Abstract
Background
Traditional endoscopic thoracic sympathicotomy is usually performed through an axillary incision with 5-mm thoracoscope under general anesthesia with endotrachea intubation. Nonintubated transareolar single-port thoracic sympathicotomy with a needle scope has rarely been attempted. The objective of this study is to evaluate the feasibility and safety of this minimally invasive technique in managing primary palmar hyperhidrosis (PPH).
Methods
From May 2012 to May 2014, a total of 85 male patients with severe PPH underwent transareolar single-port thoracic sympathicotomy by use of a 2-mm needle scope under total intravenous anesthesia without endotrachea intubation.
Results
All procedures were successfully performed with a mean operating time of 13.5 min. The palms of all patients became dry and warm as soon as the sympathetic chain was cut off. There were no sore throat, and all the patients regained consciousness rapidly after surgery. Eighty-two patients (96.5 %) were discharged from the hospital on the first postoperative day. The postoperative complications were minor, and no patients developed Horner’s syndrome. At 6 months postoperatively, there is no obvious surgical scar on the chest wall, and none of the patients complained about postoperative pain. Compensatory sweating appeared in 31 patients. No recurrent symptoms were observed in our study. One-year follow-up revealed an excellent cosmetic result and degree of satisfaction.
Conclusions
Nonintubated transareolar single-port needlescopic thoracic sympathicotomy is a safe, effective and minimally invasive therapeutic procedure, which can be performed in routine clinical practice for male PPH patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Primary palmar hyperhidrosis (PPH) is a disorder characterized by excessive perspiration beyond physiological need, leading to severe psychological, social and occupational dysfunction [1, 2]. The excessive sweating is primarily of the palms but may involve sole and axilla as well [3, 4]. The degree of sweating is variable, ranging in severity from moderate moisture to severe dripping. Patients sweat in response to thermal and emotional stimuli but also spontaneously without apparent trigger. PPH has an estimated prevalence of nearly 0.6–1.0 % in the Western population [5, 6]. In one of our nationwide survey [7], the PPH prevalence rate of adolescents was 2.08 % in mainland China, and positive family history was found in 25.40 % of PPH cases. The cause of PPH still remains unknown. Many evidences imply that PPH could be a genetic disorder with an autosomal dominant mode of transmission [8, 9]. Recently, we have identified a novel locus on chromosome 2q31.1 and have provided direct evidence that PPH is a clinically and genetically heterogenous group of disorders [10].
Endoscopic thoracic sympathicotomy is currently the only effective and sustainable surgical treatment for disabling palmar hyperhidrosis [11, 12]. Under general anesthesia with endotracheal intubation, 1–3 port thoracic sympathicotomy with 5-mm thoracoscope offers a high level of safety and has reached a high therapeutic standard. But, this traditional procedure still leaves visible scars on the chest wall as well as pain on the trocar sites, and sometimes even causes complications associated with endotracheal intubation.
Current development in video-assisted thoracoscopic surgery and anesthesia using video-assisted thoracoscopic surgery without endotracheal intubation are safe and feasible for the diagnosis and treatment of pleural diseases [13, 14], including nonintubated needlescopic thoracic surgery. Such procedures can last up to half an hour with adequate patient tolerance. This led us to question whether such a procedure could be applied to the simple surgery of thoracic sympathicotomy. Such an approach could reduce surgical invasion, produce better cosmetic results and decrease the complications associated with endotracheal intubation.
In this article, we report our initial experience with nonintubated transareolar single-port needlescopic thoracic sympathicotomy.
Patients and methods
Patient selection
Between May 2012 and May 2014, 85 male patients with severe PPH underwent nonintubated transareolar single-port thoracic sympathicotomy with needlescopic instruments (Fig. 1) exclusively at our institution. Patient characteristics are presented in Table 1. This study was approved by the institutional review board and ethics committee of The First Affiliated Hospital of Fujian Medical University (no. 2012-17). All the patients received a preoperative routine blood examination, cardiological consulting and chest computed tomography scan to exclude lung, pleural and heart diseases. A detailed medical history, the degree of symptoms and distribution of excessive sweating were also documented. All patients signed their informed consent at least 1 day before operation after careful explanation of the procedure and goals of the study. Criteria for inclusion of the study were: age ≥16 years and ≤50 years; male patients with severe palmar hyperhidrosis that significantly affects their daily life; absence of thoracic surgery history and severe chest wall deformity. Criteria for exclusion were: plantar, axillary hyperhidrosis without palmar hyperhidrosis; upper airway and maxillofacial injury or deformity, and patients who cannot cooperate with face mask ventilation; lung, pleural and heart diseases that could increase surgical risk; secondary hyperhidrosis including hyperthyroidism, acute and chronic infections, malignancy and immunologic disorders.
