Abstract
Introduction
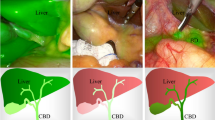
Near-infrared fluorescence cholangiography (NIRF-C) is a popular application of fluorescence image-guided surgery (FIGS). NIRF-C requires near-infrared optimized laparoscopes and the injection of a fluorophore, most frequently Indocyanine Green (ICG), to highlight the biliary anatomy. It is investigated as a tool to increase safety during cholecystectomy. The European registry on FIGS (EURO-FIGS: www.euro-figs.eu) aims to obtain a snapshot of the current practices of FIGS across Europe. Data on NIRF-C are presented.
Methods
EURO-FIGS is a secured online database which collects anonymized data on surgical procedures performed using FIGS. Data collected for NIRF-C include gender, age, Body Mass Index (BMI), pathology, NIR device, ICG dose, ICG timing of administration before intraoperative visualization, visualization (Y/N) of biliary structures such as the cystic duct (CD), the common bile duct (CBD), the CD-CBD junction, the common hepatic duct (CHD), Visualization scores, adverse reactions to ICG, operative time, and surgical complications.
Results
Fifteen surgeons (12 European surgical centers) uploaded 314 cases of NIRF-C during cholecystectomy (cholelithiasis n = 249, cholecystitis n = 58, polyps n = 7), using 4 different NIR devices. ICG doses (mg/kg) varied largely (mean 0.28 ± 0.17, median 0.3, range: 0.02–0.62). Similarly, injection-to-visualization timing (minutes) varied largely (mean 217 ± 357; median 57), ranging from 1 min (direct intragallbladder injection in 2 cases) to 3120 min (n = 2 cases). Visualization scores before dissection were significantly correlated, at univariate analysis, with ICG timing (all structures), ICG dose (CD-CBD), device (CD and CD-CBD), surgeon (CD and CD-CBD), and pathology (CD and CD-CBD). BMI was not correlated. At multivariate analysis, pathology and timing remained significant factors affecting the visualization scores of all three structures, whereas ICG dose remained correlated with HD visualization only.
Conclusions
The EURO-FIGS registry has confirmed a wide disparity in ICG dose and timing in NIRF-C. EURO-FIGS can represent a valuable tool to promote and monitor FIGS-related educational and consensus activities in Europe.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Fluorescence image-guided surgery (FIGS) defines the intraoperative use of an optical navigation imaging modality, which provides an enhanced visualization of metabolic processes and/or anatomical structures [1,2,3]. FIGS is achieved by means of near-infrared (NIR) light-powered systems and video-cameras which are able to excite, collect, and display the fluorescence signal emitted by fluorophores which can be endogenous tissue components or exogenous compounds administered to the patient [4]. FIGS has raised a substantial interest over the last decade. It is currently tested in a variety of medical fields, including digestive surgery [5, 6]. Near-infrared fluorescence cholangiography (NIRF-C) is one of the most popular and promising applications. The objective of NIRF-C is to intraoperatively highlight the biliary anatomy and, through such an enhancement, to potentially prevent bile duct injuries (BDI) [7]. The anatomical enhancement is obtained with an intravenous injection of a bile-excreted fluorophore, e.g., indocyanine green (ICG). The exposure of the operative field to an NIR light source excites the fluorophore, which flows within the main biliary ducts. The subsequent emission of energy produces the fluorescence signal and allows to visualize the content of the ducts and their anatomical profile. The main interest of using NIR light lies in the fact that it penetrates deeper into tissues when compared to white light. This property should allow to better identify and document the biliary anatomy during or even before the beginning of the surgical dissection. NIRF-C was introduced by Ishizawa et al. in [8], and so far several trials have been performed, as reported in recent review articles [9,10,11], demonstrating that NIRF-C can be a valid imaging tool to improve the identification of biliary structures. Dip et al. have recently published the first randomized multicenter clinical trial comparing the identification of the biliary anatomy with NIRF-C versus white light only, confirming the enhanced ability of recognition of biliary structures provided with fluorescence imaging [12]. The analysis of the literature has revealed a large disparity in the protocols, including major differences in terms of dosing and timing of administration of the ICG [11], which are among the controllable factors affecting the performance of NIRF-C [10]. This variability can be partly explained by the exploratory phase and the lack of guidelines. In turn, this impedes a proper evaluation of the technique and makes it even more difficult to provide evidence to recommend the introduction of NIRF-C.
