Abstract
Background
Robotic camera holders for endoscopic surgery have been available for 20 years but market penetration is low. The current camera holders are controlled by voice, joystick, eyeball tracking, or head movements, and this type of steering has proven to be successful but excessive disturbance of surgical workflow has blocked widespread introduction. The Autolap™ system (MST, Israel) uses a radically different steering concept based on image analysis. This may improve acceptance by smooth, interactive, and fast steering. These two studies were conducted to prove safe and efficient performance of the core technology.
Methods
A total of 66 various laparoscopic procedures were performed with the AutoLap™ by nine experienced surgeons, in two multi-center studies; 41 cholecystectomies, 13 fundoplications including hiatal hernia repair, 4 endometriosis surgeries, 2 inguinal hernia repairs, and 6 (bilateral) salpingo-oophorectomies. The use of the AutoLap™ system was evaluated in terms of safety, image stability, setup and procedural time, accuracy of imaged-based movements, and user satisfaction.
Results
Surgical procedures were completed with the AutoLap™ system in 64 cases (97%). The mean overall setup time of the AutoLap™ system was 4 min (04:08 ± 0.10). Procedure times were not prolonged due to the use of the system when compared to literature average. The reported user satisfaction was 3.85 and 3.96 on a scale of 1 to 5 in two studies. More than 90% of the image-based movements were accurate. No system-related adverse events were recorded while using the system.
Conclusion
Safe and efficient use of the core technology of the AutoLap™ system was demonstrated with high image stability and good surgeon satisfaction. The results support further clinical studies that will focus on usability, improved ergonomics and additional image-based features.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Visualization in endoscopic surgery is a challenge for both surgeon and camera assistant. The surgeon highly depends on the camera assistant to provide a stable, centered, and non-rotated image of the target area and has no control other than verbal commands or manual correction, which requires release of one of the instruments.
An often encountered pitfall is that the image is unstable due to tremor, unintended movements or rotation of the camera by the surgical assistant. Furthermore, manual control can also be physically demanding leading to fatigue and suboptimal control. Inexperienced or inattentive assistants may displace the camera frequently, make jerky movements, malposition the laparoscope in relation to the horizon, and point the camera outside the focus of interest more often than experienced assistants [1]. In addition, touching tissues with the camera results in image blurring and a need for frequent removal of the camera for cleaning. These parameters can cause motion sickness and surgeon distraction that may result in prolonged procedures and patient safety hazards [2].
Robotic devices have been introduced 20 years ago to overcome some of these pitfalls [2]. A robotic laparoscopic positioner can perform the task of the surgical assistant and enables the surgeon to control camera movements personally. Robotic camera holders provide a stable, non-rotated field of view with a focus based on the surgeon’s preference. In the last two decades, different robotic arms have been developed, controlled by voice, joysticks, eyeball tracking, and head movements. The various commercialized systems have proven to be reliable and efficient but market penetration is low. This might be due to excessive hindrance of surgical flow as a result of cumbersome control modalities causing surgeons to decide to rely on human assistants.
The AutoLap™ system (MST, Israel) aims to overcome unsuccessful camera control concepts by radically changing laparoscope manipulation technology. The steering concept is based on image analysis and computer-based instrument recognition instead of direct electromechanical steering of a holder that is unaware of the relation between camera position and field of view. The aim of these studies were to evaluate reliability and efficiency of the core electromechanical elements of the Autolap™ system in clinical use. The system was evaluated in terms of safety, image stability, procedural and setup time, accuracy of image-based movements and user satisfaction during general and gynecological laparoscopic surgeries in two multi-center studies, which were conducted in Israel, the Netherlands, and Italy.
