Abstract
Background
Adhesion formation remains an important issue in hernia surgery. Liquid agents were developed for easy and versatile application, especially in laparoscopy. The aim of this study was to compare the antiadhesive effect of fibrin sealant (FS, Artiss®), Icodextrin (ID, Adept®) and Polyethylene glycol (PEG, CoSeal®) alone and in combination and to evaluate the resulting effect on tissue integration of the mesh.
Methods
A total of 56 Sprague–Dawley rats were operated in open IPOM technique. A middleweight polypropylene mesh of 2 × 2 cm size was implanted and covered with 1: FS, 2: ID, 3: PEG, 4: FS + ID, 5: FS + PEG, 6: PEG + ID, 7: control group, uncovered mesh (n = 8 per treatment/control). Observation period was 30 days. Macroscopic and histological evaluation was performed.
Results
Severe adhesions were found in group 2 (ID), group 6 (PEG + ID) and the controls. Best results were achieved with FS alone or FS + ID. Mesh integration in the treatment groups was reduced in comparison with the control group. This is a new finding possibly relevant for the outcome of intraperitoneal mesh repair. Group 6 (PEG + ID) showed an impairment of tissue integration with <50 % of the mesh surface in seven samples.
Conclusion
FS alone and in combination with ID yielded excellent adhesion prevention. ID alone did not show significant adhesion prevention after 30 days. Tissue integration of FS-covered meshes was superior to ID or PEG alone or combined. PEG did show adhesion prevention comparable to FS but evoked impaired tissue integration. So Artiss® is among the most potent antiadhesive agents in IPOM repair.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Intraabdominal adhesion formation remains an important issue in hernia surgery. Among other adhesion-related problems, surgical emergencies (e.g., bowel obstruction or bowel perforation) and long lasting and complex complications like fistulation, pain and a reduced pregnancy rate can be observed [1, 2]. Visceral adhesions pose a lifetime problem. Ellis et al. [3] reported 5.7 % of hospital readmissions attributable to adhesion formation within 10 years after initial abdominal surgery. The impact of adhesions is hard to predict, as illustrated by Wassenaar et al. who found intraabdominal adhesions in 83 % of patients reoperated for various indications after laparoscopic ventral hernia repair with an ePTFE mesh. In this retrospective single center study, only 0.15 % (one patient) required an acute intervention due to an adhesion-related complication [4]. Surgery-related factors leading to adhesion formation comprise peritoneal injury (by handling, dissection or heat), ischemia, infection and foreign bodies as well as suture material, glove powder and the mesh implant itself [5]. The laparoscopic operation procedure, although less adhesiogenic than laparotomy due to its less invasive nature, was found to unfavorably influence adhesion formation. The high intraperitoneal pressure leads to cell hypoxia, and desiccation is caused by the endoscopic light and dry carbon dioxide [6, 7]. Physiologically, the mesothelial trauma leads to the buildup of a fibrin gel matrix while at the same time surgery diminishes the fibrinolytic activity [8]. In hernia surgery, antiadhesive-covered composite meshes have been introduced for adhesion prevention. Liquid antiadhesive agents can be used with any mesh of choice. Polyethylene glycol (PEG) and fibrin sealant (FS) have been described to have antiadhesive properties [9–11]. More recently, PEG is also used for mesh fixation—e.g., Adhesix®, Bard [12, 13], which for FS is already a standard application [14, 15]. Furthermore, liquid antiadhesives can easily be applied laparoscopically and not only the mesh but also fixation points can be covered, what is often not feasible with precoated mesh implants. The aim of this study was the comparison of three different liquid antiadhesives—either alone or as combinations of two of the products—when applied on an intraperitoneal polypropylene mesh. We hypothesized that combining different antiadhesive agents (varying in origin, resorption time and application method) might enhance their antiadhesive effect. To the best of our knowledge, this has never been evaluated before. The primary aim of the study was to find the most effective single agent or antiadhesive combination in terms of adhesion reduction. The secondary study aim was the evaluation of the influence on the implant’s integration. Previous observations of impaired integration of IPOM meshes by adhesion barriers sparked our interest in the topic.
