Abstract
Background
Laparoscopic surgery for gastric gastrointestinal stromal tumors (GISTs) is now widely performed. However, laparoscopic resection of GIST in the esophagogastric junction (EGJ) is technically difficult and rarely reported. Herein, we introduce four fashions of laparoscopic resection for EGJ-GIST.
Methods
A retrospective review of 42 consecutive patients with EGJ-GIST who underwent attempted laparoscopic surgery was conducted. EGJ-GIST was defined as GIST with an upper border of less than 5 cm from the esophagogastric line. Four fashions of laparoscopic resection were performed: fashion A, laparoscopic wedge resection using linear stapler; fashion B, laparoscopic complete resection by opening the stomach wall and closing with suture or linear stapler; fashion C, laparoscopic mucosa-preserving resection; and fashion D, laparoscopic proximal gastrectomy with pyloroplasty and gastric plication. Clinicopathologic characteristics, operative course, and short-term and long-term outcomes were analyzed.
Results
All procedures were completed successfully without operative complications. In 24 of 42 (57.1%) patients, tumors were located in the fundus or greater curvature. Out of those, 70.8% (17/24) received fashion A and 29.2% (7/24) received fashion B. Tumors in 16 of 42 (38.1%) patients were located in the lesser curvature. Of those, 81.3% (13/16) underwent fashion B and 18.7% (3/16) underwent fashion D. One tumor in the anterior stomach wall and one in the posterior wall received fashion C. The mean operative time was 103.8 ± 22.1 min and the mean estimated blood loss was 22.4 ± 13.5 ml. The mean time to flatus was 40.3 ± 12.9 h and the time to fluid intake was 43.2 ± 14.3 h. The mean hospital stay was 4.8 ± 2.1 days.
Conclusions
Laparoscopic surgery for EGJ-GIST is safe and feasible. The selection of various laparoscopic resection fashions should be chosen based on tumor location and the surgeon’s experience.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Gastrointestinal stromal tumors (GISTs) most commonly arise from KIT and PDGRFA activating mutations [1]. Although GISTs can be found anywhere along the digestive tract, the stomach (60%) is the most common primary site [2]. For localized gastric GIST lesions, the optimal therapy of choice and only curative treatment method is complete surgical resection. The surgical procedure should abide by two main principles: protecting the pseudocapsule integrity and achieving histologically negative margins. GISTs are mainly located in the submucosa and grow in an expansive manner rather than a diffuse infiltrative fashion. They also tend to have low incidence of lymph node involvement, so lymphadenectomy is usually not demanded [3,4,5]. Accordingly, these biological features offer favorable conditions for minimally invasive surgery.
In recent decades with the further understanding of the anatomy underlying laparoscopic conditions and improvement of minimally invasive techniques, laparoscopic resection for resectable gastric GIST is now widely performed [6, 7]. Moreover, the indications for laparoscopic surgery for gastric GIST continue to expand gradually. In the 2004 NCCN task force report, it was suggested that laparoscopic resection for gastric GISTs 5 cm in size or less can be performed safely in experienced hands [8]. In 2010, the indication for laparoscopic procedure expanded to include tumors larger than 5 cm, depending on the location and shape [9]. Notably, laparoscopic resection for non-metastatic gastric GIST must also follow the two principles of oncologic surgery mentioned above. The favorable gastric GIST locations for laparoscopic approaches are at the greater curvature and the anterior wall of the stomach.
However, laparoscopic approach for gastric GIST located in the esophagogastric junction (EGJ-GIST) is technically challenging and rarely reported because of the complex anatomical factors and the difficulty of functional preservation. Although laparoscopic wedge resection is mostly performed for gastric GISTs, it cannot be ideally used for all EGJ-GISTs. Although some fashions of laparoscopic procedures for EGJ-GISTs are depicted in literature, there is still no generally accepted technique for this condition [10, 11].
The aims of this study are to introduce four fashions of laparoscopic resection for EGJ-GIST and to report surgical outcomes based on our center’s experience.
