Abstract
Introduction
This study describes the experience with robot-assisted transanal minimally invasive surgery (rTAMIS) at a single institution. TAMIS has become a popular minimally invasive technique for local excision of well-selected rectal lesions. rTAMIS has been proposed as another option as it improves the ergonomics of conventional laparoscopic techniques.
Methods
Retrospective case series of patients with rectal lesions who underwent rTAMIS. Patient demographics, final pathology, surgical and admission details, and clinical outcomes were recorded. Successful procedures were defined as having negative margins on final pathology.
Results
A total of 16 patients underwent rTAMIS by a single surgeon between April 2018 and December 2019. Mean age of patients was 63 years. Final pathologies were negative for tumor (n = 4), tubulovillous adenoma (n = 4), tubulovillous adenoma with high-grade dysplasia (n = 4), and invasive rectal adenocarcinoma (n = 4). 43% were located in the middle rectum and 56% were located in the distal rectum. Mean maximum diameter was 4.1 cm (IQR 2–3.1 cm). Negative margins were seen in 100% of the excision cases, and 100% were intact. Mean operative time was 87 min (IQR 54.8–97.3 min), and median length of stay was 0 days (IQR 0–1 days). Postoperative complications included incontinence (n = 1) and abscess formation (n = 2). rTAMIS provided curative treatment for 12/16 patients, and the remaining 4 patients received the appropriate standard of care for their respective pathologies.
Conclusions
Robot-assisted TAMIS is a safe alternative to laparoscopic TAMIS for resection of appropriate rectal polyps and early rectal cancers. rTAMIS may provide a modality for resecting larger or more proximal rectal lesions due to the wristed instruments and superior visualization with the robotic camera. Future studies should focus on comparing outcomes between robotic and laparoscopic TAMIS, and whether rTAMIS allows for the removal of larger, more complex lesions, which may save patients from a more morbid radical proctectomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
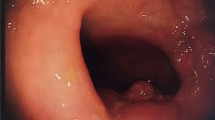
Local excision has become the standard of care for carefully selected benign and early rectal neoplasms, as it can be performed with minimal morbidity and mortality. According to the American Society of Colon and Rectal Surgeon Clinical Practice Guidelines, local excision is an appropriate treatment option in well-selected patients with favorable clinical and histological features, or as definitive treatment for those with advanced disease who are at high risk for surgery. Indications for local excision include moderately differentiated T1 cancers with the absence of lymphovascular or perineural invasion, and tumors that are less than 3 cm in diameter and are less than a third of the rectal circumference. Local excision involves a full-thickness excision down to the perirectal fat and is required to have a 1 cm margin circumferentially [1].
Local excision was first described using a transanal approach with conventional retractors. However, this conventional surgery is often limited by poor visualization and inadequate exposure. In order to gain better access to the rectum, Buess et al. were the first to describe an endoscopic approach with the transanal endomicrosurgery (TEM) platform [2, 3]. With the advancement of minimally invasive surgery, transanal minimally invasive surgery (TAMIS) was then introduced in 2010 and utilizes commercially available single-incision laparoscopic surgery ports for the procedures [4]. As such, TAMIS is thought to be more cost effective and easily accessible to colorectal surgeons [5,6,7,8].
More recently, with the development of robotic surgery, robotic TAMIS (rTAMIS) has been advocated for as an alternative to conventional TAMIS as robotic surgery can offer better surgeon ergonomics and superior optics [9,10,11]. Therefore, this case series was organized to discuss our experience at a single institution with rTAMIS, including approach, positioning, and early outcomes.
Methods
Patients who had undergone rTAMIS at a single community center were retrospectively reviewed. The patients had been previously referred for further evaluation of endoscopically unresectable rectal tumors. Patients who presented with malignant tumors had a full colonoscopy, MRI pelvis, CT chest abdomen and pelvis, and baseline CEA. Based on the results of the workup, rTAMIS was either offered for curative excision of a precancerous lesion or as diagnostic excisional biopsy as part of the patients’ workup. All rTAMIS procedures were carried out using the da Vinci Xi (Intuitive, Sunnyvale, CA) robotic system.
All patients had undergone a pre-operative flexible sigmoidoscopy or colonoscopy to determine the location of the lesion and for pre-operative planning. Patients were instructed to undergo a standard pre-operative regimen that included a mechanical bowel and pre-operative antibiotics before induction of general anesthesia. Patients were secured in operative position, prepped, and draped in standard sterile fashion. The first two patients in the series were positioned in lithotomy while the remaining 14 were placed in prone jackknife. Each patient received a digital rectal exam and a full anoscopic exam to confirm the location of the lesion.
