Abstract
Background
Transanal minimally invasive surgery (TAMIS) offers intra-luminal full-thickness excision of rectal neoplasia. Robotic TAMIS (RT) allows for greater versatility in motion while operating in the limited space of the rectum. We present our experience with this technique in practice using the DaVinci Xi™ platform.
Method
This is a multi-institutional retrospective analysis for patient undergoing Robotic TAMIS for resection of rectal lesions at two tertiary referral hospitals in the United States. Morbidity, mortality, anatomic measurement, and final pathology were analyzed.
Results
Thirty-four patients planned for Robotic TAMIS were identified. Average follow-up was 188 days. The average BMI was 29.5 ± 5.9. All patients had an American Society of Anesthesiologist (ASA) Class of 2 or greater and 21 (62%) were ASA 3 or greater. Rectal lesions located from 2 to 15 cm from the dentate line were successfully resected. Lesions up to 4.5 cm in the longest dimension were successfully resected. The average operative time was 100 ± 70 min, which correlated to a robotic console time of 76 ± 67 min. Patients were placed in Lithotomy in 32 (94%) cases and were prone in only 2 (6%) cases. There were no intraoperative complications or conversions to another technique. The only postoperative complication was a medically managed Clostridium difficile infection in 1 patient. Three patients were upstaged to T2 on final pathology and underwent successful formal resections. BMI was a statistically significant predictor of a longer operation.
Conclusions
With increased reach and operative range of motion, Robotic TAMIS is a safe and effective method for excising low-risk rectal neoplasia with a wide range of anatomical measurements. Higher BMI is a significant predictor of a longer and likely more challenging operation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In the early 1980s, Buess et al. first reported on transanal endoscopic microsurgery (TEM) as a minimally invasive procedure to remove rectal polyps and early rectal cancers through the anus [1,2,3]. This approach had been shown to achieve full-thickness resections with acceptable margins for lesions from 5 to 20 cm from the anal verge [3]. In long-term follow-up, TEM excision of rectal lesions has been shown to have favorable oncologic outcome as well as low morbidity and mortality [4,5,6,7,8]. However, general adoption of TEM has been limited in clinical practice. Several reasons contributed to this. One of which was the difficult learning curve associated with this technique. Another factor was the limited availability of training, which tended to be at selective centers. A third barrier was the availability of the specialized instruments. And finally, the TEM approach may be unsuitable for lesions closer to the anal verge [8,9,10].
To tackle these problems, transanal minimally invasive surgery (TAMIS) was introduced in 2009. This approach used traditional laparoscopic instruments placed through the anus to perform local excision. Previous studies have shown that TAMIS provided high-quality local excision, comparable to TEM [11,12,13,14]. TAMIS has the additional benefit of utilizing conventional laparoscopic instruments that are nearly ubiquitously available in the United States. However, using laparoscopic tools in TAMIS to replace TEM equipment was not without its shortcomings. Laparoscopic instruments were limited by their rigid design and inability to fully articulate. This loss of operative freedom was a great drawback, especially when performing procedures in small spaces [15]. Nowhere was this more pronounced as when operating in the confines of the rectal lumen.
In 2010, the Da Vinci Robotic Surgical System (Intuitive Surgical, Inc., Sunnyvale, CA) caught the interest of several clinicians as a possible platform for TAMIS. This was eventually termed Robotic TAMIS (R-TAMIS). Prior to this, robotic surgery had been successfully adopted in many areas of surgery with limited operative space, such as the mediastinum and pelvis. Investigators first demonstrated the feasibility of R-TAMIS in cadaver models [16, 17]. Since then, several published case reports and small series have described the R-TAMIS technique with encouraging early results [18,19,20,21].
R-TAMIS is still a novel procedure only performed in specialized centers. Because of this, operative standards and limitations have not been well defined. To make matters even more complicated, since the introduction of this technique, the makers of the DaVinci Robotics introduced the newest iteration of its technology, the DaVinci Xi™, in 2014. To our knowledge, there had been no large patient series describing the operative technique and outcomes using this platform. Here, we present the largest North American series to date on patient undergoing R-TAMIS using this new robotic surgical platform.
