Abstract
Introduction
Colonic stenting has evolved to be an alternative to emergency laparotomy in the management of acute left-sided malignant colonic obstruction. This retrospective comparative study aimed to review the outcomes of colonic stent as bridge to surgery with emergency operation in a regional hospital in Hong Kong.
Method
Consecutive patients who were admitted from January 2006 to July 2014 with diagnosis of malignant left-sided colonic obstruction (from splenic flexure to rectosigmoid colon) were included. Patients with peritonitis or disseminated disease were excluded. Colonic stenting was attempted in all eligible patients when fluoroscopy was available in the endoscopy suite during office hour. Otherwise, emergency operation was performed. For patients with clinical success in colonic stenting, interval colectomies were performed. The postoperative outcomes, including the 30-days mortality, the stoma creation rate, the complication rate as well as the survival data were analyzed on an intention-to-treat (ITT) basis.
Results
From January 2006 to July 2014, 62 patients underwent colonic stenting and 40 patients underwent emergency operations. The technical success rate and the clinical success rate of stenting were 95.2 and 83.9 %, respectively. Laparoscopic resection was achieved in 74.2 % in the stenting group. More primary anastomoses were performed in the stenting group (71.0 vs. 27.5 %, p = 0.000). The stenting group had a significantly lower permanent stoma rate (16.1 vs. 52.5 %, p < 0.000), fewer Dindo grade III to IV postoperative morbidity (16.1 vs. 40 %, p = 0.007), and the 30-day mortality rate was lower (3.2 vs. 17.5 %, p = 0.018), translating into a better overall 5-year survival rate. The disease-free survival was comparable between the two groups.
Conclusions
Colonic self-expanding metal stent is effective in the management of acute left-sided colonic obstruction. It is associated with reduced stoma creation rate and postoperative morbidity. The oncological safety is not jeopardized by stenting and the interval operation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Large bowel obstruction occurs in 10–47 % of patients with colorectal cancer [1–3]. Obstruction relief and oncological clearance remain a challenge in this group of patients. A stoma is often created surgically because of the presence of edematous colonic tissue resulting from intestinal obstruction and the poor general condition of patients [4]. Unfortunately, emergency operations are often associated with high morbidity and mortality [5–8].
The use of self-expanding metal stents (SEMS) provides an alternative in the management of acute left-sided colonic obstruction. Successful placement of SEMS temporarily relieves the obstruction, allowing the patient to undergo more thorough oncological workup and optimization of their general condition. Subsequent laparoscopic colectomy and primary anastomosis are also made possible [9]. Stenting can also be considered a palliative treatment for patients with disseminated disease [5].
A few comparative studies and randomized controlled trials examining the use of SEMS as a bridge to surgery have been published, and the literature shows diversified results in terms of stoma creation rate, mortality and morbidity rates [9–16]. In a randomized controlled trial published by van Hooft et al. [10], the high colonic perforation rate in the stenting group translated into more septic complications and a higher 30-day mortality rate. Furthermore, it is postulated that both clinical and silent perforation by SEMS cause dissemination of tumor cells and thus poorer long-term oncological outcomes [17, 18]. Therefore, this study aimed to review the perioperative outcomes and long-term overall and disease-free survival of patients treated with SEMS as a bridge to surgery, as compared with emergency operations.
Method
Between January 2006 and July 2014, patients admitted to our hospital for acute malignant left-sided colonic obstruction were retrospectively analyzed from a prospectively managed database. Patients with obstructing tumor between the splenic flexure and rectosigmoid colon were included. Patients with peritonitis, doubtful bowel viability on CT scan, recurrent colorectal cancer or evidence of disseminated disease were excluded. From January 2006 to December 2010, the diagnosis and site of obstruction were confirmed with contrast enema (n = 30); whereas from January 2011 onwards, we performed contrast computed tomography (CT) (n = 72) to diagnose patients with suspected malignant large bowel obstruction. The reason for the change in management protocol was that contrast CT scan offered a better assessment of the perfusion and viability of the bowel wall. Any extra-colonic disease could also be detected.