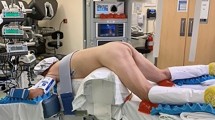
The anal dilator from the applied GelPOINT path transanal access device was first used to dilate the anus. The TAMIS gel port was then placed through the anal canal, past the sphincter muscles and secured in place. Three 8 mm working ports and a 5 mm AirSeal® port (ConMed, Utica, NY) were placed into the cap and placed over the transanal TAMIS port to create a seal (Fig. 1). The rectum was insufflated to 15 mm of Hg using the AirSeal® device. The da Vinci Xi robot was docked, and an 8 mm 30-degree camera was used for all cases to visualize the rectum.
Using electrocautery scissors, a 1 cm margin was marked circumferentially around the lesion. Precancerous lesions were removed with at least submucosal thickness, and lesions suspicious for malignancy removed full thickness to the perirectal fat. Once excised, the defects were observed for hemostasis, and bleeding was cauterized. All defects were then closed primarily with a running 3–0 V-Loc™ (Medtronic, Minneapolis, MN) suture. Lesions were removed intact through the gel port and marked for pathologic analysis. The gel port was removed, and the anal canal was packed with gel foam.
Patients were discharged on the same day, unless they had other indications for admission. Patients were then followed and surveilled according to the current National Comprehensive Cancer Network Guidelines, and appropriate follow-up was done based on their final pathologies.
After approval from the IRB, patient demographics, final pathology, surgical and admission details, and clinical outcomes were collected for this case series. The primary outcome was success of the procedure, which was defined as having negative margins on final pathology. Negative margins were defined as having no tumor on ink for adenomatous tumors and at least 10 mm margin for invasive carcinoma tumors. Numerical data are presented as means with interquartile ranges.
Results
From April 2018 to December 2019, 16 patients had robotic TAMIS performed by a single, board-certified colorectal surgeon. Details of each case are summarized in Table 1. Mean age of the patients was 63 years old. Mean operative time was 87 min (IQR 54.75–109.5minutes). The first 2 patients were placed in lithotomy position while the remaining 14 were placed in prone jackknife position. 7 (43%) of these lesions were found in the middle rectum, located between the first and second rectal folds, while 9 (56%) of the lesions were in the distal rectum, distal rectum to the first rectal fold. None of the cases required conversion to an open abdominal approach. Average blood loss was 17.5 cc (ICQ 8.75–20 cc). Median length of stay was 0 days (ICR 0–1 days). 1 patient had a total length of stay for 11 days as his lesion was biopsied using rTAMIS, and he received definitive treatment during the same admission.
A total of 3 patients had postoperative complications. One patient had reported stool incontinence at 2 weeks, which resolved spontaneously in one month. One patient had formation of a presacral abscess which was treated with a course of antibiotics. One patient required reoperation for poor wound healing and a rectal wall abscess. The patient’s wound had decreased in size significantly by postoperative week 5 and eventually healed completely.
Final pathologies for the lesions were no evidence of tumor or dysplasia found (n = 4), tubulovillous adenoma (n = 4), tubulovillous adenoma with high-grade dysplasia (n = 4), and invasive adenocarcinoma (n = 4). Mean maximum diameter of the specimens was 4.1 cm (IQR 2–3.1 cm). 100% of the specimens were intact. Excluding the biopsy specimens, 100% of the excision specimens had negative margins. 3 specimens included a total of 4 lymph nodes, all of which were negative for tumor.
Robotic TAMIS provided adequate, oncologic treatment for 12/16 of patients: 2 negative pathologies, 4 tubulovillous adenomas, 4 tubulovillous adenomas with high-grade dysplasia, and 2 pT1 invasive adenocarcinomas. 4 patients received additional therapies after their rTAMIS procedure as seen in Table 1.
Discussion
Through this case series, we describe our early experience with robotic TAMIS with the da Vinci Xi robotic system at a single community center. There was a total of 16 cases who underwent successful rTAMIS for both benign and malignant lesions. All specimens had negative margins on final pathology. rTAMIS was able to provide both curative treatment for benign and early malignant lesions as well as definitive diagnosis in patients with more advanced malignancies, allowing them to receive the appropriate oncologic treatment.
Patient positioning may serve as one of the advantages of the robotic approach over laparoscopic TAMIS. The first two patients were placed in lithotomy, but the remaining 14 were placed in prone jackknife. The primary surgeon felt the patients’ legs while in lithotomy hindered the robotic arms, and positioning the patients in prone allowed for a wider range of movement of the robotic arms. Patient positioning for laparoscopic TAMIS is critical as the procedure is much more difficult if the lesion is not located facing down towards the 6:00 position. With the articulating instruments of the robot, the location of the lesion becomes less important as all quadrants of the lumen are accessible, and therefore, even circumferential lesions can be excised intact. For example, patient 16 in our case series had a circumferential carpeting adenomatous polyp at the distal rectum which extended to the first rectal valve. A circumferential mucosal resection was performed using the robot, and the proximal mucosa was then sewn to the anus. As such, we believe robotic TAMIS will also allow for removal of larger, more proximal and complex lesions that have typically been unresectable with a laparoscopic approach in the past.