Materials and methods
This a retrospective analysis of cohorts recruited between two North America tertiary referral hospitals (University of California San Diego Healthcare System and Oklahoma Surgical Hospital). The study was approved by each hospital’s local institutional review board (IRB). All patients undergoing R-TAMIS for elective excision of tissue were included in the study. Patient information was prospectively entered into each institution’s local electronic medical record (EMR) system. This information included patient demographics, clinic visit notes, imaging reads, pathological results, intraoperative measurements, operative reports, and post-operative follow-up of up to 1 year.
Preoperative workup
All patients undergoing evaluation for R-TAMIS were subjected to preoperative colonoscopy by either the operating surgeon or a board-certified gastroenterologist. At the time of this event, anatomic measurements, tissue biopsies, and assessment of any synchronous lesions were performed. For early malignant tumors of the rectum, either endorectal ultrasound (EUS) or pelvic magnetic resonance imaging (MRI) was performed to determine preoperative staging. Patients with early-stage rectal neoplasm (uTis or uT1N0M0) and low-risk histology (no lymphovascular invasion) were considered candidates for R-TAMIS. Other indications included T1 carcinoid tumors, incompletely endoscopically resected polyps of the rectum, and in one case partial resection for palliative control of rectal bleeding in metastatic disease. Because this was only performed for early-stage disease, none of the patients underwent neoadjuvant chemoradiotherapy.
Operative intervention
All patients underwent a routine mechanical bowel preparation prior to surgery with Fleet® enema the morning of surgery. General endotracheal intubation was preformed for all patients. Cefazolin and metronidazole were given as perioperative antibiotics. Patients were placed in either lithotomy with moderate Trendelenburg or prone jack-knife position. The decision for positioning was decided by the individual surgeon’s preference at the time of surgery based on tumor positioning.
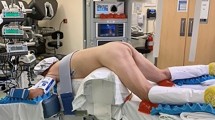
All surgery began with digital rectal examination. The GelPOINT® Path Transanal Access Platform (Applied Medical, Rancho Santa Margarita, CA) was placed into the anal canal and suture anchored to the surrounding skin. Three 8-mm robotic trocars and one 5-mm laparoscopic trocar were placed into the gel interface (Fig. 1). The rectum was insufflated with CO2 with pressure setting of 15 mmHg. The robotic system was then docked from the side of the patient. A 30° 8-mm robotic camera is placed in the middle and 2 articulated robotic instruments were used with an additional assistant trocar (Fig. 2).
The usual tools for excision used on the robotic platform include atraumatic graspers, articulated hook cautery, or robotic scissors attached to electrocautery. A laparoscopic suction catheter was used for defog during the case and is operated by the surgical assistant through the 5-mm laparoscopic trocar. Full-thickness excision was confirmed in each case with visualization of the perirectal fat. Rectal wall defect was closed with a barbed absorbable suture in a transverse manner. Successful resection was defined as the absence of a gross residual tumor in the surgical bed and pathologically negative margins. All patients were expected to stay in the hospital for observation for one night before discharge to home.
Follow-up
Patients received regular follow-up with the surgeon at 1–3 months postoperatively. All patients were expected to follow-up with their primary care provider or gastroenterologist (if benign), or the operating surgeon (if malignant) at 1 year. MRI and colonoscopy were performed annually after the surgery if they are indicated.
Statistical analysis
Statistical analysis was performed on SPSS v23 (SPSS Inc. 2017). Statistical significance was set at P < 0.05.
Results
From 2014 to 2016, we identified 34 patients undergoing elective R-TAMIS for resection of rectal neoplasms. Patient demographics were shown in Table 1. This was a mostly male (67%) population who tended to be moderately overweight (average BMI 29.4) and mostly having ASA classification of II and III. Patients were on average followed for > 6 months (188 ± 209 days) with 10 patients (29%) completing a 1-year follow-up.
