Abstract
Background
There is no consensus about the utility of using the robotic platform to perform a unilateral lateral transabdominal adrenalectomy in comparison with conventional laparoscopy. In some groups, obese patients (Body Mass Index > 30 kg/m2) and patients with tumor size > 5 cm have been considered as good candidates for robotic adrenalectomy. However, evaluation of incidence and risk factors for perioperative complications is currently lacking in large series of patients. The aim of this study was to evaluate incidence and predictive factors for intraoperative (conversion and capsular rupture) and postoperative complications (morbidity) after unilateral robotic-assisted transabdominal lateral adrenalectomy.
Methods
From 2001 to 2016, consecutive patients undergoing unilateral lateral transabdominal robotic adrenalectomy were included in a prospectively maintained database and analyzed retrospectively (clinicaltrials.gov NCT03410394).
Results
A total of 303 consecutive patients were analyzed. Between the first and last 100 of patients, mean tumor size increased from 2.9 to 4.2 cm (p < 0.001) and mean operating time decreased from 99 to 77 min (p < 0.001). Postoperative complications occurred in 28 patients (9.2%) and no postoperative death was observed. Nine patients (3%) were converted to open laparotomy and capsular rupture was observed in nine patients (3%). BMI was not a significant risk factor for conversion, capsular rupture, or postoperative complication. Tumor size > 5 cm remained the only predictive factor for conversion to laparotomy (OR 7.47, 95% CI 1.81–30.75; p = 0.005). History of upper gastrointestinal surgery was the only predictive factor for capsular rupture (OR 13.6, 95% CI 2.33–80.03; p = 0.004). Conversion to laparotomy (OR 8.35, 95% CI 1.99–35.05; p = 0.003) and patient age (OR 1.039, 95% CI 1.006–1.072; p = 0.019) remained independent predictive factors for postoperative complications.
Conclusions
This study identified independent risk factors for perioperative complications after robotic-assisted unilateral adrenalectomy. These factors should be taken into account when evaluating robotic-assisted transabdominal lateral adrenalectomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Conventional laparoscopic adrenalectomy is the procedure of choice for the surgical management of most benign unilateral adrenal tumors, with an overall postoperative complication rate around 10% [1,2,3,4,5]. Widespread adoption of robotic technology has positioned robotic adrenalectomy as an option in some medical centers with proof of feasibility and safety [6,7,8,9,10,11]. Nevertheless, no clear benefit from the use of the robotic platform has been validated in comparative studies with conventional laparoscopic adrenalectomy. Some authors have considered patients with BMI > 30 kg/m2, tumor size > 5 cm, and those with previous history of abdominal surgery as good candidates for robotic surgery [6, 12]. Others have demonstrated that a laparoscopic approach is safe, even with tumors > 8 cm [13]. However, large series evaluating intraoperative and postoperative complications after robotic adrenalectomy are currently not available.
Since 2001, we have evaluated the use of the robotic platform to perform laparoscopic surgical procedures on adrenal glands at the University of Lorraine [8, 12]. Consequently, data from a large number of consecutive patients that underwent robotic-assisted adrenalectomy were available for analysis. The aim of this study was to determine incidence and predictive factors for intraoperative (conversion and capsular rupture) and postoperative complications (morbidity) after unilateral robotic-assisted adrenalectomy.
Methods
Patients
All consecutive patients who underwent a robotic unilateral transabdominal adrenalectomy for any indication from November 2001 to September 2016 at University of Lorraine (Brabois Hospital) were entered into a prospective database and were included in this study. Patients were analyzed retrospectively. Patients who underwent conventional laparoscopic adrenalectomy, bilateral or subtotal robotic adrenalectomy, open adrenalectomy, and those with concomitant procedures at the time of adrenalectomy were excluded from analysis. The database and study design were approved by the institutional review board of Nancy Brabois Hospital. This cohort is registered under a specific CNIL French national number (Commission Nationale de l’Informatique et des Libertés) (CNIL No. 2015–34). Data from the first 100 patients included in this study were previously published [12]. This cohort study was registered at clinicaltrials.gov as NCT03410394.