A positive strategy towards a better evaluation of NIRF-C is to ensure a uniformity of practices through educational and dissemination activities. Bearing this in mind, a European registry on Fluorescence Imaging Guided Surgery (EURO-FIGS: www.euro-figs.eu) has been created, in order to obtain a snapshot of the current practices of NIRF-C across Europe, which could serve as a reference point for future consensus meetings and guidelines articles.
Additionally, we aimed to promote networking throughout European surgical departments, share experiences, and set up collaborations on FIGS-related clinical applications.
The EURO-FIGS registry is a joint effort between the IRCAD, the IHU-Strasbourg Institute of Image-Guided Surgery, and the Technology Committee of the European Association of Endoscopic Surgery (EAES). The registry is currently collecting data on 3 applications related to digestive surgery: (1) near-infrared cholangiography; (2) anastomotic perfusion evaluation, and (3) fluorescence-based lymphography. The results of a 2-year data collection on NIRF-C are presented in this manuscript.
Methods
The EURO-FIGS online platform
EURO-FIGS is a secured online database which collects cases performed using FIGS and accessible by members only. Data collected are completely anonymous. The creation of the registry was approved by the University of Strasbourg and it was communicated to the French authority protecting privacy, which translates to the National Commission of Informatics and Liberty (CNIL or Commission Nationale de l’Informatique et des Libertés), under the Reference Number 2007309v0. The registry is endorsed by the European Association of Endoscopic Surgery (EAES), which is a leading surgical society in Europe. Data collection is centralized at IHU-Strasbourg, France.
Participants were directly contacted by the PI (MD). Along with the invitation letter, participants received a specific consent form to be signed by the patients whose data would be added to the registry. The consent form was originally prepared in English, Italian, and French. When required, the contributors translated it into Spanish, German, Dutch, Romanian, and Lithuanian.
The aims of the registry are multiple, including the possibility to scrutinize differences in practice across Europe, and to collect safety and efficacy data on FIGS.
Data collected
The registry is easy to fill in and takes approximately 2 min per case. Data collected for NIRF-C include the following: (1) gender, (2) age, (3) Body Mass Index (BMI), (4) pathology, (5) NIR device used, (6) ICG dose, (7) ICG timing, (8) visualization (Y/N) of biliary structures such as cystic duct (CD), common bile duct (CBD), the CD-CBD junction, and common hepatic duct (CHD), (9) visualization scores, (10) adverse reactions to ICG, (11) operative time, and (12) surgical complications. The details about the surgical technique itself were not asked for.
Scores
A 5-point Likert scale (1 = poor, 2 = sufficient, 3 = fair, 4 = good, 5 = excellent) was used to score the quality of visualization of biliary structures before and after dissection. Additional scores were filed regarding (1) how much the was the fluorescence imaging perceived to be helpful in the specific case (HELPFUL score), and (2) how much the background fluorescence from the liver (liver-to-ducts contrast) was perceived to be disturbing (DISTURBED score, the lower the better). The helpful score was built as follows on a 0 to 3 scale: 0 = not helpful, 1 = moderately helpful (increased intraoperative confidence), 2 = very helpful (enabled safer dissection), and 3 = highly helpful (could prevent a potential biliary injury). The disturbed score was scaled from 0 to 4 as follows: 0 = absence of disturbance from liver fluorescence background, 1 = lightly disturbed (all structures were visible in the NIR mode), 2 = disturbed visualization, but the CD-CBD junction was clearly visible before dissection, (3) disturbed visualization and CD-CBD junction was only visible after dissection, (4) heavily disturbed: it was impossible to correctly visualize biliary structures.