Materials and methods
Patients
Between 2010 and 2016, a total of 42 patients with suspected EGJ-GIST underwent laparoscopic resection in our institution. The diagnostic information, including the origin, location, and size of the EGJ-GISTs, was established with preoperative computed tomography and gastrofiberscope. An ultrasonic endoscopy was also regularly performed for diagnosis since 2011. A preoperative pathological diagnosis was not normally conducted. This study protocol was approved by our institutional review board. Before surgery, all patients were offered detailed information regarding potential risks of laparoscopic technique and provided written informed consent. The following clinical data of the patients were collected and collated: age, sex, body mass index (BMI), tumor location and size, operation time, length of postoperative hospital stay, postoperative recovery and complications, and pathological results. Follow-up was carried out with telephone or through outpatient visits. The follow-up data included postoperative adjuvant therapy, survival and recurrence.
Esophagogastric junction gastrointestinal stromal tumor definition
EGJ-GIST is defined as a GIST with an upper border of less than 5 cm from the esophagogastric line [12].
Surgical technique
General surgical operations
The patients were placed in a supine position with legs separated under general anesthesia. A total of five trocars were utilized as needed. The first 12 mm trocar was inserted at the level of the umbilicus for the laparoscope. Two trocars were inserted on the left side for the operating surgeon, and two trocars were placed on the right side for the assistant surgeon. A gastrofiberscope was used to identify the anatomic location of the tumor and estimate the distance of the upper border of the tumor to the esophagogastric line before the procedure. Then an ultrasonic scalpel was used to separate the gastrocolic and gastrosplenic ligament and divide the short gastric vessels as needed. When the tumor was located in the lesser curvature, the lesser omentum was dissected to completely expose the lesion.
After mobilization, one of four fashions of laparoscopic resection for EGJ-GIST was selected according to several factors, such as tumor location and size, distance between the upper border of the tumor and esophagogastric line, manner of growth, and whether or not an ulcer was present: fashion A, laparoscopic wedge resection; fashion B, laparoscopic resection through opening the whole layers of the stomach wall and closing with suture or linear stapler; fashion C, laparoscopic mucosa-preserving resection; and fashion D, laparoscopic proximal gastrectomy with pyloroplasty.
Fashion A: laparoscopic wedge resection
Tumors located in the fundus or greater curvature of the stomach with a distance of more than 2 cm from the esophagogastric line was most suitable for laparoscopic wedge resection. After complete exposure of the tumor, an intracorporeal linear stapler was placed through a 12 mm trocar to remove the lesion. The adequate margin was confirmed using an intraluminal inspection with a gastrofiberscope. When the tumor was located in the posterior wall, the stomach was turned over for complete visualization and easier resection of the tumor. Then, the resection specimen was placed in a plastic bag and removed from the abdomen. Finally, the staple line was checked with laparoscopy and endoscopy.
Fashion B: laparoscopic resection through opening the whole layers of the stomach wall and closing with suture or linear stapler
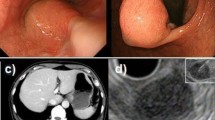
When the tumor was located in the lesser curvature, laparoscopic wedge resection was dangerous due to potential cardiac stenosis. Before performing fashion B, the lesser omentum was separated for complete exposure of the tumor. Then the whole layers of the stomach wall were opened with an ultrasonic scalpel and an adequate surgical margin was confirmed under inspection with a gastrofiberscope (Fig. 1). The stomach wall surrounding the lesion was dissected cautiously to avoid esophagogastric line damage. If EGJ invasion by the tumor was detected during operation, a conversion to laparoscopic proximal gastrectomy was inevitable. A plastic bag was used to remove the lesion to prevent spillage or seeding in the abdomen. Next, the stomach incision was closed using a continuous suture technique or a linear stapler. An examination of the suture or staple line utilizing endoscopy was imperative for detection of EGJ damage.