Materials and methods
System’s description
The AutoLap™ (MST, Israel) is an image-guided system that utilizes unique image processing algorithms to enable the motorized movement of the laparoscope at any oblique path by following the movement of a designated surgical instrument. It can be mounted on either side of the operating bed rail, providing flexibility for supporting various laparoscopic procedures and negates the need for calibration if the patient position is altered. The system comprises a robotic motion assembly unit and a processing unit, which holds the AutoLap™ unique software and algorithms (Fig. 1). The robotic motion assembly unit, which holds the laparoscope (Fig. 2), is covered by a sterile sleeve during the procedure (Fig. 3). Control over camera’s movements and operative field is enabled by a small wireless sterile disposable button, which transmits RF signals and is worn by the surgeon as a ring or is attached to the surgical tool (Fig. 4).
There are three modes of operation, manual operation with a force joystick and two modes initiated by the wireless button: “joystick mode” and “follow-me mode.” In joystick mode, the surgeon can move in four directions; up, down, left, right, and zoom in/out. In follow-me mode, the system movements are controlled by tracking the movements of a designated tool, which is selected by the surgeon. The system is able to recognize any off-the-shelf surgical tool used in laparoscopic surgery and does not need special marking or brand.
Additionally, the use of the data gathered from the video image enables additional image-based features such as performing zoom in/out movement with an angled laparoscope while maintaining the center of the displayed image, correction of directional movement in case of camera rotation, provide adaptive velocity according to the working zoom level and enable digital zoom using the AutoLap™ controller.
Study design
Two multi-center studies have been performed in five centers in three countries [Assuta Medical Center Haifa (Israel), Assuta Medical Center Tel Aviv (Israel), Meander Medical Center (the Netherlands), Niguarda Cà Granda Hospital (Italy), UMC Utrecht (the Netherlands)]. The first study was conducted in 2013–2014 to evaluate safety and performance of the AutoLap™ system (the “safety study”, Clinical Trials.gov identifier NCT01828580). The second study was conducted in 2014–2016 to evaluate the follow-me mode (the “follow-me study”, Clinical Trials.gov identifier NCT02326870). Patients who were scheduled for hiatal hernia repair/fundoplication, cholecystectomy, endometriosis surgery, (bilateral) salpingo-oophorectomy and inguinal hernia repair were included in both studies. The clinical studies were approved by the centers’s relevant Ethic Committees and if required also by the Ministry of Health.
Inclusion criteria were age > 18 and signed informed consent form for both studies. Exclusion criteria for the safety study were previous abdominal surgery and contraindications to pneumoperitoneum, pregnancy, obesity (BMI > 35 kg/m2), generalized peritonitis, septic shock from cholangitis, severe acute pancreatitis, uncorrected coagulopathy, advanced cirrhosis with failure of hepatic function, suspected gallbladder cancer, acute cholecystitis, presence of any medical or psychiatric condition or any other condition that, in the opinion of the investigator, could affect the successful participation of the patient in the study and patient participates in any other clinical study 60 days prior to the start of the study and throughout the study duration. Exclusion criteria for the follow-me study were pregnancy, American Society of Anesthesiologist (ASA) classification > 2, and extensive adhesions that preclude the standard laparoscopic surgical technique.
Primary outcomes of the both studies were adverse events and performance evaluation [operation time (skin to skin), number of successful movements]. The procedure time for each type of operation was measured; however, no comparison was made with a control group. The average percentage of successful movements was calculated by the number of successful movements divided by the total number of movements during the procedure × 100%. A movement is deemed successful if the laparoscope reached the desired position, which was verbally verified with the surgeon after each movement.
Secondary outcomes were system setup time (including installation, draping and positioning), the number of times the laparoscope was removed for cleaning and a usability evaluation (questionnaire). Usability questionnaires were completed postoperatively by the surgeon for subjective evaluation of usability aspects, specifically regarding system handling, image stability, effort, satisfaction, and the efficiency to perform the procedure with the AutoLap™ system. Answers were given on a scale ranging from one (disagree completely) to five (agree completely, the most positive response). Different usability questionnaires were used for both studies comprising of 15 questions for the safety study and 18 questions for the follow-me study.
Data analysis
In addition to the primary and secondary outcomes, demographic data were recorded including age, gender, ASA score, weight, height, and BMI. The data were analyzed with Excel (Microsoft, Redmond, Washington, 2016).