Statistical analysis
Statistics were performed using Graph-Pad Prism®, Version 6.07 (Graph Pad Software Inc.). A Pearson’s rho coefficient was calculated to evaluate the correlations between image quality score, the ICG dose and timing of ICG administration, Body Mass Index (BMI), and the pathology (cholecystitis/cholelithiasis). ANOVA followed by Tukey’s multiple comparison test were used to calculate p values for continuous variables. A Fisher’s exact test was used to calculate p values for categorical variables. A multivariate analysis was performed using the R-package (R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: https://www.R-project.org/). A p value < 0.05 was considered statistically significant.
Results
Fifteen surgeons from 12 different centers uploaded their data on fluorescence-based cholangiography (NIRF-C) on the web-based EURO-FIGS registry, from March 2017 to January 2019. During this period, 314 patients (190 female/124 male, mean age: 53.73 ± 15.51 years, mean BMI 27.78 ± 5.47 kg/m2) were included. The distribution per country was as follows: Italy (n = 198), France (n = 53), The Netherlands (n = 33), Spain (n = 20), Lithuania (n = 9), Switzerland (n = 1).
The indication for cholecystectomy was cholelithiasis (n = 249), cholecystitis (n = 58), and polyps (n = 7). The following NIR cameras were used: (1) D-Light P (Karl Storz, Germany, D1, n = 239), (2) Firefly (Surgical Intuitive, USA, D2, n = 53), (3) SPY (Stryker, USA, D3, n = 21), (4) Pinpoint (Novadaq, Canada, D4, n = 1).
A single adverse event directly related to ICG administration (self-resolving cutaneous rush) was reported. There were no bile duct injuries.
There was a large disparity in the reported doses of ICG which was administered (mean 0.28 ± 0.17 mg/kg, median 0.3 mg/kg, range from 0.02 to 0.62 mg/kg). Similarly, the timing of the fluorophore administration before intraoperative visualization varied largely (mean 217 ± 357 min; median 57 min), ranging from 1 min (direct intragallbladder injection in 2 cases) to more than 2 days (3120 min, in 2 cases) (Fig. 1).
Overall visualization results of biliary structures and related visualization scores are reported in Table 1.
At univariate linear regression, the variables that were significantly correlated to the visualization scores of relevant structures (CD, CD-CBD and HD) before dissection were the following: (1) ICG interval timing (all structures), (2) ICG dose (CD-CBD junction), (3) device (CD and CD-CBD), (4) surgeon subjective scoring (CD and CD-CBD), and (5) pathology (CD and CD-CBD) as reported in Table 2. In case of cholecystitis, the mean dose of ICG (mg/kg) allowing to obtain the best score (5 = excellent), before dissection, was 0.23 ± 0.15, 0.22 ± 0.14, and 0.2 ± 0.14 for CD , CD-CBD, and HD, respectively. There was no significant difference between the dose given in cases of score 0 (no visualization, 0.3 ± 0.17 mg/kg) and score 5 (p = 0.29) for the CD. For CD-CBD and for HD, there was a significant difference in doses (CD-CBD score 0 = 0.36 ± 0.17 mg/kg vs. score 5 = 0.22 ± 0.14 mg/kg, p = 0.005) and (HD score 0 = 0.393 ± 0.134, p = 0.006). Timing (minutes) conducive to the best score was 998.5 ± 1132, 1010 ± 1128, and 1022 ± 1123 for CD, CD-CBD and HD, respectively. Score 5 timing was significantly higher when compared to score 0 (no visualization) for CD (240 ± 208 min, p = 0.02), CD-CBD (205 ± 224 min, p = 0.005), and HD (212 ± 243 min, p = 0.001).