Fashion C: laparoscopic mucosa-preserving resection
As GIST originates from the interstitial cells of Cajal and grows in an expansive manner, laparoscopic mucosa-preserving resection may be performed. When the tumor was located near the esophagogastric line, a mucosa-preserving resection could be performed to maintain the function of the cardia. The gastric serosa around the tumor was completely exposed by mobilizing adjacent anatomic structures around the EGJ (Fig. 2). The peripheral serosa and muscle layers of the tumor were dissected with a sufficient oncologic margin using an ultrasonic scalpel. Then the tumor was separated by pulling the dissected serosa and muscle layers associated with the tumor while preserving the mucosa. During manipulation, the traction must be gentle and the pseudocapsule of the tumor should remain intact. A plastic bag was used for specimen extraction, and a leak test was performed endoscopically.
Fashion D: laparoscopic proximal gastrectomy
When the tumor invaded the esophagogastric line or if the cardia integrity could not be maintained using the previously described fashions of resection, laparoscopic proximal gastrectomy was performed with pyloroplasty and gastric plication.
Statistical analysis
All data are described as mean ± standard deviation (SD). Statistical analyses were performed using SPSS version 17.0 (SPSS, Chicago, IL, USA).
Results
Patient characteristics are shown in Table 1. Forty-two patients (26 males and 16 females) with EGJ-GISTs underwent laparoscopic resection. The mean distance between the upper border of the tumor and the esophagogastric line was 2.7 cm. Tumors in 24 of 42 (57.1%) patients were located in the fundus or the greater curvature. Of these, 70.8% (17/24) received fashion A and the remaining (7/24) underwent fashion B. Tumors in 16 of 42 (38.1%) patients were located in the lesser curvature. Of these, 81.3% (13/16) underwent fashion B and the remaining (3/16) underwent fashion D. One tumor located in the anterior stomach wall and one in the posterior wall received fashion C. In the three cases undergoing fashion D, two were planned and one was initially arranged for fashion B but converted to fashion D because cardiac stenosis was detected during operation.
Table 2 summarizes the intra-operative and postoperative outcomes. No surgical morbidity or mortality was recorded. One patient suffered from a pulmonary infection and one from a urinary tract infection, but both were cured with antibiotics. One patient undergoing fashion D presented with mild gastroesophageal reflux one month after operation and was successfully treated with proton pump inhibitors and Chinese medicine. All tumors were confirmed as GISTs and negative surgical margins were diagnosed in all cases pathologically. The mean tumor size was 3.1 ± 1.7 cm, ranging from 1.5 to 5.9 cm. According to the modified Fletcher classification, five patients were classified as very low risk of recurrence and 34 as low risk. In addition, three were classified as medium risk and received one-year adjuvant imatinib therapy [13]. Within a median follow-up period of 56.8 months (range 2–77 months), no patient experienced recurrence, as verified by computed tomography and gastrofiberscope.
Discussion
Laparoscopic gastrectomy is widely performed for benign and malignant stomach tumors because of the advantages associated with minimally invasive techniques, such as less pain, earlier recovery, and favorable cosmetic outcomes [14, 15]. Although laparoscopic resection for gastric GISTs was recently added to the ESMO and NCCN guidelines, its success depends on tumor location and size, as well as abidement of the two main oncologic surgical principles: maintanence of the tumor pseudocapsule integrity and achievement of microscopically negative margins on final pathology [16, 17]. In this study, we have reviewed four various fashions of laparoscopic resection for EGJ-GIST according to the anatomic and biologic characteristics. The acceptable operative index and satisfactory postoperative outcomes suggest that laparoscopic procedures for EGJ-GIST are safe and feasible. Thus, these may serve as a reference for other laparoscopic surgeons when handling unfavorable tumors.