Technical success using R-TAMIS was obtained in all cases without converting to another modality of surgery. Table 2 showed the operative details including operative timing, patient positioning, and anatomic descriptions of the specimens removed. The average operative time per case was 100 ± 70 min. However, this included the docking and undocking the robotic platform, which on average took 25 (± 14) min. Robotic console time was calculated by subtracting the docking and undocking time from the total operative time. Other than two patients, 94% of the patients had their procedure preformed in the lithotomy position as per experience learned from the operating surgeons throughout the study period. Lesions up to 5.5 cm in longest dimension and ranging from 2 to 15 cm from the dentate line were successfully excised. Figure 3 demonstrated the distribution of tumor size and distance from the dentate line in this series.
The final pathology of the excised lesions was listed in Table 3. Twenty-two (65%) of the patients had an adenoma, 7 (21%) had carcinoma, and 4 (12%) had carcinoid tumors (all 1.5 cm or less in the longest dimension). Full-thickness R0 resection was obtained in 33/34 (97%) patients. Three patients were noted to have microscopic tumor invasion into the muscularis propria but still within the margin of resection, and were upstaged to T2 on final pathology. They received formal low anterior resections without any positive lymph nodes and went on to complete chemo-radiation. One patient with known metastatic disease received R-TAMIS for palliative rectal bleeding (R2 resection) was found to have T3 on final pathology report. The patient’s acute bleeding resolved but was placed to comfort care and passed away in the month following his operation. Other than that patient, all other patients remained disease free and alive on their most recent follow-up appointment. Patients stayed on average 1.18 (± 0.83) days in the hospital postoperatively. There were no reports of urinary retention, post-operative bleeding needing intervention, fever, or incontinence. One patient (3%) developed medically managed Clostridium difficile diarrhea within 1 week postoperatively.
Univariate analysis of predictors of operative time is shown in Table 4. Patient BMI was a statistical significant positive predictor of both total operative time as well as robotic console time (calculated as total operative time minus time spent docking the robot). Specimen size was also a positive independent predictor of total operative time. Specifically, those with severe obesity (BMI > 35) had a significantly longer total operative time (167 ± 128 vs 86 ± 42 min, P = 0.008) as well as robotic console time (129 ± 114 vs 62 ± 41 min, P = 0.017). However, there were no differences in postoperative complications and disease-free survival in patients with varying BMI.
Discussion
The robotic approach to TAMIS is the most recent evolution of natural orifice surgery for the treatment of low-risk rectal tumors. This study presented our 2-year experience with the technique across two North American tertiary referral hospitals. This is the largest case series to date of performing RT using the DaVinci Xi™ Platform. We have adopted this technique in our own clinical practice for the treatment of low-risk rectal neoplasms. These included T1 rectal cancers without high-risk pathological characteristics and inadequately resected rectal polyps. Technical success was obtained in all cases without the need to convert to another modality of surgery.
Neoplasms of a variety of anatomic characters were resected in our practice. Although the majority of lesions were between 5 and 10 cm from the dentate line, we were able to reach tumors as low as 2 cm and as high as 14 cm from the dentate line. Similarly, although most lesions were < 4 cm in largest dimension, lesions up to 5.5 cm have been successfully excised. This may represent the physical boundaries of a feasible resection for this technique. Caution should still be exercised when closing the defect after excision of a large lesion as to not narrow the rectal lumen. Transverse closure should always be used and is technically easier to achieve robotically than laparoscopically due to the instrument’s ability to articulate.
Several factors could contribute to the difficulty of the operation. Using the operative time as a marker for case difficulty, we observed that BMI was the greatest predictor of a longer operation. This was most pronounced when looking at patients with severe obesity as defined by BMI > 35. These patients had nearly double the operative time as their less obese counterparts in both total operative time (167 vs 82 min) as well as robotic console time (129 vs 62 min). Lesion size was also a significant predictor of a longer total operative time, although this was not found to be significant when only looking at console time. Lesion distance from the dentate line, patient ASA classification, tumor pathology, and whether or not the case was performed earlier on in our experience compared to later did not affect the length of the operation. Although there was no clear explanation as to why BMI had such a pronounced effect on the length and likely difficulty of the operation, we do believe this may be related to patient positioning. Because these cases were done in the lithotomy position, the distance between the patient’s legs may be limited in the severely obese patient population. This could restrict the full range of motion for the robotic arms, increasing its operative difficulty. Despite this, all investigating surgeons of this study felt that the R-TAMIS technique was less technically difficult and had a faster learning curve compared to the conventional laparoscopic TAMIS technique.