Surgical procedure
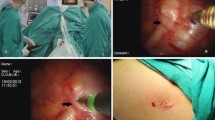
Surgery was performed under total intravenous anesthesia with face mask (Fig. 2A), which was fixed with belts to prevent air leakage. Anesthesia was induced with intravenous fentanyl (1–1.5 μg/kg to maximum of 100 μg), propofol (2–3 mg/kg) and cisatracurium (2–4 mg). Throughout the surgical procedure, oxygen was delivered via face mask ventilation, and remifentanil (4–12 μg/kg/h) and propofol (4–12 mg/kg/h) were given by target-controlled infusion. All patients were placed on the operating table in a semi-sitting position, thus exposing areola to allow a sequential bilateral procedure without the need for turning. A palmar temperature probe (Infrared Thermometer GM700, Shenzhen, China) was placed on the thenar eminence and taped in place. Before operation, palmar temperature was kept <30 °C by immersing the hand in water at 4 °C. Before skin incision, baseline palmar temperature was recorded. The transareolar incision was characterized by a 5-mm incision delineated along the areola margin on the operative side of surgery. After temporarily disconnecting the ventilation pipe of anesthesia machine, a 3-mm trocar with blunt obturator was introduced into the thoracic cavity. Open pneumothorax was achieved when the trocar’s obturator was pulled out. Ventilation stopped for 10 s, and the lung deflated from the vertex of thoracic cavity under gravity. A 2-mm 30° thoracoscope (Karl Storz, Tuttlingen, Germany) then was introduced into the thoracic cavity. When the trocar was pulled out from the incision, a 2-mm cautery hook was inserted into the thoracic cavity through the same port (Fig. 2B). The sympathetic chain was identified at the level of the crossing of the second, third and fourth costal heads. The parietal pleura was opened, and the sympathetic chain crossing the third rib (R3) was simply transected by the cautery hook (Fig. 2C). The incision was routinely extended laterally for approximately 3 cm on the corresponding costa to include any accessory nerve fibers (the nerve of Kuntz). A palmar temperature increase of 1.5 °C confirmed adequate sympathicotomy. Once sympathicotomy was completed, the cautery hook was pulled out and the trocar was inserted along the thoracoscope into the thoracic cavity. Then, the thoracoscope was removed leaving the trocar in position as a vent for retained air. The surgical procedure was completed by insertion of an 8F chest tube into the thoracic cavity through the trocar (Fig. 2D). The chest tube was aspirated while the anesthesiologist ventilated the patient manually, exerting continuous positive pressure for a few seconds, to prevent pneumothorax before the drainage was subsequently removed. At the end of the procedure, the incision was tightly pressed for a few seconds to make sure there was no active bleeding. Then, the incision was closed with Dermabond Skin Adhesive (Ethicon, LLC, USA) (Fig. 3A, B). No sutures or dressing was needed. The entire procedure was then repeated on the opposite side without changing the position of the patient or the operation setting.
Data collection and follow-up
The operating time, resuscitation time, sore throat, palmar temperature rise, resolution of palmar hyperhidrosis, hospital stay and complications were collected after surgery. The operating time was calculated from the time of skin incision to the application of the dressing over the wound. This excluded anesthesia induction and reversal time.
All patients were followed up at the time of discharge, 6 and 12 months after the operation. A detailed questionnaire (Table 2) was completed by study patients. Cosmetic results, postoperative pain, compensatory sweating (CS) and satisfaction scores were evaluated by hospital visits, telephone or e-mail.