Medical management
All patients were managed at the University of Lorraine and underwent a complete preoperative checkup with discussion of each case during multidisciplinary meetings including endocrine medical and surgical specialists (weekly multidisciplinary endocrine conference). Consequently, the decision to operate was not surgeon dependent only. Surgical indications followed international standard set of criteria for deciding whether or not to operate [1, 2, 5]. When a surgical indication was decided, patients were preferentially operated on using the robotic system irrespective of surgical indications. In our group, conventional laparoscopic approach for adrenalectomy was only used when the robotic platform was not available. Suspicion or actual visualization of invasive malignancy was considered a contraindication to robotic surgery. Patients with primary hyperaldosteronism were given potassium and antihypertensive medications preoperatively when needed. Some patients with pheochromocytoma were treated preoperatively by alpha and calcium channel blockade. Patients with hypercortisolism were supplemented postoperatively to prevent postoperative adrenal insufficiency. Nasogastric tube was used during the procedure and removed at the end of adrenalectomy. Patients were started on a clear liquid diet after surgery and advanced to regular as tolerated. Normal diet and mobilization were initiated the day of surgery.
Surgical procedure
Transabdominal lateral adrenalectomy was carried out with the patient in the lateral decubitus position. No antibiotic prophylaxis was given according to the current French guidelines for antibiotic prophylaxis [14]. Patients routinely received prophylaxis for deep vein thrombosis. The technique of robotic unilateral transabdominal adrenalectomy was described previously [15]. We used two main operating trocars (8 mm) and one optic trocar (12 mm) for robotic control. The fourth robotic arm was not used to minimize costs. One additional operating trocar was used routinely for the first assistant (5 mm). A second additional operating trocar was used when needed during the dissection to improve surgical field exposure (5 or 10 mm). When necessary, conversion to laparotomy was performed via an ipsilateral subcostal incision without patient repositioning. All surgical procedures were performed by one of the five different surgeons: three senior surgeons defined as surgeons performing more than six adrenalectomies per year (individual experience > 30 adrenalectomies) and two junior surgeons defined as surgeons supervised by a senior surgeon (individual experience < 30 adrenalectomies). Surgeon’s first assistant skills varied from 1 (junior resident) to 4 (graduated junior surgeon) [8]. Patients were ranked in two groups according to the date of surgery (from 2001 to 2010 vs. from 2011 to 2016) because half of the patients were enrolled prior to 2011.
Clinical outcomes
A postoperative complication was defined as an unexpected or undesired postoperative course. All medical or surgical complications were collected and stratified according the Clavien–Dindo classification, including complications that did not lead to re-hospitalizations [16]. Variables analyzed were demographic data, body mass index (BMI), American Society of Anesthesiologists (ASA) grade, morphologic and hormonal characteristics of adrenal tumor, surgical indications, history of previous ipsilateral upper mesocolic or retroperitoneal surgical procedure, adrenal tumor diameter on pathology, intraoperative variables, postoperative parameters and outcomes, and histological diagnosis. In patients with Cushing disease, we included patients who underwent a unilateral adrenalectomy only. Operative time was defined as duration in minutes from skin incision to skin closure, including robotic cart docking [8, 12].
Statistical analyses
Results were reported as mean + standard deviation (quantitative data) or as percentages (qualitative data). Univariate analyses and multivariable logistic regression analyses were performed to identify independent predictive factors for intraoperative (conversion, capsular rupture) and postoperative complications. Odds ratios were calculated using logistic regression. Fisher’s exact test was used to compare differences in discrete or categorical variables, and the Student’s t test was used for continuous variables. Statistical analyses were performed using R software version 3.2.5. A difference was considered statistically significant when p < 0.05.
Results
From November 2001 to September 2016, 303 consecutive patients underwent a robotic transabdominal unilateral total adrenalectomy. Flow chart of patients undergoing robotic surgery at University of Lorraine during the same period is shown in Fig. 1. Overall, adrenal surgical procedures accounted for 27% of all robotic surgical procedures performed during the same period at this institution. During the same period, 19 patients underwent a conventional laparoscopic unilateral adrenalectomy and were excluded. Similarly, 68 patients underwent an open adrenalectomy and were excluded. Mean age was 53.2 ± 13.6 (range 17–84) years. Mean BMI was 27.5 ± 5.3 (range 14–44.5) kg/m2 and 53.5% of patients were women (n = 162). ASA scores were as follows: grade I in 19 patients (6.3%), grade II in 192 patients (63.4%), grade III in 89 patients (29.4%), and grade IV in 3 patients (0.9%). Nine patients (3.0%) had a previous history of ipsilateral upper mesocolic or retroperitoneal surgical procedure: cholecystectomy in four patients, hiatal hernia repair in two patients, pancreatic necrosectomy in one patient, and laparotomy for perforated ulcer in one patient.