We performed through-the-scope stenting with Niti-S uncovered colorectal stents (10 cm × 22 mm, 12 cm × 22 mm or 14 cm × 22 mm, Taewoong Medical®) or with Cook Medical Evolution® colonic controlled-release stents (8 cm × 25 mm or 10 cm × 25 mm) under fluoroscopic guidance. Fluoroscopy was available in the endoscopy suite during office hours. The procedure was performed by three colorectal surgeons who were experienced with stenting. A routine abdominal X-ray was taken 24 h after the intervention. Patients who consented to colonic stenting when fluoroscopy was available comprised the stenting group. Clinical success was defined as the resolution of obstructive symptoms within 72 h after the procedure with passage of stool [10]. Upon resolution of the intestinal obstruction, patients underwent optimization of their medical condition and thorough oncological workup, including a CT scan of the thorax, abdomen and pelvis if these had not been performed before stenting. Interval colectomy was then performed, and the laparoscopic approach was attempted in all patients with clinical success in bowel decompression. Defunctioning stoma was created if the operating surgeons had doubts about the integrity and safety of the anastomosis. Patients who had clinical failure, failed stent placement or developed complications after stenting underwent emergency laparotomy.
For patients who refused stenting or when fluoroscopy was not available, emergency laparotomy was performed. The type of operation was determined by the patient’s clinical condition and the decision of the operating surgeons. As intestinal obstruction is considered one of the risk factors for high recurrence rate [19, 20], all patients were referred to the clinical oncologist for consideration of adjuvant chemotherapy.
All patients received follow-up in our colorectal clinic once every 12 weeks for the first 2 years and half-yearly afterward for 3 years. CEA level was monitored and an ultrasound of the abdomen was performed every 6 months to probe for liver metastasis. Patients underwent a surveillance colonoscopy at the first and fourth year after the operation.
Since this retrospective case series was a clinical review of our center’s clinical and oncological outcomes on colonic stenting as a bridge to surgery, and the presentation of data remained anonymous, it was exempted from review by our hospital’s ethics committee.
Statistics
The data were analyzed on the intention-to-treat basis. Continuous variables were tested using a Student T test. A Chi-square test was used to compare proportions between two groups, such as stoma creation, mortality and morbidity, while categorical values such as tumor grade, ASA status, T stage and N stage were tested using a Mann–Whitney U test. All p values were reported as two sided, and a value of <0.05 was considered significant. Statistical analysis was performed using SPSS software (version 20.0).
Results
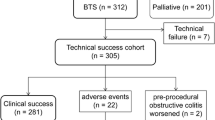
From January 2006 to July 2014, 102 patients were included in the study, with 62 patients in the stenting group and 40 patients in the emergency surgery group. The flowchart of patients at different stages of treatment is depicted in Fig. 1. There was no difference in the characteristics of the two groups in terms of patient age, sex, tumor location, ASA status and T/N stage. The median follow-up times were 21 and 25.5 months for the stenting group and emergency surgery group, respectively (Table 1). Three patients were lost to follow-up during the study period (2.9 %).
We used through-the-scope stenting with Niti-S uncovered colorectal stents (Taewoong Medical®) in 58 patients, while colonic controlled-release stents (Cook Medical Evolution®) were used in the remaining four patients. Technical success was achieved in 59 (95.2 %) patients in the stenting group. In the three cases of technical failure, complete obstruction was encountered and the guidewire could not be negotiated through the stricture. Clinical success was achieved in 52 patients (83.9 %). There were five stent perforations and one stent dislodgement. The stent dislodgement was detected on the post-procedural abdominal X-ray. This patient had persistent symptoms of intestinal obstruction, and he subsequently underwent emergency laparotomy. One patient did not have resolution of the obstructive symptoms even after successful stent deployment. The median time to operation was 29 days (18–45 days). Laparoscopic resection was completed in 46 patients in the stenting group (74.2 %). Conversion was required in five patients, due to the presence of cecal–sigmoid colon fistula in one patient and locally advanced tumor in the other four patients. Patients who had technical failure or clinical failure in the stenting group underwent emergency operations (Fig. 1).