Although laparoscopic resection for gastric GISTs has been increasingly performed in the recent decades with satisfactory short-term outcomes and similar long-term outcomes compared to open surgery, there are still many controversies regarding laparoscopic resection for EGJ-GISTs. Because the involved anatomic region is covered by the left lobe of the liver, laparoscopic manipulation and complete exposure are very difficult, which may increase the incidence of tumor rupture. Several studies have indicated the high rate of conversion from laparoscopy to laparotomy due to tumor proximity to the EGJ. Nguyen et al. reported that in 28 patients with gastric GISTs undergoing attempted laparoscopic surgery, there were three (12%) conversions to open surgery, two of which were due to tumor proximity to the EGJ. They concluded that laparoscopic resection may increase the risk of clinically significant disorders and stenosis of the EGJ region [18]. In 40 cases of attempted laparoscopy of gastric GISTs reported by Karakousis et al., there were 13 cases of conversion to laparotomy. Among these conversions, five cases were due to proximity of the tumor to the EGJ or the lesser curvature, and four were due to surgeon inexperience [19]. Moreover, Poškus et al. described the rate of conversion from laparoscopy to open surgery was 100% (3/3) when the tumor was located near the EGJ [20].
However, with the development of laparoscopic technique and the invention of minimally invasive instruments, the exposure of the EGJ area has improved. An intraperitoneal atraumatic liver retractor can now be utilized as required to elevate the left lobe of the liver [10]. In our procedures, we inserted two trocars on the right side, one serving the role of the liver retractor, for the assistant surgeon. Several scholars have also reported a novel technique of laparoscopic transgastric resection for EGJ-GISTs in which some special trocars were inserted into the gastric cavity through the stomach wall for manipulation [10, 21,22,23]. As this technique requires the special trocars and might not be appropriate for a large tumor growing outside of the stomach, its use is restricted.
In this study, we have introduced four fashions of laparoscopic resection for EGJ-GISTs according to several factors, including tumor location and size, manner of growth, relationship between the tumor and esophagogastric line, and whether or not an ulcer was present. When the tumor was located in the greater curvature or fundus and the distance between the tumor and esophagogastric line was more than 2 cm, fashion A (wedge resection) was the most simple and convenient technique to perform. This was adequate to achieve negative margins and cardia preservation. However, if wedge resection is carried out for a tumor located in the lesser curvature, this may result in excessive stomach wall resection and will likely shorten the length of the lesser curvature, leading to a structural deformity as well as abnormal gastric motility [11]. As a consequence, we more commonly chose fashion B for EGJ-GISTs located in the lesser curvature. A safe and adequate surgical margin can be confirmed through direct inspection of the tumor and cardia under laparoscopic conditions. This procedure was usually suitable for EGJ-GISTs located in the lesser curvature more than 2 cm away from the esophagogastric line as this distance was necessary for a linear stapler or a suture technique to close the stomach wall incision [11]. When an extraluminal lesion was located less than 2 cm away from the esophagogastric line but had not invaded the EGJ, fashion C was performed to preserve cardiac function. Because the mucosa needs to be preserved, an intraluminal tumor or tumor with an intraluminal ulcer are not suitable for this resection fashion. Fashion D was planned for GISTs invading the esophagogastric line based on preoperative clinical examination. In our series, however, one case planned for fashion B was converted to fashion D because during resection, a severe cardiac deformation was confirmed under inspection with endoscopy.
Notably, a retro-gastric lesion resection is relatively difficult. In our experience, when the tumor was located in the posterior wall, the lower esophagus and the upper stomach had to be mobilized completely in order to exchange the positions of the posterior and anterior stomach walls. This provided a complete exposure of the tumor for convenient resection. During operation, the manipulation and traction must be gentle to ensure the integrity of the pseudocapsule. In addition, the surgeon’s experience with laparoscopic technique must be considered. In the present study, all cases were performed by two skilled laparoscopic surgeons who regularly performed laparoscopic D2 radical gastrectomies.
Intra-operative endoscopy is serving an increasingly important role in laparoscopic resection for gastric GISTs, especially for EGJ-GISTs. Endoscopic examination not only helps to detect some small intraluminal tumors during operation, but also provides assistance for confirmation of sufficient surgical margins, as well as cardiac integrity. Thus, many scholars have paid much attention to laparoscopic endoscopic cooperative surgery [24, 25]. In our study, an intra-operative endoscopy was used for each case and provided a safe and effective method of examination for complete resection of the tumor and the integrity of the cardia. Nowadays, the long-term outcomes of gastric GISTs are satisfactory because gastric GIST is relatively inactive and the complete surgical resection and perioperative adjuvant imatinib treatment have been rapidly refined. Therefore, maximal preservation of the remnant stomach and cardiac function for EGJ-GISTs should be prioritized during resection. We mostly chose resection fashions A, B, and C for EGJ-GISTs according to tumor characteristics. If fashion D was necessary, pyloroplasty and gastric plication were normally performed.