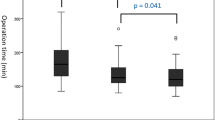
Mean tumor diameter was 3.6 ± 2.2 (range 0.3–14.5) cm and most of the resected adrenal glands (176 patients) were on the left side (58.1%). When comparing the first and last 100 of patients, we observed that mean tumor size increased significantly from 2.9 + 1.9 to 4.2 + 2.4 cm (p < 0.001). Mean adrenal weight was 45.7 ± 56.5 (range 6–550) g. Clinical and pathological data are reported in Table 1. Mean operative time was 88.9 ± 26 (range 34–310) min. Mean operating time decreased significantly from 99 + 36 min in the first 100 cases to 77 + 40 min in the final 100 cases (p < 0.001). A significant correlation was observed between tumor diameter and operative time (p < 0.001; r = 0.07). This correlation showed that for every 1 mm increase in tumor diameter, it took on average an extra 6.2 min to perform the case (total operating time) (Fig. 2). No significant correlation was observed between patient’s BMI and total operative time (p = 0.737).
Intraoperatively, capsular rupture was observed in nine patients (3.0%) and six of them (67%) were within the first 100 patients. Corresponding pathologies were mainly Conn’s adenoma on the left side (four patients) and pheochromocytoma (three patients) (Table 2). Intraoperative surgical difficulties without the need for conversion were observed in 38 patients (12.5%) and corresponding causes were diffuse bleeding (8 patients), local adhesions (6 patients), small vascular injury (6 patients), technical problems with the robotic system (5 patients), splenic small injury without splenectomy (5 patients), need for an additional trocar (2 patients), pneumoperitoneum leakage (2 patients), diaphragmatic injury (2 patients), intraoperative hemodynamic instability during pheochromocytoma resection (1 patient), and difficult surgical exposure (1 patient). Nine (3.0%) patients were converted (8 to laparotomy and 1 to laparoscopy) and main causes for conversion were intraoperative bleeding and need for adhesiolysis (Table 3).
Postoperative complications were observed in 28 patients (9.2%). Medical complications occurred in 7 patients (2.3%), and surgical complications in 21 patients (6.9%) (Table 4). A reoperation for severe complications (Clavien > 3b) was needed in 3 patients (1%). Two patients had organ injuries: one patient with splenic injury managed using hemostatic gauze application and one patient with colonic necrosis who underwent a colectomy. The last patient had a strangulated incisional hernia (trocar) with the need for an urgent reoperation after adrenalectomy. There was no postoperative death. Mean hospital stay (including the day of arrival before surgery) was 5.5 ± 1.9 days (range 2–30) in all patients.
In univariate analysis, we observed that the need for conversion was associated with larger tumor size (diameter > 5 cm) and longer mean operative time (Table 5). Similarly, we observed that capsular ruptures were more frequently observed in patients with a previous history of upper mesocolic or retroperitoneal surgical procedure (Table 6). Lastly, we observed that postoperative complications occurred more frequently in patients older than 65 years of age, or those who underwent conversion (Table 7).
In multivariable logistic regression analysis, this study showed that tumor diameter > 5 cm was the only predictive factor for the need for conversion (OR 7.47, 95% CI 1.81–30.75; p = 0.005); while history of upper gastrointestinal surgery was the only remaining predictive factor for capsular rupture (OR 13.6, 95% CI 2.33–80.03; p = 0.004). The need for conversion (OR 8.35, 95% CI 1.99–35.05; p = 0.003) and patient age (OR 1.039, 95% CI 1.006–1.072; p = 0.019) remained independent predictive factors for overall postoperative complications (Clavien grade 1–4), as well surgery-specific complications (OR 17.3, 95% CI 2.5–119.2; p = 0.004) and (OR 1.089, 95% CI 1.023–1.159; p = 0.007), respectively. However, no significant factor was associated with postoperative medical complications.