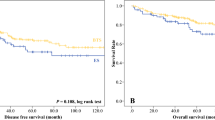
One-staged operation, without creation of stoma, was more commonly performed in the stenting group (71.0 vs. 27.5 %, p = 0.000). The temporary stoma was subsequently reversed in three patients in the stenting group and eight patients in the emergency surgery group. Significantly fewer permanent stomas were created in the stenting group (16.1 vs. 52.5 %, p = 0.000). The total hospital stay after operation was comparable between the two groups (13 vs. 12 days, p = 0.457) (Table 2). The complications were graded according to the Clavien–Dindo classification [21]; the total number of complications was greater in the emergency surgery group, with significantly fewer grade III to IV complications observed in the stenting group (16.1 vs. 40 %, p = 0.007) (Table 3). The stenting group also had a significantly lower 30-day mortality rate (3.2 vs. 17.5 %, p = 0.018) and a better overall survival (45.1 vs. 37.8 months, p = 0.012). A total of thirty-nine patients (38.2 %) received adjuvant chemotherapy, and there was no significant difference between the two groups (45.2 vs. 27.5 %, p = 0.073). The disease-free survival was comparable between the two groups (39.3 vs. 37.8 months, p = 0.134) (Figs. 2, 3).
Discussion
Left-sided colorectal tumors can present as an emergency with large bowel obstruction, which often result in massive colonic distension, bacterial translocation and electrolyte and fluid imbalance. Relief of obstruction, resection of the obstructing pathology and restoration of bowel continuity are therefore the main management goals. While staged operation with resection and colostomy followed by subsequent reversal is often practised, the one-staged procedure (resection and primary anastomosis with on-table lavage) has become increasingly popular. Despite the advances in surgical techniques and perioperative care, emergency operations still carry high morbidity and mortality rates [5–8].
Colonic stenting was first introduced in the 1990s as a bridge to surgery used to restore the luminal patency in patients with malignant left-sided colonic obstruction. In a randomized controlled trial, Van Hooft et al. [10] achieved clinical and technical success rates of only 70 %. Together with a stent perforation rate of 12.8 %, colonic stenting as a bridge to surgery has not been shown to be superior to emergency operations. In fact, stent placement is often difficult in patients with total obstruction or with tumors at an acute angle in relation to the lumen; these are also identified as risk factors for stent-related complications [22, 23]. For the benefits of colonic stenting to be observed, the skills and experience of the surgeon in guidewire cannulation and gentle stent deployment are of paramount importance. More thorough study of the anatomy and relationship between the tumor and the colon on the contrast CT scan or fluoroscopy is also important in planning the procedure. We achieved a technical success rate of 95.2 % for stenting, which compares favorably with the pooled data reported in a recent meta-analysis [11, 12]. The cannulation rate and technical success rate have improved as our colorectal surgeons gain experience and become more familiar with the stent deployment system. Although the definition of clinical success varies in the literature, our clinical success rate (83.9 %) is still comparable with other published data [12, 15, 16]. However, there were five stent perforations in our study (8.0 %), which is higher when compared with the reported perforation rate of 4 % [13–16]. This may be due to misplacement of the guidewire, leading to subsequent bowel perforation. Overzealous pushing and deployment of the stent across a tight stricture can also cause perforation. During the study period, the same type of stent was used in 96.1 % of patients. In fact, we experienced two stent perforations when a different stent deployment system was used. Therefore, the familiarity of the surgeon with the stent deployment system is of paramount importance to technical success without complications.