Materials and methods
Male Sprague–Dawley rats, weighing 400–500 g were obtained from the Institut fuer Labortierkunde und—genetik der Medizinischen Fakultaet der Universitaet Wien (Himberg, Austria). The City of Vienna animal committee granted permission to conduct this study. The antiadhesive agents and the hernia mesh were kindly provided by the manufacturers. All reagents used were of analytical grade.
Mesh
A macroporous, monofilament polypropylene mesh of 2.4 mm pore size and a thickness of 0.25 mm (PP, Vitamesh®, Proxy biomedical, Spiddal, Ireland) was cut to pieces of 2 cm side length before implantation.
Antiadhesive treatment
Fibrin sealant
The fibrin sealant used in this study was Artiss® (FS, Baxter, Vienna), a two component product consisting of human fibrinogen and 4IU of thrombin. The FS was applied using the EasySpray® System (Baxter Bioscience, Vienna, Austria) which allows the delivery of a consistently thin layer of sealant. Primary clotting occurs within 30–60 s. The FS layer is resorbed within 10–14 days.
Polyethylene glycol
CoSeal® (PEG, Baxter, Vienna) is composed of two synthetic polyethylene glycols. The powder is to be dissolved in hydrochloric acid solution and reacted with a sodium phosphate/sodium carbonate solution. Prior to application, the PEG powder is mixed with the liquid components in a syringe. CoSeal® is degraded and absorbed within approximately 5–7 days.
Icodextrin
Adept® (ID, Baxter, Vienna) is a clear solution containing 4 % icodextrin and provided in a sterile pouch of 1000 ml. Icodextrin is an α-1-4-linked glucose polymer. The solution is instilled into the abdominal cavity. The liquid separates implant and tissue by hydroflotation and should remain in the abdominal cavity during the first days after surgery. Adept® is absorbed by the lymphatic system within 4 days and is metabolized by alpha-amylase to lower molecular weight oligosaccharides.
Randomization
Prior to surgery, 56 rats were randomized by draw to one of the six study groups or the control group (n = 8). One mesh with or without antiadhesive treatment was implanted per animal. Observation time comprised 30 days.
Study groups
-
1: PP + FS
-
2: PP + PEG
-
3: PP + ID
-
4: PP + FS + PEG
-
5: PP + FS + ID
-
6: PP + PEG + ID
-
7: PP, no antiadhesive (control group)
Rationale for observation period
A follow-up of 30 days allowed the assessment of adhesion formation when wound healing was completed and collagen remodeling within the fibrin clots as well as soft tissue integration was detectable. The liquid antiadhesive agents had been fully resorbed at this time point, and a chronic situation of adhesion formation was established.
Experimental procedure
The operation was performed according to the well-established IPOM model [11, 16].
Rats were anaesthetized with an intraperitoneal injection of Ketavet® 110 milligrams per kilogram bodyweight (mg/kg BW) (Ketamine-hydrochloride 100 mg/ml, Pharmacia, Germany) and Rompun® 12 mg/kg BW (Xylazine-Hydrochloride, Bayer, Germany). A subcutaneous injection of 1.25 mg/kg Butomidor® (Butorphanol, Richter Pharma, Austria) was applied preoperatively to reduce visceral pain.
Surgery was performed under sterile conditions at the Ludwig Boltzmann Institute for Experimental and Clinical Traumatology (LBI).
After shaving and disinfection of the abdomen, the skin was incised longitudinally. The subcutaneous tissue was bluntly detached from the abdominal muscles, and a U-shaped laparotomy was made beginning and ending at the lateral lower abdomen, traversing 0.5 cm below the rib cage. The full thickness muscular including the peritoneum was flipped caudally and the peritoneum exposed. Centrally a peritoneal defect of 0.5 cm in diameter was made. A polypropylene mesh of 2 × 2 cm size was placed on the defect and fixed with four permanent sutures (Synthofil® 4/0, Ethicon, Germany) at the corners.