Results
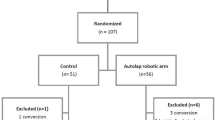
From January 2013 to October 2015, 66 patients were enrolled to participate in two multi-center studies in five centers. A total of nine surgeons participated in the studies. The demographic data of the patients are shown in Table 1. The number of procedures performed per center is shown in Table 2.
Setup and operation time
The mean setup time was 4 min (4:08 ± 0.1). In most cases, the system setup was performed while the patient was being prepared for surgery. Draping of the system was mostly done during insufflation of the abdomen, integrating the setup of the system in the normal OR setup time. The mean operative time in minutes for the different procedures are descripted in Table 3.
Laparoscope cleaning
The laparoscope was not removed at all in most of the procedures. An average of one (0.72 range 0–5) removal per procedure was recorded. In some cases, the need to clean the scope was due to fogging that resulted from temperature differences and not due to smearing of the lens of the laparoscope.
Number of successful movements
On average, 99 joystick- and 12.8 follow-me movements were made during a procedure. More than 90% of these movements were successful in both studies. Most of the surgeons used the joystick mode for small movements close to the tissue and the follow-me mode for larger movements, further away from the tissue.
Safety and usability evaluation
No system-induced complications occurred during all the performed procedures. The usability questionnaires demonstrated adequate satisfaction of all surgeons in all of the procedures with a median score of four (on a scale of 1–5) in both studies. The average usability score of the safety study was 3.85 (± 0.37) and 3.96 (± 0.54) for the follow-me study. The satisfaction specifically related to the usage of the follow-me mode was 3.92. All surgeons expressed satisfaction from the high image stability provided by the system (4.41) as well as the advantage of full control over the field of view (3.72), intuitive use (3.76), and anticipated short learning curve (3.97). Less satisfaction was expressed from the release of the laparoscope for cleaning, which had the lowest average usability score of 3.1.
Discussion
Minimal invasive surgery has become a leading surgical modality. Every year, the rate of procedures performed is growing. However, laparoscopy requires a high demand of skill, concentration, and maneuverability leading to increased fatigue and physical discomfort for primary surgeons and assistants [3]. Operating in the small pelvis and the upper abdomen is especially physically challenging and burdensome. These complex surgeries require the assistant to hold the camera for a long period of time at the same, frequently angled, position. The resulting uncomfortable static working postures can lead to increased strain to different parts of the musculoskeletal system [4,5,6]. By improving ergonomics, primary surgeons and assistants may greatly benefit, allowing them to perform more complex and lengthy procedures with ease.
Different robotic active camera holders have been introduced in order to improve ergonomics and overcome some disadvantages of laparoscopy. One of the first systems on the market was the AESOP™ system (Intuitive Surgical, Inc.), which was controlled by voice or manually. The LapMan™ (Medsys S.A, Belgium) uses a joystick that is connected to the surgical tool to steer and the EndoAssist™ (Armstrong Healthcare LLC, UK), which is the first version of FreeHand™, uses a combination of a headset with motion sensor and a foot pedal to control the laparoscope’s movements. Smaller systems include the ViKY™ system (Endocontrol, France), FreeHand™ system (OR Productivity plc, UK) and the Soloassist™ (Actormed, Barbing, Germany). The Soloassist™ controls the scope with a joystick and the ViKY™ system with voice control in combination with a foot pedal to ensure that movement is only possible when the pedal is pressed.
Market penetration of active robotic camera holders is low. Despite the fact that several types of movement controls have proven to be successful, excessive disturbance of the surgical workflow has blocked widespread introduction. The main difference of the AutoLap™ system with other systems on the market is the usage of a different steering mechanism by image processing software. The AutoLap™ system is not limited to perpendicular movements alone, as can only be done by other systems in the market, but enables the surgeon to move the laparoscope in any direction and path. Acquiring the video images and using image processing algorithms enables the AutoLap™ to continuously detect the instruments within the field of view and control the laparoscope’s movements according to the instruments’ movements, as commanded by the surgeon.