The median time interval from stent placement to definitive surgery in our study was 29 days, which is comparable with other studies in the literature [11–13]. The optimal timing for elective surgery after stenting is still controversial. A 2-week interval is suggested for the obstructed colon to be fully decompressed and tissue edema to subside. This also provides an opportunity to optimize the patient’s nutritional status before embarking on definitive surgery. By so doing, primary anastomosis is considered safe without the need for stoma creation. In fact, the creation of stoma, either permanent or temporary, has been shown to negatively impact the patients’ quality of life and psychosocial well being [24]. In our center, we performed significantly fewer stomas in the stenting group. This is in contrast to the findings in previous studies [10, 14].
Minimally invasive colonic surgery has been widely performed for colorectal malignancy in the elective setting. In the presence of intestinal obstruction, laparoscopy is difficult to perform within the limited working space and there is a higher risk of causing inadvertent injury to the dilated bowel. With successful decompression using stenting, laparoscopic bowel resection is made possible. In this study, laparoscopic colectomy was successfully performed in 46 stented patients (74.2 %). Although this is slightly lower than the rate reported in a randomized controlled trial by Cheung [9], our conversion rate is comparable to the color group and classic group, which are quoted as 17 and 25 % for colon cancer, respectively [25, 26]. We also reported more advanced T staging of the tumor, and three patients (3/62, 4.8 %) even required enbloc resection of adjacent organs (e.g., small bowel and cecum) in order to achieve negative margins. Nonetheless, laparoscopic surgery is a safe and feasible option after successful stenting and colonic decompression.
The mortality rate reported for emergency colorectal surgery was once higher than its elective counterpart [5–8]. However, recent randomized controlled trials have shown no significant differences in the 30-day mortality and the overall mortality rate between the two procedures [27, 28]. In our study, the 30-day mortality rate was significantly lower in the stenting group (1.6 vs. 15.5 %, p = 0.018), which is comparable with other series in the literature [29, 30]. Two patients in the stenting group died from anastomotic leakage after emergency open sigmoid colectomy following technical failure of stenting. There was no difference in the total number of morbidities between the two groups. However, significantly more patients suffered from Clavien–Dindo grade III or IV complications in the emergency surgery group, and patients in the stenting group also had fewer medical complications. There was no significant difference in the total length of hospital stay between the two groups, suggesting that the use of SEMS as a bridge to surgery does not prolong the whole course of patient management.
With a median follow-up of 23 months, the overall survival was significantly better in the stenting group. This can be partly explained by their lower 30-day mortality rate. In the literature, clinically silent tumor micro-perforations are observed in about 13 % of stented patients on histology [17, 18]. Concerns over tumor micro-perforations causing tumor seeding have been discussed in the literature. However, in our study, we did not observe any silent tumor perforation on histology and there was no difference in the disease-free survival upon follow-up. All our patients were referred to the clinical oncologist after the operation as obstruction is considered a risk factor for higher recurrence rates [19, 20]. However, only 39 patients (38.2 %) received adjuvant chemotherapy, either due to patients’ refusal or their poor general condition and advanced age, which rendered them unfit for chemotherapy.
Our study design has a few shortcomings. As a retrospective comparative study, it would be difficult for us to standardize the patient selection and management protocol during the study period. Since fluoroscopy in the endoscopy suite was only available during office hours, randomization of patients was difficult. In our unit, all elective colorectal surgeries are performed by colorectal surgeons, whereas emergency colonic surgeries are performed by specialists in general surgery. The experience of the two groups of surgeons is different, making standardization of the surgical approach difficult as the decision to create stomas is individualized, depending on the patients’ factor and the surgeons’ preference. Furthermore, the rate of stent-related perforation in our study was higher than other centers, subjecting more patients in this group to emergency operations. As surgeons at our center have gained experience, the number of stent-related complications has decreased over the years. We believe that successful stent deployment without complications is one of the most important contributing factors to the benefits of the use of SEMS in the management of malignant left-sided colonic obstruction. Given the potential benefits demonstrated in this retrospective study, future prospective or randomized controlled trials are needed to confirm the role of self-expanding metal stents as a bridge to surgery in patients with colorectal cancer-related large bowel obstructions.