According to randomization, the antiadhesive treatment was applied: 0.2 ml of FS was sprayed on the mesh, 0.2 ml of PEG was applied with the syringe onto the mesh surface, or the abdominal cavity was filled with 10 ml of ID. In case of combination of FS and PEG the longer lasting FS was applied directly onto the mesh surface, and the PEG was spread on the FS layer. When ID was combined with FS or PEG, the mesh surface was treated first and ID was instilled into the abdominal cavity thereafter.
Finally, the muscle flap was flipped back into the original position. The laparotomy was closed in layers, using resorbable suture material (Vicryl® 3.0, Ethicon, Germany) for muscle adaptation and subcutaneous sutures and non-resorbable material (Synthofil® 4/0) for adaptation of the skin.
Postoperative care
Metacam® 0.15 mg/kg BW (subcutaneously applied) was routinely administered for pain management once daily for 3 days postoperatively. Rats were checked daily for signs of pain and infection and received an additional dose of 1.25 mg/kg Butomidor® once daily when pain was presumed.
Autopsy
For autopsy, animals were euthanized in deep anesthesia with an intracardial overdose of barbiturate (Thiopental®, Sandoz, Austria).
Postmortem all meshes were assessed macroscopically evaluating adhesion formation, tissue integration, dislocation, seroma formation, inflammation and mesh shrinkage.
Macroscopic evaluation criteria
Each mesh was rated by two investigators unaware of the randomization at evaluation (SGB, JB). In case of discrepancies between the observers, the worse score was accepted.
Adhesion formation
Adhesions were assessed qualitatively and semiquantitatively according to the Vandendael score [17]. Its design allows for reliable and precise description of the local situation. Grade I describes mild, grade II moderate and grade III severe adhesion formation (Table 1).
Mesh surface covered by adhesions (MSA)
Additional information was gathered by evaluating the percentage of adhesion-covered mesh surface in situ by placing a grid of 2 × 2 cm side length and 25 squares of 0.16 cm2 each over the mesh and counting the number of squares covering adhesions.
Tissue integration
Ingrowth of the mesh into the abdominal wall was assessed by lifting the center of the mesh with a forceps and sliding the back of a second forceps between mesh and abdominal wall. Areas of full tissue integration were transferred to a grid of 2x2 cm side length and 25 squares as described above. The number of squares with complete mesh integration was counted. Excellent integration into the abdominal wall (tissue ingrowth of >75 % of mesh) was scored as A, a well-integrated implant (up to 75 % of surface area integrated) was scored as B, whereas moderate integration (e.g., no tissue ingrowth through perforation holes and less than 50 % of mesh surface integrated) was scored as C.
Dislocation
Meshes found in their initial position and fully adherent to the peritoneum at autopsy were scored as A, whereas meshes found to be dislocated but still attached to the abdominal wall, with a failure of sutures or meshes freely floating in the abdominal cavity, were scored as F.
Seroma formation
No seroma was scored as A; a seroma (encapsulation with fluid) directly adjacent to the implant (verified by needle aspiration) was scored F.
Local inflammation
Absence of macroscopic inflammation (defined as unfavorable inflammation with pus and debris) was scored as A. Macroscopically visible debris, pus or abscess formation was scored as F.
Following macroscopic evaluation, samples were harvested and prepared for histological evaluation. They were fixated in 4 % buffered formaldehyde solution (Merck, Austria) and conserved in a solution of 70 % ethanol (Merck, Austria) after 24 h. Samples were sent to the histopathologist where they were dehydrated and embedded in paraffin according to standard procedures. The 5-mm sections were stained with hematoxylin/eosin (H&E).
Histological evaluation criteria
The blinded analysis of histological samples included the detection of antiadhesive remnants; the presence of macrophages; lymphocytes and plasma cells; foreign body reaction (defined as prolonged neutrophil response; foreign body giant cells and necrosis as sign of impaired biocompatibility) and bacterial colonization.
Histological grading was performed according to detected alteration in comparison with tissue of native rats [18]. Tissue alterations were rated as none (0), moderate [1], severe [2] or, as [3] for maximum alterations.