Results
During the operation, the vital signs of all patients were stable, and none of them needed conversion to endotracheal intubation. All patients regained consciousness rapidly (the mean resuscitation time was 4.5 ± 0.8 min) and did not complain about sore throat after surgery. All procedures were successfully performed with a mean operating time of 13.5 min. The palms of all patients became dry and warm immediately after surgery. The average rise in probe temperature after nerve ablation was 2.2 °C. It usually occurred 1–5 min after the ablation. There were no operative mortality and conversion to open procedure. Hospital stay was very short, with 82 patients (96.5 %) discharged on the first postoperative day, and the remainder discharged on postoperative day 2. Postoperative pneumothorax was found on the chest X-ray in 5 patients, 2 of them resolved with pleural drainage, and others did not required further intervention. No patients had intraoperative bleeding. Horner’s syndrome was not observed in the study. None of the patients had an infection of his wound.
Follow-up was 100 % completed. The great majority of the patients returned the questionnaires immediately, and the remainder returned the questionnaires after a reminder or repeated mailing. The response rates at the time of discharge, 6 and 12 months postoperatively were 100 %. The effectiveness of the surgery is presented in Table 3. No recurrent symptoms were observed in our study. Postoperative pain at the time of discharge affected 33 patients (38.8 %). According to visual analogue scale (VAS) pain score, 30 cases (90.9 %) were considered mild (the mean VAS score was 2.03 ± 0.85) and 3 cases (9.1 %) were considered moderate (the mean VAS score was 5.67 ± 0.58). No patient required analgesia. None of the patients complained about postoperative pain 6 months after surgery. CS occurred in 31 patients (36.5 %). Among them, 25 cases (80.6 %) were mild and 6 cases (19.4 %) were moderate. The most frequent locations were the back (54.8 %), abdomen (48.4 %), thorax (32.3 %) and lower extremities (25.8 %). As far as the evolution over time, 5 patients (16.1 %) reported improvement, 24 (77.4 %) had no change and 2 patients (6.5 %) got worse. Our procedure had an excellent cosmetic result (Table 3; Fig. 3C, D) and degree of satisfaction (Table 3) during follow-up.
Discussion
Thoracoscopic sympathetic ganglion resection is an effective treatment for patients suffering from PPH. Its therapeutic mechanism might be to interrupt the transmission of impulses from sympathetic ganglia to eccrine sweat glands [15]. Under general anesthesia with endotracheal intubation, conventional endoscopic thoracic sympathicotomy is usually employed in the treatment of patients with severe PPH. Although widely accepted, the procedure has some shortcomings to be improved. To begin with, endotracheal intubation is easy to cause postoperative sore throat, dysphagia, and even possible cardiopulmonary complications. Next, the cost of anesthesia with endotracheal intubation can run as high as 5000 RMB, which is more than nonintubated one. Finally, traditional endoscopic thoracic sympathicotomy via 2–3 incisions still leaves visible scars on the chest wall that results in a permanent cosmetic defect, postoperative wound-related pain, numbness or paresthesia. It is reported that the frequency of dysesthesias and pain in the thoracic wall is variable (5–78 %) and the main reason for these chronic postoperative problems is intercostal nerve injury when the trocars are introduced into the intercostal space [16, 17]. As PPH is prevalent in young people, cosmetic outcome is an important consideration in ETS. Attempts at further reducing access trauma and improving cosmetic outcome have resulted in the development of a series of minimally invasive surgical procedures, including transaxillary single-port endoscopic thoracic sympathicotomy [18] and transumbilical endoscopic thoracic sympathicotomy [19]. These surgical approaches enable a smaller wound size or allow for the positioning of the wound in areas of cosmetic benefit. However, the transareolar approach has a better cosmetic result by hiding a surgical scar in the areola and has rarely been reported in endoscopic thoracic sympathicotomy.