In conclusion, laparoscopic resection for EGJ-GIST can be safe and feasible and the choice of resection fashion must be based on tumor factors and the surgeon’s experience.
References
Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad Tunio G, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y (1998) Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 279(5350):577–580
Miettinen M, Lasota J (2006) Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 23(2):70–83
DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF (2000) Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 231(1):51–58
Kong SH, Yang HK (2013) Surgical treatment of gastric gastrointestinal stromal tumor. J Gastric Cancer 13(1):3–18
Woodall CE 3rd, Brock GN, Fan J, Byam JA, Scoggins CR, McMasters KM, Martin RC 2nd (2009) An evaluation of 2537 gastrointestinal stromal tumors for a proposed clinical staging system. Arch Surg 144(7):670–678
Khoo CY, Goh BK, Eng AK, Chan WH, Teo MC, Chung AY, Ong HS, Wong WK (2016) Laparoscopic wedge resection for suspected large (≥5 cm) gastric gastrointestinal stromal tumors. Surg Endosc. doi:10.1007/s00464-016-5229-7
Goh BK, Goh YC, Eng AK, Chan WH, Chow PK, Chung YF, Ong HS, Wong WK (2015) Outcome after laparoscopic versus open wedge resection for suspected gastric gastrointestinal stromal tumors: a matched-pair case-control study. Eur J Surg Oncol 41(7):905–910
Demetri GD, Benjamin R, Blanke CD, Choi H, Corless C, DeMatteo RP, Eisenberg BL, Fletcher CD, Maki RG, Rubin BP, Van den Abbeele AD, von Mehren M, Force NGT (2004) NCCN task force report: optimal management of patients with gastrointestinal stromal tumor (GIST)–expansion and update of NCCN clinical practice guidelines. J Natl Compr Canc Netw 2(Suppl 1):S1–S26 quiz 27–30
Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG, Pisters PW, Raut CP, Riedel RF, Schuetze S, Sundar HM, Trent JC, Wayne JD (2010) NCCN task force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw 8(Suppl 2):S1–S41 quiz S42–44
Privette A, McCahill L, Borrazzo E, Single RM, Zubarik R (2008) Laparoscopic approaches to resection of suspected gastric gastrointestinal stromal tumors based on tumor location. Surg Endosc 22(2):487–494
Sakamoto Y, Sakaguchi Y, Akimoto H, Chinen Y, Kojo M, Sugiyama M, Morita K, Saeki H, Minami K, Soejima Y, Toh Y, Okamura T (2012) Safe laparoscopic resection of a gastric gastrointestinal stromal tumor close to the esophagogastric junction. Surg Today 42(7):708–711
Siewert JR, Hölscher AH, Becker K, Gössner W (1987) Cardia cancer: attempt at a therapeutically relevant classification. Chirurg 58(1):25–32
Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O’Leary TJ, Remotti H, Rubin BP, Shmookler B, Sobin LH, Weiss SW (2002) Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol 33(5):459–465
Chen K, Zhou YC, Mou YP, Xu XW, Jin WW, Ajoodhea H (2015) Systematic review and meta-analysis of safety and efficacy of laparoscopic resection for gastrointestinal stromal tumors of the stomach. Surg Endosc 29(2):355–367
Hu Y, Huang C, Sun Y, Su X, Cao H, Hu J, Xue Y, Suo J, Tao K, He X, Wei H, Ying M, Hu W, Du X, Chen P, Liu H, Zheng C, Liu F, Yu J, Li Z, Zhao G, Chen X, Wang K, Li P, Xing J, Li G (2016) Morbidity and mortality of laparoscopic versus open D2 distal gastrectomy for advanced gastric cancer: a randomized controlled trial. J Clin Oncol 34(12):1350–1357
Group ESESNW (2014) Gastrointestinal stromal tumours: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 25(Suppl 3):iii21–iii26