Statistical analysis
GraphPad Software (GraphPad Software Inc., USA) was used for statistical analysis. A one-way analysis of variance (ANOVA) calculation was used to check for differences in adhesion formation (Vandendael score, MSA) between the seven groups. The Bonferroni correction was used to control the familywise error rate. Post hoc pairwise comparisons were performed using the Dunnett’s multiple comparison test. Differences in tissue integration were calculated by the Chi-square test. A p value p < 0.05 was considered to indicate statistical significance.
For analysis of the correlation between Vandendael score and MSA, Pearson’s coefficient r was evaluated.
Results
All animals were included in evaluation as no dropouts were occurred.
Macroscopy
Adhesion formation
Vandendael score
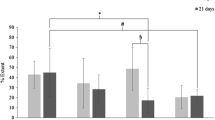
Figure 1 shows the qualitative and semiquantitative adhesion formation of the six groups and the control group evaluated by the Vandendael score after 30 days. Severe adhesions were found in group 3 (ID), PEG + ID group and the controls. In FS and FS + ID groups, the best adhesion reduction was seen compared to the control group (both p < 0.001). These groups were also statistically significantly different from ID + PEG (p < 0.05). PEG-coated samples showed significantly milder adhesion formation in comparison with the control group (p < 0.05). Adhesions seen in ID, FS + PEG and ID + PEG were moderate without statistically significant difference from the control group.
Mesh surface covered by adhesions (MSA)
On evaluation in groups FS, PEG and FS + ID the mesh surface area covered by adhesions was significantly smaller than in the control group (p < 0.05) (Fig. 2.)
A positive correlation between Vandendael Score and MSA was seen, reflected by a Pearson’s coefficient of r = 0.95 and p = 0.0006.
The macroscopic results of adhesion formation are depicted in Fig. 3.
Macroscopic results after 30 days are depicted. A significant adhesion reduction was seen in FS, PEG and FS + ID samples, what was thwarted by a significantly impaired tissue integration in PEG, FS + ID and PEG + ID groups. A adhesion reduction on mesh surface, T tissue integration. “+” indicates significantly favorable results, “−” indicates significantly unfavorable results, and “−” indicates results without significant difference from favorable or unfavorable values
Tissue integration
Detailed results are listed in Table 2. The evaluation of mesh integration into the abdominal wall showed statistically significant differences between groups:
Samples of the non-treated control group were integrated best with seven graded A and one graded B. Tissue integration of FS + ID and PEG + ID group meshes was significantly impaired in comparison with the control group (p < 0.05). Best tissue integration within the treatment groups was observed in FS group where four samples were graded A and four B. Mesh ingrowth of FS, FS + PEG and non-coated samples was significantly better in comparison with PEG + ID-coated ones (p < 0.05).
Samples in the ID group were significantly better integrated than in PEG and in ID + PEG group with p < 0.05.
Dislocation
At the time of explantation, all meshes and sutures were in place and no mesh dislocation was observed in any sample or group (score A).
Seroma formation
There was no seroma formation traceable in any sample (score A).
Local inflammation
There were no macroscopic signs of inflammation like pus or abscess formation in any group (all score A). No bacterial colonization was detected in histology in any group.
Histology
Analysis of H&E-stained samples revealed physiological scar tissue in all groups with regard to the presence of macrophages, lymphocytes and plasma cells as depicted in Fig. 4. There was no significant difference in foreign body reaction detectable between treatment groups and the control group. In samples with impaired mesh integration, a lack of cell transgression was observed histologically and a neomesothelial cell layer was surrounding abdominal wall and mesh separately as depicted in Fig. 4.
Sample of CoSeal®-treated polypropylene mesh, H&E staining, magnification ×1.25. A mild foreign body reaction can be seen around mesh fibers. Asterisks mark the area of absent cell transgression. Here, a neomesothelial cell layer is surrounding abdominal wall and mesh separately. At the non-integrated mesh margin, an adhesion strand has formed
Antiadhesive agents were fully degraded and no longer histologically traceable after 30 days.