Local excision has been proven to provide curative oncologic treatment for benign rectal lesions and carefully selected, T1N0 cancers with histologically favorable features. Transanal minimally invasive surgery (TAMIS) was first described in 2009 as an alternative to local excision and transanal endoscopic microsurgery (TEM). It has since become a popular approach for rectal lesions in the mid and distal rectum, especially since it utilizes widely available laparoscopy instruments instead of the less accessible TEM platform [12,13,14,15,16,17]. As such, it is thought to be more cost effective and allows surgeons to take advantage of their already established laparoscopic skillset. Albert et al. reviewed 50 patients who had undergone TAMIS for benign neoplasms and early-stage rectal cancer, and concluded that it was a safe and effective platform. While 6% had microscopically positive margins, it was still favorable compared to TEM, which has a reported rate of up to 17%. None of their patients had long-term complications [16]. Similarly, in a larger, follow-up study, Lee et al. (2017) reported on clinical outcomes of 200 patients who had undergone local excision of benign and malignant rectal lesions via TAMIS. 7% of their cases had positive margins, and there was a 5% fragmentation rate. However, given the location and small surgical field, access and visualization of rectal lesions for adequate excision have remained a significant challenge [17].
More recently, the da Vinci robotic surgery system has been used to facilitate many surgical procedures including TAMIS. Robotic surgery has recently been shown to enhance surgeon comfort and ergonomics, which can lower stress and increase efficiency [18,19,20,21,22,23]. Lee et al. (2014) compared physical and cognitive ergonomic workload between robotic and laparoscopic surgeries in 13 minimally invasive trained surgeons using electromyography and NASA-TLX questionnaire. They concluded that while surgeon skill played a role in several ergonomic components, overall physical and cognitive ergonomics were significantly easier with robotic surgery [23]. While limited studies have applied robotic surgery to TAMIS, early results indicate that rTAMIS has the advantaged of better visualization and access to the rectum. Tomassi et al. (2019) described 58 cases of rTAMIS with full-thickness rectal resections. Their results showed a 5.2% positive margin rate and 1.8% fragmentation rate. 5.5% of their patients developed local recurrences with 100% undergoing successful salvage surgery. They concluded that rTAMIS is a safe, effective organ preserving approach, while improving surgeon ergonomics [11].
While our study is limited by small sample size with short-term follow-up, our early results reflect those seen in similar studies. In addition to improved visualization and surgeon ergonomics, the robotic platform allows access to larger more proximal and complex lesions and is less dependent on patient positioning. The da Vinci robot instruments provide seven degrees of movement which mimic articulated wrist movement in contrast to conventional laparoscopic instruments which only have four. We have successfully resected circumferential rectal lesions with negative margins with the help of the robot, something that would not have been feasible with conventional TAMIS.
Further studies should focus on directly comparing robotic and laparoscopic TAMIS in terms of long-term oncologic outcome, postoperative recovery, and ease of procedure for the surgeon. We should also determine the safety of performing this procedure for larger, more difficult rectal lesions that have not been possible with a traditional laparoscopic TAMIS approach, thus, possibly saving patients from a more morbid radical proctectomy.
Conclusions
Robotic TAMIS is a safe and feasible alternative to laparoscopic TAMIS and can be successfully performed in a community, nonacademic medical center. It provides better visualization and access to rectal lesions. Further studies should include direct comparison of oncologic outcomes between laparoscopic and robotic TAMIS.