Discussion
Laparoscopic adrenalectomy is regarded as the preferred surgical approach for the management of most adrenal surgical disorders [1,2,3, 5]. However, adoption of robotic technology has positioned robotic adrenalectomy as an option in some medical centers. Robotic adrenalectomy is not a new surgical procedure but consists of placing a computer interface between patient and surgeon in order to optimize the feasibility and quality of conventional laparoscopic adrenalectomy [6, 7, 11, 12, 15, 17]. Assessment of new surgical practices is complex and challenged by factors depending on operator, team, learning curves, quality variations, and perception of clinical utility [18, 19]. In this matter, international recommendations for the assessment of surgical innovations encourage the use of prospective databases first. Randomized trials are implemented later to investigate efficacy, but adequate pre-trial data are essential to allow power calculations, clarify indications, and develop quality measures [19]. This monocentric series of 303 consecutives patients who underwent robotic-assisted unilateral adrenalectomy is the largest to date and provides data about intraoperative complications (capsular rupture, conversion) and risk factors for postoperative complications. In order to avoid potential bias, all patients with bilateral or subtotal robotic adrenalectomy as well as patients with concomitant procedures at the time of adrenalectomy were excluded from analysis.
Although previous series are scarce, this study confirmed that the use of the robotic system does not preclude the incidence of capsular rupture [11, 20]. The observed rate of capsular rupture was around 3% and was similar to previous large series evaluating conventional laparoscopic adrenalectomy [1, 2, 21]. We found that surgeons early in their robotic learning curve, left Conn’s adenoma (small tumor on the left side) and large tumor were frequently observed factors in patients with capsular rupture. We have shown that history of previous ipsilateral upper mesocolic or retroperitoneal surgical procedure is an independent predictor for this intraoperative complication, in contrast to data suggesting no increase in complications of laparoscopy for patients with prior abdominal surgery [22]. Limiting the risk of capsular rupture is essential in cases of both benign and malignant lesions, especially adrenocortical carcinoma [5, 23, 24]. Although no capsular rupture and no conversion was observed among the eight patients with adrenocortical carcinoma, we acknowledge that this study was not designed to address this specific issue and did not allow to compare with conventional laparoscopy. A robotic-assisted approach can be used in patients with preoperative determined tumors < 10 cm without evidence of local invasion or enlarged lymph nodes, as shown in a recent multicenter study [21].
Here, we have confirmed that robotic-assisted lateral transabdominal adrenalectomy procedures were completed with no mortality, and low rates of conversion to open surgery and postoperative morbidity, emphasizing the safety and efficacy of this approach [11]. However, we described 47 intraoperative incidents corresponding to 38 intraoperative surgical difficulties without the need for conversion (12.5%) and 9 conversions (3.0%). Bleeding and the need for adhesiolysis were two main indications for conversion during robotic-assisted adrenalectomy, similar to those reported during conventional laparoscopic adrenalectomy [1,2,3]. Notably, the lesion diameter was identified as an independent predictor of outcome. There was a significant positive correlation observed between tumor diameter and operative time. Furthermore, this study showed for the first time that larger tumors (diameter > 5 cm) are an independent predictor for conversion during robotic-assisted adrenalectomy. As reported with conventional laparoscopic adrenalectomy, converted patients in this study had longer mean operative time and higher rate of postoperative complications [1,2,3]. Additionally, we believe that these data about capsular rupture and conversion support the fact that patients with adrenal lesions larger than 5 cm should be managed in an expert center with or without the use of a robotic platform.
In terms of morbidity, this study showed that postoperative complications rate was about 10%. This was similar to recent large studies (> 300 patients) evaluating conventional laparoscopic adrenalectomy (from 4.6 to 11.5%) [1,2,3, 5, 25]. Consequently, this study data do not support previous meta-analyses concluding that robotic group patients may be associated with lower postoperative complication rate [7, 11]. Furthermore, this study showed that patient’s age > 65 years and the need for conversion remained two independent predictors for postoperative complications after robotic adrenalectomy. Four independent predictors for intraoperative and postoperative complications were identified in this study (history of previous ipsilateral surgical procedure, tumor size, patient’s age, and conversion): we believe that these criteria should be controlled in future studies to avoid potential bias.