Discussion
Despite the introduction of minimally invasive operation techniques, adhesion formation remains an important issue in hernia surgery [1, 6, 7]. Along with numerous surgery-related factors, the mesh implant triggers adhesion formation due to the foreign body reaction and has to be shielded from visceral organs. Different matrices have been developed for this application, often already linked to the mesh as in composite meshes [16, 19–21]. Advantages of liquid antiadhesives are the possibility to use them in different open and laparoscopic abdominal surgical procedures with an intraperitoneal implant or extensive peritoneal injury. They can be applied on any mesh of choice with the option of tailoring the mesh before implantation. Furthermore, fixation points and mesh margins—known trigger points of adhesion formation—can be covered after mesh implantation. In this study, three different liquid antiadhesives were compared to an intraperitoneally placed polypropylene mesh and to a combination of two different products in order to define the most effective antiadhesive treatment.
The three antiadhesive products differed substantially in terms of origin, application method and resorption time. Whereas according to the manufacturer PEG and ID had entirely disappeared within days after surgery, FS was effective for approximately 2 weeks. Therefore, in combined groups an enhanced adhesion reduction was hypothesized, assuming a summarizing effect of the single antiadhesive agents. The most effective adhesion reduction of approximately 50 % after 30 days was achieved by fibrin sealant to median 35.1 %, and the combination of FS with ID to 33.6 % of the mesh surface covered by adhesions in comparison with the control group (median 65.8 % adhesion coverage). PEG-coated samples also yielded an effective adhesion reduction. FS and PEG are known to have an antiadhesive effect on polypropylene meshes [10, 11]. Interestingly a combination of the two as well as a combination of PEG with ID did not result in an enhanced but in a reduced antiadhesive effect. The underlying mechanisms remain unclear, as FS and PEG alone were effective. An interference of the different agents may accordingly be assumed. ID did not influence adhesion formation in combination with the polypropylene mesh. In a large randomized controlled trial, ID showed significantly higher adhesion reduction compared with lactated Ringer’s solution [22, 23]. The ineffectiveness in the presence of a hernia mesh has also been described by van t’ Riet et al. [24]. In this publication, the effect of hydroflotation might have been outweighed by a strong foreign body reaction triggered by the mesh material.
Impaired tissue integration has been described for antiadhesive products including composite meshes and antiadhesive barriers [16, 21]. This is an unfavorable finding possibly leading to recurrences, incarceration and mesh migration. So the second focus of the study was on the evaluation of tissue integration after antiadhesive treatment of the polypropylene mesh. Findings varied dramatically within the treatment groups. Tissue integration was significantly impaired in FS + ID, PEG and PEG + ID groups. In the latter, an integration of <50 % of the mesh surface was seen in seven of eight samples. The most complete tissue integration was seen in FS only covered samples and in untreated meshes of the control group. A direct correlation between antiadhesive effect and loss in tissue integration could not be found. Each antiadhesive agent and every combination of products had its very own characteristics. No cumulative effect of neither adhesion reduction or impairment of tissue integration was observed when two agents were combined. For example, FS alone featured good adhesion reduction with the desirable mesh integration, while ID treatment caused a poor antiadhesive effect and a tissue integration of <75 % of the mesh surface in half of the samples. But FS + ID samples showed a good antiadhesive effect combined with significant impairment of tissue integration. So complex mechanisms of interaction must be suspected when different antiadhesive products are applied to the peritoneal mesh in combination with each other. In our opinion, a modern antiadhesive agent has to provide a balance of efficient protection and little/no impact on tissue integration. The only substance fulfilling both criteria in any of the treatment groups was FS.
The inhibition of tissue integration in PEG-covered samples may potentially also serve as an explanation for the problem of mesh dislocation in polyethylene glycol polyvinyl pyrrolidone fixed autoadhesive meshes [13].
In summary Artiss® alone and in combination with Adept® yielded excellent adhesion prevention. Tissue integration of FS-covered meshes was superior to ID or PEG alone or combined. CoSeal® did show adhesion prevention comparable to FS but evoked impaired tissue integration. ID alone did not show significant adhesion prevention in intraperitoneal hernia repair after 30 days. According to our study, it is not advisable to combine liquid antiadhesive products, as an inhibiting interaction rather than an enhancement of adhesion reduction was observed. The good results of FS alone and FS + ID indicate that the mechanical barrier formed by FS over a period of 2 weeks might actually be the decisive factor in effective adhesion prevention. It is well known by now that the acute phase of adhesion formation triggered by mesothelial attachment and fibrinolytic activity takes place in the first 10 days [8], when FS is still stable but ID and PEG are already degraded. In addition, the unfavorable findings on tissue integration with PEG, ID alone or combined, support the clinical recommendation derived from this experimental work to probably use FS as monotherapy.