The current literature has not agreed on the most optimal positioning for patients undergoing R-TAMIS. Nearly all the patients in this study were placed in the lithotomy position. Initial reports in 2014 utilized prone or left lateral decubitus positioning for this operation [19]. However, in the years following, multiple case reports and case series of lithotomy position for R-TAMIS were also reported [22]. In our experience, either position was technically feasible. This is due to the robotic platform’s ability to invert camera view as well as having 360° articulation in the instruments. As a result, whether the tumor is above or below the field of view did not negatively impact the technical aspect of surgery. However, the surgeons of this study preferred lithotomy because it was faster and easier to position than placing the patient in prone positioning. Additionally, the airway tended to be more stable for anesthesia to monitor when the patient is not prone.
There were very few compilations to report in this patient cohort. Only one patient returned to the emergency room with post-operative C. difficile infection that was managed medically. There were three patients who had upstaged to T2 disease on final pathology. These patients all successfully underwent formal low anterior resections for their disease and are currently disease free. We did not have any patients experience issues of incontinence in the postop period. We believe this is mainly due to careful positioning of the robotic instruments. The fulcrum of the robotic arms should experience almost no lateral displacement. Thus, if care is taken to ensure the fulcrum of each instrument is located at the level of the sphincter muscles, there should be minimal radial force applied, thus preventing stretch-related injury.
As the evolution of technology phases out older generations, it is expected that with time, the newest robotic platform will overtake the previous one (DaVinci Si™) and become the mainstay model. Several advantages of the newer platform had been observed in literature, the most common of which relates to shorter console times attributed to improved ease of use [23]. Although this study is not designed to test the benefits between the two versions of the robotic surgical platform in R-TAMIS, we did note that the ability adjust external robotic arms to reduce collision was useful when operating on patients in the lithotomy position where the external space is limited by the location of the patient’s legs.
The main limitation of this study is in its retrospective design, which does not allow comparison between different rectal resection techniques compare outcomes. Additionally, our average follow-up is about 6 months for all patients, which may not be able to capture true oncologic outcomes. As time progresses, we hope to expand on our collaborative database for continued review of R-TAMIS in the treatment of rectal neoplasms.
In conclusion, this is the largest series to date of patients undergoing R-TAMIS for the purpose of rectal neoplasms. Successful resection can be achieved safely in a wide anatomic range of lesions up to 15 cm from the dentate line and up to 5.5 cm in diameter. BMI, especially when > 35, was a significant predictor of a longer and likely more challenging operation technically.
References
Buess G, Kipfmuller K, Hack D et al (1988) Technique of transanal endoscopic microsurgery. Surg Endosc 2:71–75
Buess G, Hutterer F, Theiss J et al (1984) A system for a transanal endoscopic rectum operation. Chirurg 55(10):677–680 (in German)
Allaix ME, Arezzo A, Caldart M et al (2009) Transanal endoscopic microsurgery for rectal neoplasms: experience of 300 consecutive cases. Dis Colon Rectum 52:1831–1836
Guerrieri M, Baldarelli M, Organetti L et al (2008) Transanal endoscopic microsurgery for the treatment of selected patients with distal rectal cancer: 15 years experience. Surg Endosc 22(9):2030–2035