Conclusions
The use of colonic self-expanding metallic stents as a bridge to surgery is feasible and effective in the management of acute left-sided colonic obstruction. It was associated with a reduced stoma creation rate, fewer severe morbidities, lower 30-day mortality and better overall survival. The disease-free survival in the stenting group was comparable to the emergency surgery counterpart. These clinical benefits are best seen in centers that possess the expertise to achieve a high technical success and a low stent-related complication rate. After satisfactory colonic decompression, subsequent laparoscopic colectomy is made possible in an elective setting. Future prospective studies will be helpful in defining the role of colonic stenting as a bridge to surgery.
References
Pommergaard HC, Vilmann P, Jakobsen HL, Achiam MP (2009) A clinical evaluation of endoscopically placed self expanding metallic stents in patients with acute large bowel obstruction. Scand J Surg 98:143–147
Fan YB, Cheng YS, Chen NW, Xu HM, Yang Z, Wang Y, Huang YY, Zheng Q (2006) Clinical application of self expanding metallic stent in the management of acute left sided colorectal malignant obstruction. World J Gastroenterol 12:755–759
Jiang JK, Lan YT, Lin TC, Chen WS, Yang SH, Wang HS, Chang SC, Lin JK (2008) Primary versus delayed resection for obstructive left sided colorectal cancer: impact of surgery on patient outcome. Dis Colon Rectum 51:306–311
Small AJ, Baron TD (2008) Comparison of Wallstent and Ultraflex stents for palliation of malignant left sided colonic obstruction: a retrospective, case matched analysis. Gastrointest Endosc 67:478–488
Law WL, Choi HK, Chu KW (2003) Comparison of stenting with emergency surgery as palliative treatment for obstructing primary left-sided colorectal cancer. Br J Surg 90:1429–1433
Saida Y, Sumiyama Y, Nagao J, Uramatsu M (2003) Long term prognosis of preoperative “bridge to surgery” expandable metallic stent insertion for obstructive colorectal cancer: comparison with emergency operation. Dis Colon Rectum 46:S44–S49
Runkel NS, Schlag P, Schwarz V, Herfarth C (1991) Outcome after emergency surgery for cancer of the large intestine. Br J Surg 78:183–188
Smothers L, Hynan L, Fleming J, Turnage R, Simmang C, Anthony T (2003) Emergency surgery for colon carcinoma. Dis Colon Rectum 46:24–30
Cheung HYS, Chung CC, Tsang WWC, Wong JCH, Yau KKK, Li MKW (2009) Endolaparoscopic approach vs. conventional open surgery in the treatment of obstructing left-sided colon cancer. A randomized controlled trial. Arch Surg 144:1127–1132
Van Hooft JE, Bemelman A, Oldenburg B, Marindlli AW, Holzik MF, Grubben MJ, Sprangers MA, Dijkgraaf MG, Fockens P (2011) Colonic stenting versus emergency surgery for acute left-sided malignant colonic obstruction: a multicentre randomized trial. Lancet Oncol 12:344–352
Tan CJ, Dasari BVM, Gardiner K (2012) Systematic review and meta-analysis of randomized clinical trials of self expanding metallic stents as a bridge to surgery versus emergency surgery for malignant left sided large bowel obstruction. Br J Surg 99:469–476
Sebastian S, Johnston S, Geoghegan T, Torreggiani W, Buckley M (2004) Pooled analysis of the efficacy and safety of self-expanding metal stenting in malignant colorectal obstruction. Am J Gastroenterol 99(10):2051–2057
Foo CC, Poon JT, Law WL (2011) Self-expanding metallic stents for acute left sided large bowel obstruction: a review of 130 patients. Colorectal Dis 13:549–554
Pirlet IA, Slim K, Kwiatkowski F, Michot F, Millat BL (2011) Emergency preoperative stenting versus surgery for acute left sided malignant colonic obstruction: a multicenter randomized controlled trial. Surg Endosc 25:1814–1821