In order to overcome the shortcomings mentioned above, under total intravenous anesthesia without intubation, we performed transareolar single-port thoracic sympathicotomy with needlescopic instruments. The results are in line with previous reports of endoscopic thoracic sympathicotomy [20–22]. All patients waked up quickly from anesthesia, and none of them complained about sore throat after surgery. The effectiveness of palmar hyperhidrosis was 100 % with a rate of total satisfaction (satisfied plus very satisfied) of 97.6 % at the time of 12 months postoperatively. Complications were minor, and none of them required an open surgery. Pneumothorax was the most common early postoperative complication, and only 2 cases required a pleural drainage for 1 day. However, an exertion of continuous positive pressure for a few seconds in coordination with the anesthesiologist and the application of a mild suction to the temporary chest tube before the closing of the skin incision are essential to prevent pneumothorax [23, 24]. The frequency of Horner’s syndrome is variable (0.5–17 %), and its pathogenesis is direct or indirect lesions of the stellate ganglion [25, 26]. In our study, no patients developed Horner’s syndrome, and the possible reason was that the sympathetic level of R3 transection is far from the stellate ganglion. Incidence rates of recurrence vary considerably and have been described as ranging 1–27 % [27]. The most common causes are incomplete thoracic sympathicotomy, anatomic variability of the sympathetic chain or nerve regeneration [28]. In our series, there was no recurrence observed during the follow-up period. The reason was that the sympathetic ganglion was completely ablated. CS is the most common and undesirable side effect of thoracic sympathicotomy and is characterized by excessive sweating from some regions of the body where it had not been observed preoperatively. The incidence of CS varies between 4.9 and 100 % [29–33]. This wide variability may be attributable to heterogeneous patient populations, emotional stress, climatic conditions or, more importantly, different surgical procedures. In our experience, CS occurred in 36.5 % of patients and most of them are mild or moderate. But the symptoms were not severe enough to interfere with lifestyle, and this required no further treatment. The pathogenesis of CS is still unknown. It is postulated that this phenomenon is caused by a temperature-regulating compensatory mechanism in the body and is correlated with the extent of sympathectomy [15].
In recent years, thoracic natural orifice transluminal endoscopic surgery (NOTES) has attracted great attention for its potential to avoid chest wall incisions, reduce postoperative wound-related pain and improve cosmetic results. In 2013, Zhu et al. [19] reported his initial experience of transumbilical thoracic sympathicotomy in a series of 38 patients. However, concerns about longer operative time, complicated surgical skills and increased risk of diaphragmatic hernia impede the widespread adoption of this new procedure. So far, most of thoracic NOTES procedures are still in the early stages of development and limited to animal experiments [12, 34–36]. Our transareolar approach avoids the risk of esophageal puncture-site fistula, mediastinitis or diaphragmatic hernia—the shortcomings of thoracic NOTES—and could work as a viable intermediate step before thoracic NOTES.
Additionally, our procedure has several potential advantages. Firstly, an analgesic and an amnestic, remifentanil and propofol, are used in total intravenous anesthesia without endotracheal intubation, and patients did not experience or remember pain. After surgery, most of the patients regained consciousness quickly from anesthesia, and none of them complained about sore throat. Therefore, the procedure could greatly reduce the costs of anesthesia and analgesia for patients. Secondly, with a single 5-mm incision on the areola margin hidden in the color of the areola, transareolar single-port endoscopic thoracic sympathicotomy is nearly scarless and achieves an excellent cosmetic effect. Thirdly, this procedure with needlescopic instruments only needs a single small transareolar incision, which greatly reduces postoperative wound-related pain. Lastly, this newly designed transareolar incision avoids the defect that the trocar restricts the operative direction of thoracoscope and electrocautery in other type of single-port endoscopic thoracic sympathicotomy during operation. As a mutual fulcrum, the transareolar single incision could work as a role of two incisions and make the operation more convenient. As a result, our technique could simplify the surgical procedure and offer a high level of safety.
While an improvement over the traditional method, the described procedure still has some flaws. First of all, as this was the primary evaluation of such a technique, we restricted the criteria for inclusion to male patients. Moreover, 2-mm needle scope is comparatively fragile and easily to be broken during the operation. Care must be taken to avoid damaging the fragile endoscope. Finally, this study has a small number of patients and a short follow-up time. More patients with a longer follow-up are needed to evaluate the long-term outcomes of this procedure.
Conclusions
This study demonstrates that nonintubated transareolar single-port needlescopic thoracic sympathicotomy is a safe, effective and minimally invasive therapeutic procedure for PPH, which gives an excellent cosmetic and clinical outcome. This procedure is a promising approach and can be performed in routine clinical practice for male patients.