In case of cholelithiasis, the mean dose of ICG (mg/kg) allowing to obtain the best score (excellent) was 0.26 ± 0.18, 0.26 ± 0.175, and 0.235 ± 0.167 for CD, CD-CBD, and HD, respectively, before dissection. Concerning CD-CBD visualization, the dose was significantly higher when compared to the dose achieving score 0 (no visualization, 0.159 ± 0.201 mg/kg, p = 0.009), but significantly lower when compared to the dose achieving a score of 3 (0.394 ± 0.17 mg/kg, p = 0.04) and 4 (0.358 ± 0.163 mg/kg, p = 0.002). Similarly, for the visualization of HD, the dose providing excellent visualization was significantly lower when compared to the dose achieving score 2 (0.433 ± 0.115 mg/kg, p = 0.049), score 3 (0.4 ± 0.13 mg/kg, p = 0.016) and score 4 (0.321 ± 0.147 mg/kg, p = 0.01). Missing scores were not provided by the users. In the same conditions, the associated mean timing (minutes) conducive to the best score was 382.8 ± 377, 413 ± 379, and 484 ± 379 for CD, CD-CBD, and HD, respectively. Score 5 timing was significantly higher when compared to score 0 (no visualization) for CD (61.4 ± 34.8 min, p = 0.001), for CD-CBD (51.976 ± 27.3 min, p < 0.0001) and for HD (56.4 ± 50.1 min, p < 0.0001).
BMI had no influence on visualization scores. Lower visualization quality scores in near-infrared before dissection were reported in the case of cholecystitis for CD (2.76 ± 1.9 vs. 3.54 ± 1.6, p = 0.001) and for the CD-CBD junction (2.43 ± 2 vs. 3 ± 1.9, p = 0.04) but not for HD (2.12 ± 2.14 vs. 2.7 ± 2.2, p = 0.08), when compared to cholelithiasis.
Importantly, 3 surgeons (contributing with a total of 43 cases) were identified as being strong outliers and responsible for the correlation with visualization scores at univariate analysis. When these 3 outliers were removed, the variable “surgeon” was no longer significant. For this reason, coupled with the fact that three additional surgeons have only provided one case each, the variable “surgeon” was not included in the multivariate analysis. Similarly, the distribution of cases per device was too unbalanced and no cases of cholecystitis were performed with D2 (n = 53 cholelithiasis, from one center) and D4 (n = 1 cholelithiasis). Additionally, mean timing (min) was significantly different between the devices used: D1 247.7 ± 399 versus D2 47.7 ± 25.2 versus D3 28.05 ± 8.32 (D1 versus D2: p < 0.0001, D1 versus D3: p = 0.006, D2 versus D3: p = 0.001). For those reasons, the “device” variable was also excluded from the multivariate linear regression model which included dose, timing, and pathology.
At multivariate linear regression, pathology and timing remained significant factors affecting the visualization scores of all three structures, whereas the ICG dose was significantly correlated with HD visualization only (Table 3).
ICG guidance was considered to be highly helpful in 100/314 (31.84%) cases, very helpful in 101/314 (32.16%), moderately helpful in 61/314 (19.42%), and not helpful in 52/314 (16.56%) cases (score mean value 1.8 ± 1.06).
Liver background fluorescence was considered to be not disturbing in 200/314 (63.7%) cases. It was considered lightly disturbing in 44/114 (38.6%) cases, disturbing but the CD-CBD junction was clearly visible before dissection in 32/114 (28.07%) cases, disturbing and the CD-CBD junction was only visible after dissection in 32/114 (28.07%) cases, heavily disturbing and it was impossible to correctly visualize the critical structures in 6/114 (5.3%) cases. The mean disturbed score was 0.72 ± 1.11 and was not related to BMI nor to ICG dose, but significantly correlated to the timing of injection (Pearson’s: p = 0.0005) and strongly dependent on the device used [(D1 = 0.33 ± 0.78, D2 = 1.19 ± 1.47, D3 = 2.28 ± 0.74), D1 vs. D2 = p < 0.0001, D1 vs. D3 = p < 0.0001, D2 vs. D3 = p < 0.0001].
Discussion
NIRF-C can significantly enhance the visualization of the biliary tree [12] and has the advantages of being relatively inexpensive, radiation free, and perfectly integrated to the surgical workflow, with virtually no extra operative time when compared to X-ray intraoperative cholangiography (IOC) [13].
Drawbacks of NIRF-C lie in the need to inject a fluorophore, the inability to detect retained stones, and the noise fluorescence signal from the liver. In a comparative study, the relatively high fluorescence liver background led surgeons to assign a lower score to the image quality obtained with NIRF-C, when compared to X-ray IOC [13].