In these studies, the accuracy of the image processing algorithms was 90%. Failure of detecting the tip of the tool is caused by several factors. First of all, the image quality needs to be sufficient in order for the algorithm to perform properly. Factors that adversely affect the image quality are fogging, blurring, and smearing of the lens. The amount of light in the field of view is also an important factor. A dark image due to poor equipment or a narrow and deep working space like the pelvis can contribute to poorer detection of the tip. Additionally, user errors were made. For instance, activating the detection algorithm when the tip of the instrument is not within the field of view. Lastly, this was the first clinical experience with the image processing algorithms. Small bugs and glitches were detected and corrected during the clinical work, resulting in a new software version.
The AutoLap™ system demonstrated to be an effective and safe system for robotic camera control during different types of abdominal procedures in our studies. High image stability leading to high surgeons’ satisfaction was reported in the questionnaires. No system-induced complications occurred and in most procedures the laparoscope did not need to be cleaned due to unintended contact with tissue. The average setup time of the AutoLap™ system is found to be comparable with other active camera holders. Wagner et al. reported an average setup time for the Endoassist™ and the AESOP™ of 2 ± 0.8 and 5.3 ± 2.4 min, respectively [7]. Kommu et al. also measured the setup time for the Endoassist™, varying from 5.1 ± 1.2 to 6.8 ± 2.3 min depending on the type of procedure [8]. The average setup time for the SoloAssist™ was reported to be 7 min by Beckmeier et al. [9] There was a significant learning curve of 20 procedures regarding the mounting of the system [9]. Maheswari et al. reported an average setup time of 3–5 min using the ViKY™ system in a small study of three patients [10].
During conventional laparoscopy, about 7% of the OR time is spent on cleaning the scope according to a recent article of Yong et al. [11]. Besides the fact that this is a considerable amount of valuable OR time, cleaning of the scope can disrupt the flow of the operation and be very frustrating. In our AutoLap™ trials, the scope was cleaned only once per operation on average. Other trials on robotic active camera holders also demonstrated less frequent cleaning of the scope and a reduction or equal total OR time [1, 12,13,14]. However, a longer OR time was demonstrated in a study by Gillen et al. using the Soloassist™ system, favoring the human assistant [15]. Kommu et al. reported more frequent cleaning of the scope during nephrectomies using the EndoAssist™, which was probably related to the product design [8].
Although there is no comparison with human assistance in our studies, the measured operational times when using the AutoLap™ system are in line with the literature [16,17,18,19,20]. Comparative studies between human- and robotic camera assistance demonstrated similarity in operational procedure times.[12, 21,22,23] However, the transabdominal preperitoneal hernia procedure (TAPP) took longer compared to literature due to hindrance of system, limiting the movement range of the surgeon’s tool. A new curved design of the bar solves this problem, and successful TAPP procedures are already performed with the system.
The follow-me mode is based on instrument recognition, followed by dragging the camera to the new focus point by a smooth instrument movement. An alternate mode of the follow-me, which has been developed following these studies, is the go-to mode. This mode enables the surgeon to use an instrument to tag the new desired center field of view. The surgeon moves the tagging tool to the new desired center field of view and releases the button of the remote controller. The field of view will then be centered around the virtually marked new position, at a comparable distance from the tissue. Thus, without losing grip of the surgical instrument, the surgeon only needs to point and click to adjust his desired field of view.
The Autolap™ system may prove economic value by reducing the number of surgical team members. Several procedures in these studies were performed as solo surgery, i.e., without the need of a surgical assistant. The operation was performed with a surgeon and scrub nurse alone. Greater involvement of the scrub nurse may facilitate fewer OR personnel—a factor that may have an important economic impact where skilled staff shortage is an obstacle to the performance of laparoscopic surgery.
The main limitations regarding the performed studies must be noted. There is a potential risk of bias due to heterogeneity; a total of nine surgeons, in five different centers, performed six different types of procedures. Moreover, the learning curve was not measured. It is possible that some surgeons were still in their learning process, which can influence the length of the procedures.