References
Monson JRT, Weiser MR, Buie WD, Chang GJ, Rafferty JF (2013) Practice Parameters for the Management of Rectal Cancer (Revised). Dis Colon & Rectum 56(6):535–550
Buess G, Theiss R, Hutterer F, Pichlamaier H, Pelz C, Holfeld T, Isselhard W (1983) Transanal endoscopic surgery of the rectum - testing a new method in animal experiments [in German]. Leber Magen Darm 13(2):73–77
Buess G, Kipfmüller K, Ibald R et al (1988) Clinical results of transanal endoscopic microsurgery. Surg Endosc 2(4):245–250
Attalah S, Albert M, Larach S (2010) Transanal minimally invasive surgery: a giant leap forward. Surg Endosc 24:2200–2205
deBeche-Adams T, Nassif G (2015) Transanal Minimally Invasive Surgery. Clin Colon Rectal Surg 28:176–180
Lim SB, Seo SI, Lee JL, Kwak JY, Jang TY, Kim CW, Yoon YS, Yu CS, Kim JC (2012) Feasibility of Transanal Minimally Invasive Surgery for Mid-Rectal Lesions. Surg Endosc 26(11):3127–3132
Barendse RM, Doornebosch PG, Bemelman WA, Fockens P, Dekker E, de Graaf EJ (2012) Transanal employment of single access ports is feasible for rectal surgery. Ann Surg 256(6):1030–1033
Thompson EV, Bleier JIS (2017) Transanal Minimally Invasive Surgery. Clin Colon Rectal Surg 30:112–119
Attalah SB, Albert MR, DeBeche-Adams TH, Larach SW (2011) Robotic TransAnal Minimally Invasive Surgery in a cadaveric model. Tech Coloproctol 15(4):461–464
Hompes R, Rauh SM, Ris F, Tuynman JB, Mortensen NJ (2014) Robotic transanal minimally invasive surgery for local excision of rectal neoplasms. Br J Surg 101(5):578–581
Tomassi MJ, Janos T, Yuhan R, Ruan JH, Klaristenfeld DD (2019) Robotic transanam minimally invasive surgery for the excision of rectal neoplasia: Clinical experience with 58 consecutive patients. Dis Colon Rectum 62:279–285
Keller DS, Tahilramani RN, Flores-Gonzalez JR, Mahmood A, Haas EM (2016) Transanal minimally invasive surgery: Review of indications and outcomes from 75 consecutive patients. J Am Coll 222(5):814–822
Martin-Perez B, Andrade-Ribeiro GD, Hunter L, Atallah, (2014) A systematic review of Transanal Minimally Invasive Surgery (TAMIS) from 2010–2013. Tech Coloproctol 18(9):775–788
McLemore EC, Weston LA, Coker AM, Jacobsen GR, Talamini MA, Horgan S, Ramamoorth SL (2014) Transanal Minimally Invasive Surgery for benign and malignant rectal neoplasia. Am J Surg 208(3):372–381
Rimonda R, Arezzo A, Arolfo S, Salvai A, Morino M (2013) TransAnal Minimally Invasive Surgery (TAMIS) with SILS Port versus Transanal Endoscopic Microsurgery (TEM): a comparative experimental study. Surg Endosc 27:3762–3786
Albert MR, Atallah SB, deBeche-Adams TC, Izfar S, Larach SW (2013) Transanal minimally invasive surgery (TAMIS) for local excision of benign neoplasms and early-stage rectal cancer: Efficacy and outcomes in the first 50 patients. Dis Colon Rectum 56(3):301–306
Lee L, Burke JP, DeBeche-Adams T, Nassif G, Martin-Perez B, Monson JRT, Albert MR, Attalah SB (2018) Transanal minimally invasive surgery for local excision of benign and malignant rectal neoplasia: outcomes from 200 consecutive cases with midterm follow up. Ann Surg 267(5):910–916
Huang YJ, Huan YM, Wang WL, Tong YS, Hsu W, Wei PL (2020) Surgical outcomes of robotic transanal minimally invasive surgery for selected rectal neoplasms: A single-hospital experience. Asian J Surg 43(1):290–296
Baik SH, Kwon HY, Kim JS, Hur H, Sohn SK, Cho HC, Kim H (2009) Robotic versus laparoscopic low anterior resection of rectal cancer: Short-term outcome of a prospective comparative study. Ann Surg Oncol 16:1480–1487
Van der Schatte Oliver RH, Van’t Hullenaar CD, Ruurda JP, Broeders IA, (2009) Ergonomics, user comfort, and performance in standard and robot-assissted laparoscopic surgery. Surg Endosc 23:1365–1371
Tarr ME, Brancato SJ, Cunkelman JA, Polcari A, Nutter B, Kenton K (2015) Comparison of postural ergonomics between laparoscopic and robotic sacrocolpopexy: a pilot study. J Minim Invasive Gynecol 22:234–238
Lee SG, Russ AJ, Casillas MA Jr (2019) Laparsocopic transanal minimally invasive surgery (L-TAMIS) versus robotic TAMIS (R-TAMIS): short-term outcomes and costs of a comparative study. Surg Endosc 33:1981–1987
Lee GI, Lee MR, Clanton T, Sutton E, Park AE, Marohn MR (2013) Comparative assessment of physical and cognitive ergonomics associated with robotic and traditional laparscopic surgeries. Surg Endosc 28:456–465
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Karina W Lo, MD, David N Blitzer, MD, Sami Shoucair, MD, and David M Lisle, MD have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lo, K.W., Blitzer, D.N., Shoucair, S. et al. Robotic transanal minimally invasive surgery: a case series. Surg Endosc 36, 793–799 (2022). https://doi.org/10.1007/s00464-020-08257-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-020-08257-1