endovasc Neurosurg 15:76–84
Li H, Zhou J, Chi C, Mao Y, Yang F, Tian J, Wang J (2016) Clinical application of near-infrared thoracoscope with indocyanine green in video-assisted thoracoscopic bullectomy. J Thorac Dis 8:1841–1845
Paiella S, De Pastena M, Landoni L, Esposito A, Casetti L, Miotto M, Ramera M, Salvia R, Secchettin E, Bonamini D, Manzini G, D’Onofrio M, Marchegiani G, Bassi C (2017) Is there a role for near-infrared technology in laparoscopic resection of pancreatic neuroendocrine tumor? Results of the COLPAN “colour-and-resect the pancreas” study. Surg Endosc 31:4478–4484
Barnes TG, Penna M, Hompes R, Cunningham C (2017) Fluorescence to highlight the urethra: a human cadaveric study. Tech Coloproctol 21:439–444
Vignali A, Gianotti L, Braga M, Radaelli G, Malvezzi L, Di Carlo V (2000) Altered microperfusion at the rectal stump is predictive for rectal anastomotic leak. Dis Colon Rectum 43:76–82
Kologlu M, Yorganci K, Renda N, Sayek I (2000) Effect of local and remote ischemia-reperfusion injury on healing of colonic anastomoses. Surgery 128:99–104
Sheridan WG, Lowndes RH, Young HL (1987) Tissue oxygen tension as a predictor of colonic anastomotic healing. Dis Colon Rectum 30:867–871
Ris F, Liot E, Buchs NC, Kraus R, Ismael G, Belfontali V, Douissard J, Cunningham C, Lindsey I, Guy R, Jones O, George B, Morel P, Mortensen NJ, Hompes R, Cahill RA, Near-Infrared Anastomotic Perfusion Assessment Network VOIR (2018) Multicentre phase II trial of near-infrared imaging in elective colorectal surgery. Br J Surg 105(10):1359–1367
Wada T, Kawada K, Takahashi R, Yoshitomi M, Hida K, Hasegawa S, Sakai Y (2017) ICG fluorescence imaging for quantitative evaluation of colonic perfusion in laparoscopic colorectal surgery. Surg Endosc 31:4184
Son GM, Kwon MS, Kim Y, Kim J, Kim SH, Lee JW (2018) Quantitative analysis of colon perfusion pattern using indocyanine green (ICG) angiography in laparoscopic colorectal surgery. Surg Endosc 33(5):1640–1649
Kamiya K, Unno N, Miyazaki S, Sano M, Kikuchi H, Hiramatsu Y, Ohta M, Yamatodani T, Mineta H, Konno H (2015) Quantitative assessment of the free jejunal graft perfusion. J Surg Res 194:394–399
Sherwinter DA, Gallagher J, Donkar T (2013) Intra-operative transanal near infrared imaging of colorectal anastomotic perfusion: a feasibility study. Colorectal Dis 15:91–96
Acknowledgements
The authors thank Chiba Medical Journal for providing the figure data (Fig. 1). We thank Editage (www.editage.jp) for English language editing. This work was supported by JSPS KAKENHI Grant Number JP 18K16343.
Funding
This work was supported by JSPS KAKENHI Grant Number JP 18K16343.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Hiroyuki Amagai, Hideaki Miyauchi, Yorihiko Muto, Masaya Uesato, Gaku Ohira, Shunsuke Imanishi, Tetsuro Maruyama, Toru Tochigi, Koichiro Okada, Michihiro Maruyama, and Hisahiro Matsubara have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Amagai, H., Miyauchi, H., Muto, Y. et al. Clinical utility of transanal indocyanine green near-infrared fluorescence imaging for evaluation of colorectal anastomotic perfusion. Surg Endosc 34, 5283–5293 (2020). https://doi.org/10.1007/s00464-019-07315-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-019-07315-7