Doornebosch PG, Tollenaar RA, Gosselink MP et al (2007) Quality of life after transanal endoscopic microsurgery and total mesorectal excision in early rectal cancer. Colorectal Dis 9(6):553–558
Lin GL, Meng WC, Lau PY et al (2006) Local resection for early rectal tumours: comparative study of transanal endoscopic microsurgery (TEM) versus posterior trans-sphincteric approach (Mason’s operation). Asian J Surg 29(4):227–232
Winde G, Nottberg H, Keller R et al (1996) Surgical cure for early rectal carcinomas (T1). Transanal endoscopic microsurgery vs. anterior resection. Dis Colon Rectum 39(9):969–976
Middleton PF, Sutherland LM, Maddern GJ (2005) Transanal endoscopic microsurgery: a systematic review. Dis Colon Rectum 48(2):270–284
Papagrigoriadis S (2006) Transanal endoscopic micro-surgery (TEMS) for the management of large or sessile rectal adenomas: a review of the technique and indications. Int Semin Surg Oncol 3:13
Maslekar S, Pillinger SH, Sharma A et al (2007) Cost analysis of transanal endoscopic microsurgery for rectal tumours. Colorectal Dis 9(3):229–234
Atallah S, Albert M, Larach S (2010) Transanal minimally invasive surgery: a giant leap forward. Surg Endosc 24(9):2200–2205
Lim SB, Seo SI, Lee JL et al (2012) Feasibility of transanal minimally invasive surgery for mid-rectal lesions. Surg Endosc 26(11):3127–3132
Barendse RM, Doornebosch PG, Bemelman WA et al (2012) Transanal employment of single access ports is feasible for rectal surgery. Ann Surg 256(6):1030–1033
Lorenz C, Nimmesgern T, Back M et al (2010) Transanal single port microsurgery (TSPM) as a modified technique of transanal endoscopic microsurgery (TEM). Surg Innov 17(2):160–163
Hideki K, Chiharu I (2012) Mechanical analysis of the formation of forceps and scope for single-port laparoscopic surgery. Surg Laparosc Endosc Percutan Tech 22(4):e168–e175
Hompes R, Rauh SM, Hagen ME et al (2012) Preclinical cadaveric study of transanal endoscopic da Vinci® surgery. Br J Surg 99(8):1144–1148
Atallah SB, Albert MR, deBeche-Adams TH et al (2011) Robotic transanal minimally invasive surgery in a cadaveric model. Tech Coloproctol 15(4):461–464
Bardakcioglu O (2013) Robotic transanal access surgery. Surg Endosc 27(4):1407–1409
Hompes R, Rauh SM, Ris F et al (2014) Robotic transanal minimally invasive surgery for local excision of rectal neoplasms. Br J Surg 101(5):578–581
Atallah S, Quinteros F, Martin-Perez B et al (2014) Robotic transanal surgery for local excision of rectal neoplasms. J Robot Surg 8(2):193–194
Vallribera Valls F, Espín Bassany E, Jiménez-Gómez LM et al (2014) Robotic transanal endoscopic microsurgery in benign rectal tumour. J Robot Surg 8(3):277–280
Ruiz MG, Parra IM, Palazuelos CM et al (2015) Robotic-assisted laparoscopic transanal total mesorectal excision for rectal cancer: a prospective pilot study. Dis Colon Rectum 58(1):145–153. https://doi.org/10.1097/DCR.0000000000000265
Patel MN, Hemal A (2017) Does advancing technology improve outcomes? Comparison of the da Vinci Standard/S/Si to the Xi robotic platforms during robotic nephroureterectomy. J Endourol 27157(336):end.2017.0477. https://doi.org/10.1089/end.2017.0477
Author information
Authors and Affiliations
Contributions
Study conception and design—SL, TS, BWM, LP, CSJ, SH, SR, SE. Data acquisition and analysis—SL, TS, BWM, LP, CSJ, SH, SR, SE. Drafting or revising the article—SL, TS, BWM, LP, CSJ, SH, SR, SE. Final approval—SL, TS, BWM, LP, CSJ, SH, SR, SE. Agreement to be accountable for work—SL, TS, BWM, LP, CSJ, SH, SR, SE.
Corresponding author
Ethics declarations
Disclosures
Drs. Shanglei Liu, Toshiaki Suzuki, Bryce W. Murray, Lisa Parry, Craig S. Johnson, Santiago Horgan, Sonia Ramamoorthy, and Samuel Eisenstein have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Liu, S., Suzuki, T., Murray, B.W. et al. Robotic transanal minimally invasive surgery (TAMIS) with the newest robotic surgical platform: a multi-institutional North American experience. Surg Endosc 33, 543–548 (2019). https://doi.org/10.1007/s00464-018-6329-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-018-6329-3