There are also some limitations regarding the AutoLap™ system. Correct positioning of the system on the bedrail is very important to have maximum movement range. Sometimes, adjustments to the positioning have to be made by a non-sterile OR personnel during the procedure. Although the system main compartments are rather large in appearance, positioning of the system is quite flexible. Various types of complex procedures have been performed successfully with the system outside the studies, including colectomies, surgery in the pelvis and gastric surgery. Also, using the wireless disposable button without losing grip of the instrument takes some practice. Improvements of these limitations are being made and will be implemented soon. Examples are a new command unit design, easy release of the laparoscope and the possibility to reposition the system by single button activation by a sterile person. Future perspectives of the system are development of the next generation system including a smaller footprint and the expansion of the image-based properties, in search for the most effective image-based steering technology without disturbance of surgical flow.
In conclusion, the AutoLap™ system is an effective, safe and easy to use system for robotic camera driving during a variety of abdominal procedures. Future studies will focus on evaluating additional image-based features, ergonomic and economic advantages while using the AutoLap™ system.
References
Dunlap KD, Wanzer L (1998) Is the robotic arm a cost-effective surgical tool? AORN J 68:265–272
Ballantyne GH (2002) The pitfalls of laparoscopic surgery: challenges for robotics and telerobotic surgery. Surg Laparosc Endosc Percutan Tech 12:1–5. https://doi.org/10.1097/00129689-200202000-00001
Berguer R (1999) Surgery and ergonomics. Arch Surg 134:1011–1016. https://doi.org/10.1001/archsurg.134.9.1011
Hu C-L, Yang C-Y, Lin Z-S, Yang S-Y, Kuo C-H, Lin M-T (2013) An interactive method for achieving ergonomically optimum conditions during laparoscopic surgery. J Robot Surg 7:125–130. https://doi.org/10.1007/s11701-012-0353-4
Miller K, Benden M, Pickens A, Shipp E, Zheng Q (2012) Ergonomics principles associated with laparoscopic surgeon Injury/Illness. Hum Factors J Hum Factors Ergon Soc 54:1087–1092. https://doi.org/10.1177/0018720812451046
Van Der Schatte Olivier RH, Van’t Hullenaar CDP, Ruurda JP, Broeders IAMJ (2009) Ergonomics, user comfort, and performance in standard and robot-assisted laparoscopic surgery. Surg Endosc Other Interv Tech 23:1365–1371. https://doi.org/10.1007/s00464-008-0184-6
Wagner AA, Varkarakis IM, Link RE, Sullivan W, Su LM (2006) Comparison of surgical performance during laparoscopic radical prostatectomy of two robotic camera holders, EndoAssist and AESOP: a pilot study. Urology 68:70–74. https://doi.org/10.1016/j.urology.2006.02.003
Kommu SS, Rimington P, Anderson C, Rané A (2007) Initial experience with the EndoAssist camera-holding robot in laparoscopic urological surgery. J Robot Surg 1:133–137. https://doi.org/10.1007/s11701-007-0010-5
Beckmeier L, Klapdor R, Soergel P, Kundu S, Hillemanns P, Hertel H (2014) Evaluation of active camera control systems in gynecological surgery: construction, handling, comfort, surgeries and results. Arch Gynecol Obstet 289:341–348. https://doi.org/10.1007/s00404-013-3004-8
Maheshwari M, Ind T (2015) Concurrent use of a robotic uterine manipulator and a robotic laparoscope holder to achieve assistant-less solo laparoscopy: the double ViKY. J Robot Surg 9:211–213. https://doi.org/10.1007/s11701-015-0518-z
Yong N, Grange P, Eldred-Evans D (2016) Impact of laparoscopic lens contamination in operating theaters: a study on the frequency and duration of lens contamination and commonly utilized techniques to maintain clear vision. Surg Laparosc Endosc Percutan Tech 26:286–289. https://doi.org/10.1097/SLE.0000000000000289