References
Sato K, Kang WH, Saga K, Sato KT (1989) Biology of sweat glands and their disorders. II. Disorders of sweat gland function. J Am Acad Dermatol 20:713–726
De Campos JR, Kauffman P, Werebe Ede C, Andrade Filho LO, Kusniek S, Wolosker N, Jatene FB (2003) Quality of life, before and after thoracic sympathectomy: report on 378 operated patients. Ann Thorac Surg 76:886–891
Adar R, Kurchin A, Zweig A, Mozes M (1977) Palmar hyperhidrosis and its surgical treatment: a report of 100 cases. Ann Surg 186:34–41
Friedel G, Linder A, Toomes H (1993) Selective video-assisted thoracoscopic sympathectomy. Thorac Cardiovasc Surg 41:245–248
Malone PS, Cameron AE, Rennie JA (1986) Endoscopic thoracic sympathectomy in the treatment of upper limb hyperhidrosis. Ann R Coll Surg Engl 68:93–94
Heckmann M, Plewig G, Hyperhidrosis Study Group (2005) Low-dose efficacy of botulinum toxin a for axillary hyperhidrosis: a randomized, side-by-side, open-label study. Arch Dermatol 141:1255–1259
Lai FC, Tu YR, Li YP, Li X, Lin M, Chen JF, Lin JB (2015) Nation wide epidemiological survey of primary palmar hyperhidrosis in the People‘s Republic of China. Clin Auton Res 25:105–108
Ro KM, Cantor RM, Lange KL, Ahn SS (2002) Palmar hyperhidrosis: evidence of genetic transmission. J Vasc Surg 35:382–386
Kaufmann H, Saadia D, Polin C, Hague S, Singleton A, Singleton A (2003) Primary hyperhidrosis—evidence for autosomal dominant inheritance. Clin Auton Res 13:96–98
Chen J, Lin M, Chen X, Cao Z, Tan Z, Xiong W, Tu Y, Yang J (2015) A novel locus for primary focal hyperhidrosis mapped on chromosome 2q31.1. Br J Dermatol 172:1150–1153
Krasna MJ (2008) Thoracoscopic sympathectomy: a standardized approach to therapy for hyperhidrosis. Ann Thorac Surg 85:S764–S767
Cerfolio RJ, De Campos JR, Bryant AS, Connery CP, Miller DL, DeCamp MM, McKenna RJ, Krasna MJ (2011) The society of thoracic surgeons expert consensus for the surgical treatment of hyperhidrosis. Ann Thorac Surg 91:1642–1648
Tseng YD, Cheng YJ, Hung MH, Chen KC, Chen JS (2012) Nonintubated needlescopic video-assisted thoracic surgery for management of peripheral lung nodules. Ann Thorac Surg 93:1049–1054
Mineo TC, Sellitri F, Tacconi F, Ambrogi V (2014) Quality of life and outcomes after nonintubated versus intubated video-thoracoscopic pleurodesis for malignant pleural effusion: comparison by a case-matched study. J Palliat Med 17:761–768
Li X, Tu YR, Lin M, Lai FC, Chen JF, Dai ZJ (2008) Endoscopic thoracic sympathectomy for palmar hyperhidrosis: a randomized control trial comparing T3 and T2-4 ablation. Ann Thorac Surg 85:1747–1751
Sihoe AD, Cheung CS, Lai HK, Lee TW, Thung KH, Yim AP (2005) Incidence of chest wall paresthesia after needlescopic video-assisted thoracic surgery for palmar hyperhidrosis. Eur J Cardiothorac Surg 27:313–319
Dumont P, Denoyer A, Robin P (2004) Long-term results of thoracoscopic sympathectomy for hyperhidrosis. Ann Thorac Surg 78:1801–1807
Lardinois D, Ris HB (2002) Minimally invasive video-endoscopic sympathectomy by use of a transaxillary single port approach. Eur J Cardiothorac Surg 21:67–70
Zhu LH, Wang W, Yang S, Li D, Zhang Z, Chen S, Cheng X, Chen L, Chen W (2013) Transumbilical thoracic sympathectomy with an ultrathin flexible endoscope in a series of 38 patients. Surg Endosc 27:2149–2155
Doolabh N, Horswell S, Williams M, Huber L, Prince S, Meyer DM, Mack MJ (2004) Thoracoscopic sympathectomy for hyperhidrosis: indications and results. Ann Thorac Surg 77:410–414
Jeganathan R, Jordan S, Jones M, Grant S, Diamond O, McManus K, Graham A, McGuigan J (2008) Bilateral thoracoscopic sympathectomy: results and long-term follow-up. Interact Cardiovasc Thorac Surg 7:67–70