von Mehren M, Randall RL, Benjamin RS, Boles S, Bui MM, Conrad EU 3rd, Ganjoo KN, George S, Gonzalez RJ, Heslin MJ, Kane JM 3rd, Koon H, Mayerson J, McCarter M, McGarry SV, Meyer C, O’Donnell RJ, Pappo AS, Paz IB, Petersen IA, Pfeifer JD, Riedel RF, Schuetze S, Schupak KD, Schwartz HS, Tap WD, Wayne JD, Bergman MA, Scavone J (2016) Soft tissue sarcoma, version 2.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 14(6):758–786
Nguyen SQ, Divino CM, Wang JL, Dikman SH (2006) Laparoscopic management of gastrointestinal stromal tumors. Surg Endosc 20(5):713–716
Karakousis GC, Singer S, Zheng J, Gonen M, Coit D, DeMatteo RP, Strong VE (2011) Laparoscopic versus open gastric resections for primary gastrointestinal stromal tumors (GISTs): a size-matched comparison. Ann Surg Oncol 18(6):1599–1605
Poskus E, Petrik P, Petrik E, Lipnickas V, Stanaitis J, Strupas K (2014) Surgical management of gastrointestinal stromal tumors: a single center experience. Wideochir Inne Tech Maloinwazyjne 9(1):71–82
Tagaya N, Mikami H, Kogure H, Kubota K, Hosoya Y, Nagai H (2002) Laparoscopic intragastric stapled resection of gastric submucosal tumors located near the esophagogastric junction. Surg Endosc 16(1):177–179
Hwang SH, Park DJ, Kim YH, Lee KH, Lee HS, Kim HH, Lee HJ, Yang HK, Lee KU (2009) Laparoscopic surgery for submucosal tumors located at the esophagogastric junction and the prepylorus. Surg Endosc Other Interv Techn 23(9):1980–1987
Ghushe ND, Dulai PS, Trus TL (2012) Laparoendoscopic transgastric resection of a submucosal mass at the gastroesophageal junction. J Gastrointest Surg 16(12):2321
Balde AI, Chen T, Hu Y, Redondo NJ, Liu H, Gong W, Yu J, Zhen L, Li G (2017) Safety analysis of laparoscopic endoscopic cooperative surgery versus endoscopic submucosal dissection for selected gastric gastrointestinal stromal tumors: a propensity score-matched study. Surg Endosc 31(2):843–851
Kikuchi S, Nishizaki M, Kuroda S, Tanabe S, Noma K, Kagawa S, Shirakawa Y, Kato H, Okada H, Fujiwara T (2016) Nonexposure laparoscopic and endoscopic cooperative surgery (closed laparoscopic and endoscopic cooperative surgery) for gastric submucosal tumor. Gastric Cancer. doi:10.1007/s10120-016-0641-1
Acknowledgements
The authors would like to give special thanks to two medical students, Kuangda Shan, and Nancy Ha, from the University of Iowa, Carver College of Medicine for their valuable assistance in the English rewriting of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosure
Wenjun Xiong, Jiaming Zhu, Yansheng Zheng, Lijie Luo, Yaobin He, Hongming Li, Dechang Diao, Liaonan Zou, Jin Wan, and Wei Wang have no conflicts of interest or financial ties to disclose.
Additional information
Wenjun Xiong and Jiaming Zhu contributed equally to this work in the design of the study and preparation of the manuscript and should be both considered as co-first authors.
Jin Wan and Wei Wang contributed equally to this work in the technique design, materials collection, analysis, interpretation of data and revising the article, they should be the co-correspondence authors.
Rights and permissions
About this article
Cite this article
Xiong, W., Zhu, J., Zheng, Y. et al. Laparoscopic resection for gastrointestinal stromal tumors in esophagogastric junction (EGJ): how to protect the EGJ. Surg Endosc 32, 983–989 (2018). https://doi.org/10.1007/s00464-017-5776-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-017-5776-6