This study showed that mean tumor size significantly increased while mean operative time decreased during this study period. This observation could be explained by patient’s selection during early experience, learning curve, and increased regional referral pattern over time. The role of robotic adrenalectomy in obese patients is controversial [6, 9]. In this study, 82 patients (28%) had a BMI > 30 kg/m2 and obesity was not significantly correlated with operative time or perioperative complications. While this could be considered an advantage of the robotic approach, it is important to note that a recent large series evaluating conventional laparoscopic adrenalectomy reported similar patterns [2]. Lastly, mean total operative time observed in the last 100 of patients (77 min) does not support previous series concluding that robotic adrenalectomy had a significantly longer operative time [11, 17]. Difficult dissections during left robotic adrenalectomy have also been reported but was not supported by this study data [26]. Overall, we believe that this study is unique in that it includes surgeons both early and late in their robotic experience and learning curves, eliminating potential biases. Also, this large study includes more than 300 consecutive patients, calling into question some previous studies with much smaller sample size, including the only available randomized trial, which reported a conversion rate of 40% [27].
This study had several limitations. This was a retrospective review of a non-randomized series of patients, and a large, prospective randomized trial is necessary to definitively compare robotic and laparoscopic outcomes. Surgeons are forced by the nature of their discipline to remain aware of all new developments because the very history of their profession consists of a succession of innovations that have survived the test of time and scientific evaluation [28]. Secondly, the higher cost of robotic surgery is a documented drawback of the procedure [11]. The cost of the robotic platform was not addressed here, but we previously calculated that robotic adrenalectomy was 2.3 times more expensive in Europe than conventional laparoscopic adrenalectomy [8]. A study from the US showed that robotic adrenalectomy added about $900 extra cost compared to laparoscopic adrenalectomy [17]. Third, included patients may have been selected and this selection could have biased this study’s conclusions.
In conclusion, this single-institution study identified independent predictive factors for capsular rupture, conversion, and postoperative complications after unilateral robotic-assisted adrenalectomy. These factors should be taken into account when evaluating robotic-assisted transabdominal lateral adrenalectomy.
References
Bittner JG, Gershuni VM, Matthews BD, Moley JF, Brunt LM (2013) Risk factors affecting operative approach, conversion, and morbidity for adrenalectomy: a single-institution series of 402 patients. Surg Endosc 27:2342–2350
Coste T, Caiazzo R, Torres F, Vantyghem MC, Carnaille B, Pattou F et al (2017) Laparoscopic adrenalectomy by transabdominal lateral approach: 20 years of experience. Surg Endosc 31:2743–2751
Gaujoux S, Bonnet S, Leconte M, Zohar S, Bertherat J, Bertagna X et al (2011) Risk factors for conversion and complications after unilateral laparoscopic adrenalectomy. Br J Surg 98:1392–1399
Hattori S, Miyajima A, Maeda T, Hasegawa M, Takeda T, Kosaka T et al (2012) Risk factors for perioperative complications of laparoscopic adrenalectomy including single-site surgery. J Endourol 26:1463–1467
Shen WT, Sturgeon C, Duh QY (2005) From incidentaloma to adrenocortical carcinoma: the surgical management of adrenal tumors. J Surg Oncol 89:186–192
Aksoy E, Taskin H, Aliyev S, Mitchell J, Siperstein A, Berber E (2013) Robotic versus laparoscopic adrenalectomy in obese patients. Surg Endosc 27:1233–1236
Brandao LF, Autorino R, Laydner H, Haber GP, Ouzaid I, De Sio M et al (2014) Robotic versus laparoscopic adrenalectomy: a systematic review and meta-analysis. Eur Urol 65:1154–1161
Brunaud L, Ayav A, Zarnegar R, Rouers A, Klein M, Boissel P, Bresler L (2008) Prospective evaluation of 100 robotic-assisted unilateral adrenalectomies. Surgery 144:995–1001