Conclusion
Artiss® is among the most potent antiadhesive agents in IPOM repair in a rat model using an uncovered polypropylene mesh. It is cost effective, easy to use in open and laparoscopic procedures and can be combined with a macroporous polypropylene mesh. In this trial, it was clearly superior to CoSeal®. The use of PEG-based products, which are increasingly promoted for adhesion prevention by various manufacturers (e.g., Progel®, Bard and Sprayshield®, Covidien), warrants further research.
References
Jenkins ED, Yom V, Melman L, Brunt LM, Eagon JC, Frisella MM et al (2010) Prospective evaluation of adhesion characteristics to intraperitoneal mesh and adhesiolysis-related complications during laparoscopic re-exploration after prior ventral hernia repair. Surg Endosc 24(12):3002–3007
ten Broek RPG, Issa Y, van Santbrink EJP, Bouvy ND, Kruitwagen RFPM, Jeekel J et al (2013) Burden of adhesions in abdominal and pelvic surgery: systematic review and met-analysis. BMJ 347:f5588
Ellis H (1982) The causes and prevention of intestinal adhesions. Br J Surg 69(5):241–243
Wassenaar EB, Schoenmaeckers EJP, Raymakers JTFJ, Rakic S (2010) Subsequent abdominal surgery after laparoscopic ventral and incisional hernia repair with an expanded polytetrafluoroethylene mesh: a single institution experience with 72 reoperations. Hernia 14(2):137–142
Zhang Z, Zhou X, Ru J, Jiang S, Du H, Ni Y et al (2006) Characteristics of genesis and development of peritoneal adhesion by different causes: experiment with rats. Zhonghua Yi Xue Za Zhi 86(46):3285–3289
Molinas CR, Mynbaev O, Pauwels A, Novak P, Koninckx PR (2001) Peritoneal mesothelial hypoxia during pneumoperitoneum is a cofactor in adhesion formation in a laparoscopic mouse model. Fertil Steril 76(3):560–567
Ott DE (2008) Laparoscopy and adhesion formation, adhesions and laparoscopy. Semin Reprod Med 26(4):322–330
diZerega GS, Campeau JD (2001) Peritoneal repair and post-surgical adhesion formation. Hum Reprod Update 7(6):547–555
Dasiran F, Eryilmaz R, Isik A, Okan I, Somay A, Sahin M (2015) The effect of polyethylene glycol adhesion barrier (Spray Gel) on preventing peritoneal adhesions. Bratisl lekárske List 116(6):379–382
Martins e Quinino R, Araújo-Filho I, Lima FP, Barbosa ALC, de Maia TC, Goldenberg A (2013) Adhesion prevention in reabsorbable polyethylene glycol hydrogel (Coseal®) coated polypropylene mesh in rabbits. Acta cirúrgica Bras/Soc Bras para Desenvolv Pesqui em Cir 28(12):807–814
Petter-Puchner AH, Walder N, Redl H, Schwab R, Öhlinger W, Gruber-Blum S et al (2008) Fibrin sealant (Tissucol) enhances tissue integration of condensed polytetrafluoroethylene meshes and reduces early adhesion formation in experimental intraabdominal peritoneal onlay mesh repair. J Surg Res 150(2):190–195
Champault G, Torcivia A, Paolino L, Chaddad W, Lacaine F, Barrat C (2011) A self-adhering mesh for inguinal hernia repair: preliminary results of a prospective, multicenter study. Hernia 15(6):635–641
Gruber-Blum S, Riepl N, Brand J, Keibl C, Redl H, Fortelny RH et al (2014) A comparison of Progrip(®) and Adhesix (®) self-adhering hernia meshes in an onlay model in the rat. Hernia 18(5):761–769
Jenkins ED, Lerdsirisopon S, Costello KP, Melman L, Greco SC, Frisella MM et al (2011) Laparoscopic fixation of biologic mesh at the hiatus with fibrin or polyethylene glycol sealant in a porcine model. Surg Endosc 25(10):3405–3413