Liu Y, Yang J, Liu J, Yang F, Jiang G, Li J, Huang Y, Wang J (2009) Surgical treatment of primary palmar hyperhidrosis: a prospective randomized study comparing T3 and T4 sympathicotomy. Eur J Cardiothorac Surg 35:398–402
Fredman B, Olsfanger D, Jedeikin R (1997) Thorascopic sympathectomy in the treatment of palmar hyperhidrosis: anaesthetic implications. Br J Anaesth 79:113–119
Conacher ID (2002) Anaesthesia for thoracoscopic surgery. Best Pract Res Clin Anaesthesiol 16:53–62
Kwong KF, Cooper LB, Bennett LA, Burrows W, Gamliel Z, Krasna MJ (2005) Clinical experience in 397 consecutive thoracoscopic sympathectomies. Ann Thorac Surg 80:1063–1066
Singh B, Moodley J, Allopi L, Cassimjee HM (2006) Horner syndrome after sympathectomy in the thoracoscopic era. Surg Laparosc Endosc Percutan Tech 16:222–225
Rodríguez PM, Freixinet JL, Hussein M, Valencia JM, Gil RM, Herrero J, Caballero-Hidalgo A (2008) Side effects, complications and outcome of thoracoscopic sympathectomy for palmar and axillary hyperhidrosis in 406 patients. Eur J Cardiothorac Surg 34:514–519
Kim DH, Paik HC, Lee DY (2005) Video assisted thoracoscopic re-sympathetic surgery in the treatment of re-sweating hyperhidrosis. Eur J Cardiothorac Surg 27:741–744
Schmidt J, Bechara FG, Altmeyer P, Zirngibl H (2006) Endoscopic thoracic sympathectomy for severe hyperhidrosis: impact of restrictive denervation on compensatory sweating. Ann Thorac Surg 81:1048–1055
Miller DL, Force SD (2007) Outpatient microthoracoscopic sympathectomy for palmar hyperhidrosis. Ann Thorac Surg 83:1850–1853
Katara AN, Domino JP, Cheah WK, So JB, Ning C, Lomanto D (2007) Comparing T2 and T2–T3 ablation in thoracoscopic sympathectomy for palmar hyperhidrosis: a randomized control trial. Surg Endosc 21:1768–1771
Licht PB, Pilegaard HK (2004) Severity of compensatory sweating after thoracoscopic sympathectomy. Ann Thorac Surg 78:427–431
Yano M, Kiriyama M, Fukai I, Sasaki H, Kobayashi Y, Mizuno K, Haneda H, Suzuki E, Endo K, Fujii Y (2005) Endoscopic thoracic sympathectomy for palmar hyperhidrosis: efficacy of T2 and T3 ganglion resection. Surgery 138:40–45
Liu YH, Liu HP, Wu YC, Ko PJ (2010) Feasibility of transtracheal thoracoscopy (natural orifice transluminal endoscopic surgery). J Thorac Cardiovasc Surg 139:1349–1350
Rolanda C, Silva D, Branco C, Moreira I, Macedo G, Correia-Pinto J (2011) Peroral esophageal segmentectomy and anastomosis with single transthoracic trocar: a step forward in thoracic NOTES. Endoscopy 43:14–20
Ko PJ, Chu Y, Wu YC, Liu CY, Hsieh MJ, Chen TP, Chao YK, Wu CY, Yuan HC, Liu YH, Liu HP (2012) Feasibility of endoscopic transoral thoracic surgical lung biopsy and pericardial window creation. J Surg Res 175:207–214
Acknowledgments
This study was supported by Natural Science Foundation of China (Grant 81070906), Natural Science Foundation of Fujian Province (Grant 2013J01303) and Key Program of Scientific Research of Fujian Province (Grant 2015-ZQN-ZD-22).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Jian-Feng Chen, Jian-Bo Lin, Yuan-Rong Tu, Min Lin, Xu Li, Fan-Cai Lai, Quan Du and Yuan-Da Dai have no conflicts of interest or financial ties to disclose.
Additional information
Jian-Feng Chen and Jian-Bo Lin contributed equally to this study, and both should be considered first author.
Rights and permissions
About this article
Cite this article
Chen, JF., Lin, JB., Tu, YR. et al. Nonintubated transareolar single-port thoracic sympathicotomy with a needle scope in a series of 85 male patients. Surg Endosc 30, 3447–3453 (2016). https://doi.org/10.1007/s00464-015-4628-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-015-4628-5