As previously stated, a wide disparity in NIRF-C protocols has been pointed out in review articles [9,10,11], particularly in terms of dosing and timing of ICG administration. The optimization of those two major controllable factors influences the noise liver signal and the image quality and, consequently the performances of NIRF-C. Other non-controllable factors include pathology (inflammatory status) [14], BMI [15], and sensitivity of the NIR device [16].
Data from the EURO-FIGS registry allowed to confirm the large differences of NIRF-C practices in several European centers, particularly regarding the dose of ICG and the interval timing between ICG administration and intraoperative imaging, as reported in Fig. 1.
The overall binary (y/n) visualization results reported by EURO-FIGS members (Table 1) are slightly lower but still congruent with other published studies [9,10,11, 17,18,19,20,21,22,23,24]. The significant higher rate of positive visualization (and higher scores) after dissection is obvious and has been reported for the sake of completeness.
The univariate analysis of EURO-FIGS data confirmed the influence of most of the abovementioned factors (the ICG dose and timing, the device, the pathology) on the quality of visualization. The BMI was not correlated to the visualization scores. On the other hand, the surgeon, being the score a subjective metric, was significantly correlated at the univariate analysis. However, as reported in the results, 3 surgeons (43 cases) were providing consistently extreme visualization scores. When these 3 outliers were removed, the variable “surgeon” was no longer significant at the univariate. Additionally, three other surgeons have only provided one case each. The device itself plays a role in the quality of visualization [16]. The various commercially available systems are equipped with different light sources, including filtered xenon lamps, laser diodes, and light-emitting diodes (LEDs) to excite the fluorophores [25]. Those different technologies influence the sensitivity of the devices. However, the distribution of the devices was unbalanced in the EURO-FIGS registry, at this stage. For those reasons, both variables “surgeon” and device were not included in the multivariate model.
ICG timing was strongly correlated to the visualization scores of all three target biliary structures, CD, CD-CBD, and HD, as presented to EURO-FIGS users. A prolonged time interval provided higher scores, since the fluorophore washout reduces the fluorescence noise from the liver, as previously reported [26].
At univariate analysis, the ICG dose was significantly (negatively) correlated to the CD-CBD junction visualization scores only whereas the ICG dose influence was no longer significant for the CD-CBD junction but was significant for HD visualization at the multivariate model. Timing and pathology seemed to have more impact on visualization determination.
In recent reviews of the literature, the reported ICG doses range from 2.5 mg in a single IV administration to 0.5 mg/kg [9,10,11]. In a study by Zarrinpar et al., the best biliary ducts to liver fluorescence ratio was obtained with 0.25 mg/kg of ICG, administered at least 45 min before the images were acquired [27]. The relationship between the visualization scoring system and the ICG dose and timing (Fig. 2) confirmed that higher scores were achieved with prolonged time interval and with an optimal dose. As an example, the mean dose associated with a score of 5 (excellent visualization), limited to the visualization of the CD-CBD junction in cholelithiasis, was very close to the findings of Zarrinpar et al. (0.26 ± 0.175). It was significantly higher when compared to the dose achieving a score of 0 (no visualization, 0.159 ± 0.201 mg/kg, p = 0.009), but significantly lower when compared to the one achieving a score of 3 (0.394 ± 0.17 mg/kg, p = 0.04) and 4 (0.358 ± 0.163 mg/kg, p = 0.002). Concerning timing, the sweet spot seems to be much higher than the “at least 45 min” (despite such timing is the most practical one, meaning immediately after patient intubation). Timing resulted in being even more relevant than the dose based on the data and the methodology of the assessment used in the EURO-FIGS registry, and was certainly limited by the subjectivity of the scoring. In case of score 0, timing was mostly found in the 50- to 60-min range (Fig. 2). In the registry, one could find an optimal time-dose combination (0.3 mg/kg administered approximately 6 h before intraoperative visualization) as the converging point to obtain a score of 5 (excellent visualization).
However, timing presented a wide standard deviation and more entries and/or a reasonable consensus are probably required to reduce the disparity.