Morelli L, Tartaglia D, Bronzoni J, Palmeri M, Guadagni S, Di Franco G et al (2016) Robotic assisted versus pure laparoscopic surgery of the adrenal glands: a case-control study comparing surgical techniques. Langenbecks Arch Surg 401:999–1006
Tang K, Li H, Xia D, Yu G, Guo X, Guan W, Xu H, Ye Z (2015) Robot-assisted versus laparoscopic adrenalectomy: a systematic review and meta-analysis. Surg Laparosc Endosc Percutan Tech 25:187 – 95
Economopoulos KP, Mylonas K, Stamou AA, Theocharidis V, Sergentanis TN, Psaltopoulou T, Richards ML (2017) Laparoscopic versus robotic adrenalectomy: a comprehensive meta-analysis. Int J Surg 38:95–104
Brunaud L, Bresler L, Ayav A, Zarnegar R, Raphoz AL, Levan T, Weryha G, Boissel P (2008) Robotic-assisted adrenalectomy: what advantages compared to lateral transperitoneal laparoscopic adrenalectomy? Am J Surg 195:433–438
Zografos GN, Farfaras A, Vasiliadis G, Pappa T, Aggeli C, Vassilatou E, Kaltsas G, Piaditis G (2010) Laparoscopic resection of large adrenal tumors. JSLS 14:364–368
Societe francaise d’anesthesie et de réanimation (SFAR) (2011) Antibioprophylaxis in surgery and interventional medicine (adult patients). Ann Fr Anesth Reanim 30:168–190
Nomine-Criqui C, Brunaud L, Germain A, Klein M, Cuny T, Ayav A, Bresler L (2015) Robotic lateral transabdominal adrenalectomy. J Surg Oncol 112:305–309
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Kahramangil B, Berber E (2017) Comparison of posterior retroperitoneal and transabdominal lateral approaches in robotic adrenalectomy: an analysis of 200 cases. Surg Endosc. https://doi.org/10.1007/s00464-017-5894-1
Wang TS, Duh QY (2018) Volume-outcome relationship in adrenal surgery. Surgery 163:165–166
McCulloch P, Altman DG, Campbell WB, Flum DR, Glasziou P, Marshall JC et al (2009) No surgical innovation without evaluation: the IDEAL recommendations. Lancet 374:1105–1112
Giulianotti PC, Buchs NC, Addeo P, Bianco FM, Ayloo SM, Caravaglios G, Coratti A (2011) Robot-assisted adrenalectomy: a technical option for the surgeon? Int J Med Robot 7:27–32
Lee CW, Salem AI, Schneider DF, Leverson GE, Tran TB, Poultsides GA et al (2017) Minimally invasive resection of adrenocortical carcinoma: a Multi-Institutional Study of 201 patients. J Gastrointest Surg 21:352–362
Morris L, Ituarte P, Zarnegar R, Duh QY, Ahmed L, Lee J, Inabnet W 3rd, Meyer-Rochow G, Sidhu S, Sywak M, Yeh M (2008) Laparoscopic adrenalectomy after prior abdominal surgery. World J Surg 32:897–903
Gaujoux S, Mihai R, Joint Working Group of ESES and ENSAT (2017) European Society of Endocrine Surgeons (ESES) and European Network for the Study of Adrenal Tumours (ENSAT) recommendations for the surgical management of adrenocortical carcinoma. Br J Surg 104:358–376
Li ML, Fitzgerald PA, Price DC, Norton JA (2001) Iatrogenic pheochromocytomatosis: a previously unreported result of laparoscopic adrenalectomy. Surgery 130:1072–1077
Lairmore TC, Folek J, Govednik CM, Snyder SK (2016) Improving minimally invasive adrenalectomy: selection of optimal approach and comparison of outcomes. World J Surg 40:1625–1631
Maker AV, Maker VK (2017) Techniques to perform robotic left adrenalectomy in the obese patient. Surg Endosc 31:950–951
Morino M, Beninca G, Giraudo G, Del Genio GM, Rebecchi F, Garrone C (2004) Robot-assisted vs laparoscopic adrenalectomy: a prospective randomized controlled trial. Surg Endosc 18:1742–1746
Brunaud L (2013) Should all new surgical procedures be published ? J Visc Surg 150:163–164
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Laurent Bresler is proctor for Intuitive Surgical Inc (pelvic floor procedures). Tristan Greilsamer, Claire Nomine-Criqui, Michaël Thy, Timothy Ullmann, Rasa Zarnegar, and Laurent Brunaud declare that they have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Greilsamer, T., Nomine-Criqui, C., Thy, M. et al. Robotic-assisted unilateral adrenalectomy: risk factors for perioperative complications in 303 consecutive patients. Surg Endosc 33, 802–810 (2019). https://doi.org/10.1007/s00464-018-6346-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-018-6346-2