Stolzenburg JU, Franz T, Kallidonis P, Minh D, Dietel A, Hicks J, Nicolaus M, Al-Aown A, Liatsikos E (2011) Comparison of the FreeHand?? Robotic camera holder with human assistants during endoscopic extraperitoneal radical prostatectomy. BJU Int 107:970–974. https://doi.org/10.1111/j.1464-410X.2010.09656.x
Aiono S, Gilbert JM, Soin B, Finlay PA, Gordan A (2002) Controlled trial of the introduction of a robotic camera assistant (EndoAssist) for laparoscopic cholecystectomy. Surg Endosc Other Interv Tech 16:1267–1270. https://doi.org/10.1007/s00464-001-9174-7
Geis WP, Kim HC, Brennan EJ, McAfee PC, Wang Y (1996) Robotic arm enhancement to accommodate improved efficiency and decreased resource utilization in complex minimally invasive surgical procedures. Stud Health Technol Inform 29:471–481
Gillen S, Pletzer B, Heiligensetzer A, Wolf P, Kleeff J, Feussner H, Fürst A (2014) Solo-surgical laparoscopic cholecystectomy with a joystick-guided camera device: a case-control study. Surg Endosc Other Interv Tech 28:164–170. https://doi.org/10.1007/s00464-013-3142-x
Shushan A, Mohamed H, Magos AL (1999) How long does laparoscopic surgery really take? Lessons learned from 1000 operative laparoscopies. Hum Reprod 14:39–43. https://doi.org/10.1093/humrep/14.1.39
Minutolo V, Licciardello A, Arena M, Nicosia A, Stefano BDI (2014) Laparoscopic cholecystectomy in the treatment of acute cholecystitis: comparison of outcomes and costs between early and delayed cholecystectomy. Eur Rev Med Pharmacol Sci 18:40–46
Morino M, Pellegrino L, Giaccone C, Garrone C, Rebecchi F (2006) Randomized clinical trial of robot-assisted versus laparoscopic Nissen fundoplication. Br J Surg 93:553–558. https://doi.org/10.1002/bjs.5325
Keus F, de Jong J, Gooszen HG, Laarhoven CJ (2006) Laparoscopic versus open cholecystectomy for patients with symptomatic cholecystolithiasis. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD006231
Memon MA, Subramanya MS, Hossain MB, Yunus RM, Khan S, Memon B (2015) Laparoscopic anterior versus posterior fundoplication for gastro-esophageal reflux disease: a meta-analysis and systematic review. World J Surg 39:981–996. https://doi.org/10.1007/s00268-014-2889-0
Proske JM, Dagher I, Franco D (2004) Comparative study of human and robotic camera control in laparoscopic biliary and colon surgery. J Laparoendosc Adv Surg Tech A 14:345–348. https://doi.org/10.1089/lap.2004.14.345
Kalteis M, Pistrich R, Schimetta W, Polz W (2007) Laparoscopic cholecystectomy as solo surgery with the aid of a robotic camera holder: a case-control study. Surg Laparosc Endosc Percutan Tech 17:277–282. https://doi.org/10.1097/SLE.0b013e31806030ae
Kavoussi LR, Moore RG, Adams JB, Partin AW (1995) Urologists at work comparison of robotic versus human laparoscopic camera control. J Urol 154:2134–2136
Acknowledgements
The study was sponsored by Medical Surgery Technologies ltd (M.S.T). The company paid the required fees to the ethics committee and all other relevant study-related expenses. No other benefits were received by participating in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Paul Wijsman is a Clinical Field Engineer of Medical Surgery Technologies ltd (M.S.T) since 2016. Ivo Broeders, Amir Szold, and Yuval Kaufman are the paid members of the Clinical Advisory Board of M.S.T. Hylke Brenkman, Antonello Forgione, Henk Schreuder, Esther Consten, Werner Draaisma, Paul Verheijen, and Jelle Ruurda have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Wijsman, P.J.M., Broeders, I.A.M.J., Brenkman, H.J. et al. First experience with THE AUTOLAP™ SYSTEM: an image-based robotic camera steering device. Surg Endosc 32, 2560–2566 (2018). https://doi.org/10.1007/s00464-017-5957-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-017-5957-3