A strategy which allows to overcome the drawback of liver noise fluorescence is to inject ICG directly into the gallbladder [28]. This is particularly interesting in case of cholecystitis which has been managed with percutaneous drainage. The drain left in place can be used to inject the dye during interval cholecystectomy. Alternatively, it is possible to simply puncture the gallbladder intraoperatively, but there is some risk of dye spillage, which could impair the visibility by contaminating the operative field [14]. Additionally, avoiding systemic ICG injection, intragallbladder administration allows for a micro-dosing of the fluorophore. The performances of direct injection were more relevant in case of cholecystitis, which is a strong point, considering that the presence of inflammation affects the visualization of biliary structures negatively, as reported in the literature and in EURO-FIGS data. This strategy was successfully applied to two cases which were uploaded to the registry.
The EURO-FIGS registry has some limitations. First, due to the network of the promoter and also to some specific regulations in some European countries, the geographical span is relatively limited and this could hinder the proper understanding of the use of FIGS in Europe. Therefore, extrapolating and generalizing registry data is reductive at this stage. However, the data seem to be congruent with the literature. Additionally, the majority of active members are individually active in the field and, consequently, are an appropriate and representative sample. Secondly, the data of the two-year collection were unbalanced in terms of the NIR devices used, which limits the possibility of testing the device sensitivity impact on performances. With longer data collection with diversified devices, we could expect the registry to provide indications for a tailored device-specific dose/timing combination. Paradoxically, at least based on a subjective perception, it seems that a lower device sensitivity is a positive feature for this specific application of fluorescence cholangiography, yielding a lower disturbance from the background fluorescence. Thirdly, there are no comparative data of patients operated on without the use of the fluorescence imaging uploaded to the registry. The absence of a comparative group is preventing us from obtaining more robust data on the efficacy of the NIRF-C beyond the subjective perception of the users. As the surgeon has an impact on visualization scores, the subjective nature of the scoring systems proposed in the registry is another structural limitation, which is however common to other imaging modalities. It is hard to assess whether the surgeon’s scoring is linked to his/her experience with the technology or linked to a personal interpretation of the items proposed on a Likert scale. Finally, there is a risk of case selection. Nevertheless, the registry seems to be a powerful tool to promote educational activities in order to homogenize the practices and share knowledge. As per NIRF-C, a follow-up will consist in a consensus activity. The registry could help monitor the uptake of potential guidelines and also help evaluate the impact of practice changes on technology performances. The next sensible step is to expand the FIGS registry initiative outside Europe in collaboration with surgical societies.
Conclusions
The EURO-FIGS registry on fluorescence-guided surgery confirmed a wide disparity in terms of protocols for near-infrared cholangiography, across several European surgical centers, particularly in terms of ICG dose and timing of administration. The registry can represent a valuable tool to promote and monitor FIGS-related educational and consensus activities in Europe.
References
Mascagni P, Longo F, Barberio M, Seeliger B, Agnus V, Saccomandi P, Hostettler A, Marescaux J, Diana M (2018) New intraoperative imaging technologies: innovating the surgeon’s eye toward surgical precision. J Surg Oncol 118:265–282