Tilney HS, Lovegrove RE, Purkayastha S, Sains PS, Weston-Petrides GK, Darzi AW, Tekkis PP, Heriot AG (2007) Comparison of colonic stenting and open surgery for malignant large bowel obstruction. Surg Endosc 21:225–233
Ng KC, Law WL, Lee YM, Choi HK, Seto CL, Ho JW (2006) Self-expanding metallic stent as a bridge to surgery versus emergency resection for obstructing left-sided colorectal cancer: a case-matched study. J Gastrointest Surg 10(6):798–803
Maruthachalam K, Lash GE, Shenton BK, Horgan AF (2007) Tumor cell dissemination following endoscopic stent insertion. Br J Surg 94:1151–1154
Small AJ, Coelho-Prabhu N, Baron TH (2010) Endoscopic placement of self-expandable metal stents for malignant colonic obstruction: long term outcomes and complication factors. Gastrointest Endosc 71:560–572
Steinberg SM, Barkin JS, Kaplan RS, Stablein DM (1986) Prognostic indicators of colon tumors. The Gastrointestinal Tumor Study group experience. Cancer 57(9):1866–1870
Hatano S, Ischida H, Ischibashi K, Kumamoto K, Haga N, Miura I (2013) Identification of risk factors for recurrence in high risk stage II colon cancer. Int Surg 98(2):114–121
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications—a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Cirocchi R, Farinella E, Trastulli S, Desiderio J, Listorti C, Boselli C, Parisi A, Noya G, Sagar J (2013) Safety and efficacy of endoscopic colonic stenting as a bridge to surgery in the management of intestinal obstruction due to left colon and rectal cancer: a systematic review and meta-analysis. Surg Oncol 22:14–21
Targownik LE, Spiegel BM, Sark J, Hines OJ, Dulai GS, Gralnek IM, Farrell JJ (2004) Colonic stent versus emergency surgery for management of acute left sided malignant colonic obstruction: a decision analysis. Gastrointest Endosc 60:865–874
Karadag A, Mentes BB, Uner A, Irkorucu O, Ayaz S, Ozkan S (2003) Impact of stomatherapy on quality of life in patients with permanent colostomies or ileostomies. Int J Colorectal Dis 18:234–238
Hazelbrook EJ (2002) Color Study Group COLOR: a randomized clinical trial comparing laparoscopic and open resection for colon cancer. Surg Endosc 16:949–953
Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM (2005) Short-term endpoints of conventional versus laparoscopic assisted surgery in patients with colorectal cancer (MRC CLASSIC trial): multicentre, randomized controlled trial. Lancet 365:1718–1726
Quereshy FA, Poon JT, Law WL (2014) Long term outcome of stenting as a bridge to surgery for acute left-sided malignant colonic obstruction. Colorectal Dis 16:788–793
Breitenstein S, Rickenbacher A, Berdajs D, Puhan M, Clavien PA, Demartines N (2007) Systemic evaluation of surgical strategies for acute malignant left-side colonic obstruction. Br J Surg 94:1451–1460
Kim JS, Hur H, Min BS, Sohn SK, Cho CH, Kim NK (2009) Oncological outcomes of self expanding metallic stent insertion as a bridge to surgery in the management of left sided colon cancer obstruction: a comparison with nonobstructing elective surgery. World J Surg 33:1281–1286
Kavanagh DO, Nolan B, Judge C, Hyland JMP, Mulcahy HE, O’Connell PR, Winter DC, Doherty GA (2013) A comparative study of short and medium term outcomes comparing emergent surgery and stenting as a bridge to surgery in patients with acute malignant colonic obstruction. Dis Colon Rectum 56:433–440
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Dr. Ho, Dr. Chan, Dr. Kwok and Dr. Lau have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Ho, Km., Chan, Km., Kwok, Sy. et al. Colonic self-expanding metal stent (SEMS) as a bridge to surgery in left-sided malignant colonic obstruction: an 8-year review. Surg Endosc 31, 2255–2262 (2017). https://doi.org/10.1007/s00464-016-5227-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-016-5227-9