Fortelny RH, Petter-Puchner AH, Redl H, May C, Pospischil W, Glaser K (2014) Assessment of pain and quality of life in Lichtenstein hernia repair using a new monofilament PTFE mesh: comparison of suture vs. fibrin-sealant mesh fixation. Front Surg 1:45
Gruber-Blum S, Petter-Puchner AH, Brand J, Fortelny RH, Walder N, Oehlinger W et al (2011) Comparison of three separate antiadhesive barriers for intraperitoneal onlay mesh hernia repair in an experimental model. Br J Surg 98(3):442–449
Vandendael A, Struwig D, Nel JT, Kruger TF, Lombard CJ (1996) Efficacy of fibrin sealant in prevention of adhesion formation on ovar surgical wounds in rabbit model. Gyn End 5:169–172
Petter-Puchner AH, Fortelny RH, Mittermayr R, Walder N, Ohlinger W, Redl H (2006) Adverse effects of porcine small intestine submucosa implants in experimental ventral hernia repair. Surg Endosc 20(6):942–946
Mutter D, Jamali FR, Moody DL, Rodeheaver GT, Thérin M, Marescaux J (2000) The concept of protected mesh to minimize adhesion formation in intraperitoneat abdominal wall reinforcement. Preclinical evaluation of a new composite mesh. Hernia 4(S1):S3–S9
Hanna EM, Byrd JF, Moskowitz M, Mann JWF, Stockamp KT, Patel GN et al (2014) Outcomes of a prospective multi-center trial of a second-generation composite mesh for open ventral hernia repair. Hernia 18(1):81–89
Schreinemacher MHF, Emans PJ, Gijbels MJJ, Greve J-WM, Beets GL, Bouvy ND (2009) Degradation of mesh coatings and intraperitoneal adhesion formation in an experimental model. Br J Surg 96(3):305–313
Brown CB, Luciano AA, Martin D, Peers E, Scrimgeour A, DiZerega GS et al (2007) Adept (icodextrin 4% solution) reduces adhesions after laparoscopic surgery for adhesiolysis: a double-blind, randomized, controlled study. Fertil Steril 88(5):1413–1426
diZerega GS, Verco SJS, Young P, Kettel M, Kobak W, Martin D et al (2002) A randomized, controlled pilot study of the safety and efficacy of 4% icodextrin solution in the reduction of adhesions following laparoscopic gynaecological surgery. Hum Reprod 17(4):1031–1038
van’t Riet M, de Vos van Steenwijk PJ, Bonthuis F, Marquet RL, Steyerberg EW, Jeekel J et al (2003) Prevention of adhesion to prosthetic mesh: comparison of different barriers using an incisional hernia model. Ann Surg 237(1):123–128
Acknowledgments
The authors wish to thank K. Brenner for animal care, K. Kropik for technical support, W. Öhlinger for the histological analysis and I. Jung for statistical analysis. SGB was awarded the runner-up Andrew Kingsnorth Prize for presentation of the given data at the 36th International Congress of the European Hernia Society 2014 in Edinburgh, UK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
S. Gruber-Blum, J. Brand, C. Keibl and M. Lechner have no conflicts of interest or financial ties to disclose. H. Redl and R. H. Fortelny worked as consultant for Baxter Biosciences during the conduction of the study. A. H. Petter-Puchner has received travel grants from Baxter.
Rights and permissions
About this article
Cite this article
Gruber-Blum, S., Fortelny, R.H., Keibl, C. et al. Liquid antiadhesive agents for intraperitoneal hernia repair procedures: Artiss® compared to CoSeal® and Adept® in an IPOM rat model. Surg Endosc 31, 4973–4980 (2017). https://doi.org/10.1007/s00464-016-5277-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-016-5277-z