Diana M (2017) Enabling precision digestive surgery with fluorescence imaging. Transl Gastroenterol Hepatol 2:97
Diana M (2017) Fluorescence-guided surgery applied to the digestive system: the cybernetic eye to see the invisible. Cir Esp 96:65–68
Gioux S, Choi HS, Frangioni JV (2010) Image-guided surgery using invisible near-infrared light: fundamentals of clinical translation. Mol Imaging 9:237–255
van Manen L, Handgraaf HJM, Diana M, Dijkstra J, Ishizawa T, Vahrmeijer AL, Mieog JSD (2018) A practical guide for the use of indocyanine green and methylene blue in fluorescence-guided abdominal surgery. J Surg Oncol 118:283–300
Baiocchi GL, Diana M, Boni L (2018) Indocyanine green-based fluorescence imaging in visceral and hepatobiliary and pancreatic surgery: state of the art and future directions. World J Gastroenterol 24:2921–2930
Pesce A, Diana M (2018) Critical view of safety during laparoscopic cholecystectomy: from the surgeon’s eye to fluorescent vision. Surg Innov 25:197–198
Ishizawa T, Tamura S, Masuda K, Aoki T, Hasegawa K, Imamura H, Beck Y, Kokudo N (2009) Intraoperative fluorescent cholangiography using indocyanine green: a biliary road map for safe surgery. J Am Coll Surg 208:e1–e4
Pesce A, Piccolo G, La Greca G, Puleo S (2015) Utility of fluorescent cholangiography during laparoscopic cholecystectomy: a systematic review. World J Gastroenterol 21:7877–7883
van den Bos J, Wieringa FP, Bouvy ND, Stassen LPS (2018) Optimizing the image of fluorescence cholangiography using ICG: a systematic review and ex vivo experiments. Surg Endosc 32:4820–4832
Conrad C, Wakabayashi G, Asbun HJ, Dallemagne B, Demartines N, Diana M, Fuks D, Gimenez ME, Goumard C, Kaneko H, Memeo R, Resende A, Scatton O, Schneck AS, Soubrane O, Tanabe M, van den Bos J, Weiss H, Yamamoto M, Marescaux J, Pessaux P (2017) IRCAD recommendation on safe laparoscopic cholecystectomy. J Hepatobiliary Pancreat Sci 24:603–615
Dip F, LoMenzo E, Sarotto L, Phillips E, Todeschini H, Nahmod M, Alle L, Schneider S, Kaja L, Boni L, Ferraina P, Carus T, Kokudo N, Ishizawa T, Walsh M, Simpfendorfer C, Mayank R, White K, Rosenthal RJ (2019) Randomized trial of near-infrared incisionless fluorescent cholangiography. Ann Surg. https://doi.org/10.1097/SLA.0000000000003178
Diana M, Soler L, Agnus V, D’Urso A, Vix M, Dallemagne B, Faucher V, Roy C, Mutter D, Marescaux J, Pessaux P (2017) prospective evaluation of precision multimodal gallbladder surgery navigation: virtual reality, near-infrared fluorescence, and X-ray-based intraoperative cholangiography. Ann Surg 266:890–897
Liu YY, Liao CH, Diana M, Wang SY, Kong SH, Yeh CN, Dallemagne B, Marescaux J, Yeh TS (2017) Near-infrared cholecystocholangiography with direct intragallbladder indocyanine green injection: preliminary clinical results. Surg Endosc 32:1506–1514
Igami T, Nojiri M, Shinohara K, Ebata T, Yokoyama Y, Sugawara G, Mizuno T, Yamaguchi J, Nagino M (2016) Clinical value and pitfalls of fluorescent cholangiography during single-incision laparoscopic cholecystectomy. Surg Today 46:1443–1450
Kono Y, Ishizawa T, Tani K, Harada N, Kaneko J, Saiura A, Bandai Y, Kokudo N (2015) Techniques of fluorescence cholangiography during laparoscopic cholecystectomy for better delineation of the bile duct anatomy. Medicine 94:e1005
Buchs NC, Hagen ME, Pugin F, Volonte F, Bucher P, Schiffer E, Morel P (2012) Intra-operative fluorescent cholangiography using indocyanin green during robotic single site cholecystectomy. Int J Med Robot 8:436–440
Buchs NC, Pugin F, Azagury DE, Jung M, Volonte F, Hagen ME, Morel P (2013) Real-time near-infrared fluorescent cholangiography could shorten operative time during robotic single-site cholecystectomy. Surg Endosc 27:3897–3901
Spinoglio G, Priora F, Bianchi PP, Lucido FS, Licciardello A, Maglione V, Grosso F, Quarati R, Ravazzoni F, Lenti LM (2013) Real-time near-infrared (NIR) fluorescent cholangiography in single-site robotic cholecystectomy (SSRC): a single-institutional prospective study. Surg Endosc 27:2156–2162
Ishizawa T, Kaneko J, Inoue Y, Takemura N, Seyama Y, Aoki T, Beck Y, Sugawara Y, Hasegawa K, Harada N, Ijichi M, Kusaka K, Shibasaki M, Bandai Y, Kokudo N (2011) Application of fluorescent cholangiography to single-incision laparoscopic cholecystectomy. Surg Endosc 25:2631–2636
Dip FD, Asbun D, Rosales-Velderrain A, Lo Menzo E, Simpfendorfer CH, Szomstein S, Rosenthal RJ (2014) Cost analysis and effectiveness comparing the routine use of intraoperative fluorescent cholangiography with fluoroscopic cholangiogram in patients undergoing laparoscopic cholecystectomy. Surg Endosc 28:1838–1843
Dip F, Roy M, Lo Menzo E, Simpfendorfer C, Szomstein S, Rosenthal RJ (2015) Routine use of fluorescent incisionless cholangiography as a new imaging modality during laparoscopic cholecystectomy. Surg Endosc 29:1621–1626
Dip F, Nguyen D, Montorfano L, Szretter Noste ME, Lo Menzo E, Simpfendorfer C, Szomstein S, Rosenthal R (2016) Accuracy of near infrared-guided surgery in morbidly obese subjects undergoing laparoscopic cholecystectomy. Obes Surg 26:525–530
Boni L, David G, Mangano A, Dionigi G, Rausei S, Spampatti S, Cassinotti E, Fingerhut A (2015) Clinical applications of indocyanine green (ICG) enhanced fluorescence in laparoscopic surgery. Surg Endosc 29:2046–2055
Sajedi S, Sabet H, Choi HS (2019) Intraoperative biophotonic imaging systems for image-guided interventions. Nanophotonics 8:99–116
Verbeek FP, Schaafsma BE, Tummers QR, van der Vorst JR, van der Made WJ, Baeten CI, Bonsing BA, Frangioni JV, van de Velde CJ, Vahrmeijer AL, Swijnenburg RJ (2014) Optimization of near-infrared fluorescence cholangiography for open and laparoscopic surgery. Surg Endosc 28:1076–1082
Zarrinpar A, Dutson EP, Mobley C, Busuttil RW, Lewis CE, Tillou A, Cheaito A, Hines OJ, Agopian VG, Hiyama DT (2016) Intraoperative laparoscopic near-infrared fluorescence cholangiography to facilitate anatomical identification: when to give indocyanine green and how much. Surg Innov 23:360–365
Liu YY, Kong SH, Diana M, Legner A, Wu CC, Kameyama N, Dallemagne B, Marescaux J (2016) Near-infrared cholecysto-cholangiography with indocyanine green may secure cholecystectomy in difficult clinical situations: proof of the concept in a porcine model. Surg Endosc 30:4115–4123
Acknowledgements
The EURO-FIGS registry is a joint effort between the IRCAD, IHU-Strasbourg, and the Technology Committee of the European Association of Endoscopic Surgeons (EAES). The authors are grateful to Camille Goustiaux, Guy Temporal, and Christopher Burel, professionals in Medical English proofreading, for their valuable assistance.
Funding
The EURO-FIGS registry is funded by a grant from the ARC Foundation for Cancer Research (9, rue Guy Môquet; 94803 Villejuif Cedex, France, www.fondation-arc.org), within the framework of the ELIOS (Endoscopic Luminescent Imaging for precision Oncologic Surgery) project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Michele Diana is PI and the recipient of the ELIOS grant. Jacques Marescaux is President of both the IRCAD and IHU Institutes, which are partly funded by KARL STORZ, Medtronic, and Siemens Healthcare. Luigi Boni reports consulting position with various companies outside the submitted work. Vincent Agnus, Antonio Pesce, Jacqueline Van Den Bos, Salvador Morales-Conde, Alessandro Paganini, Silvia Quaresima, Andrea Balla, Gaetano La Greca, Haralds Plaudis, Gianluigi Moretto, Maurizio Castagnola, Caterina Santi, Lorenzo Casali, Luciano Tartamella, Alend Saadi, Andrea Picchetto, Alberto Arezzo have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Agnus, V., Pesce, A., Boni, L. et al. Fluorescence-based cholangiography: preliminary results from the IHU-IRCAD-EAES EURO-FIGS registry. Surg Endosc 34, 3888–3896 (2020). https://doi.org/10.1007/s00464-019-07157-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-019-07157-3