Abstract
Objective
Use of surgical energy is integral to laparoscopic surgery (LS). Energized dissection (ED) has a potential to impact the biomolecular expression of inflammation due to ED-induced collateral inflammation. We did this triple-blind randomized controlled (RCT) study to assess this biomolecular footprint in an index LS, i.e., laparoscopic cholecystectomy (LC).
Methods and procedures
This RCT was conducted in collaboration with tertiary-level institutions, from January 2014 to December 2014 with institutional review board clearance. Consecutive, unselected, consenting candidates for LC were randomized (after anesthesia induction) into group I (ED) and group II (non-ED). They were managed with compliance to universal protocols for ethics, informed consent, anesthesia, drug usage and clinical pathway with blinded observers. Biomolecular inflammatory markers, i.e., interleukin 6 (IL-6), tumor necrosis factor-alpha (TNF-α) and highly sensitive CRP (HS-CRP), were measured with blood drawn juxta-preoperatively (H0), at 4 h (H4) and at 24 h (H24). The quantitative changes induced by ED on IL-6, TNF-α and HS-CRP at H0, H4 and H24 with their kinetic behavior were the study endpoint. Prospective data were analyzed statistically with a p value of <0.05 being significant.
Results
Two cases from the ED group had biliary injury and hence were withdrawn from analysis. The ED (n = 49) and non-ED (n = 51) groups had similar demographic, clinical and H0 biomolecular variables. There was a significant increase in IL-6, TNF-α and HS-CRP from H0 to H4 in both the groups (p values <0.001). From H4 to H24, all three cytokines showed significant increase in ED group (p < 0.05), whereas in the non-ED group, IL-6 showed significant fall (p = 0.004) and TNF-α showed no significant change (p = 0.063). Both the groups showed H4–H24 elevation of HS-CRP (p = 0.000).
Conclusion
Energized dissection adds to the cytokine-mediated postoperative inflammation. The additional ED-induced inflammation can be measured objectively by IL-6 and TNF-α levels.
Clinical trials registry
Clinical Trials Registry, India (REF/2014/06/007153).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Laparoscopic surgery (LS) has achieved universal popularity and acceptance. This progressive march of LS has been credited to its clinical equivalence to conventional surgery coupled with remarkably better patient-reported outcomes (PROs). Increasingly complex procedures are now being done with laparoscopic approach. This has been possible due to developments in technologies such as endooptics, imaging and surgical energy sources. The technological advances, while making LS easier, have also created concerns specific to potential challenges arising from a progressively complex “man–machine Interface” [1]. Use of surgical energy is considered a fundamental necessity in LS. Irrespective of the source and nature, energy acts via its thermal expression causing tissue necrosis. The thermal process proceeds irreversibly along a cascade of macromolecular aggregation, collagen helical disintegration, protein denaturation, coagulation, cellular desiccation, cell vaporization and finally tissue carbonization [2]. Energy usage cascade is a valuable aid in performing LS. Ideally, such a cascade would be precise resulting in perfect hemostasis without any collateral tissue insult [2]. But this ideal objective eludes our practices, which have been incorporating surgical energy-based devices at an exponential rate with the advances in LS [3]. Concerns have been raised about our gap in knowledge and application of surgical energy, leading to institution of structured programs to bridge the gap [4–6]. Absolute avoidance of surgical energy will obviously abolish these concerns, but that is practically hard to envisage. Hence, along with narrowing our knowledge and application gap as advised [4–6], an objective assessment of the invisible collateral insult is necessary. This will provide a judicious insight to limit surgical energy for hemostatic application as much as possible and provide a metric for evaluation of newer energy devices.
Such an objective assessment of invisible insult is possible, given our understanding of surgical intervention-induced changes in the peritoneal biomolecular environment [7]. The changes in biomolecular peritoneal environment are quantitatively proportional to the insult and have been characterized by the release of cytokines such as interleukin 6 (IL-6) and tumor necrosis factor-alpha (TNF-α) [8]. The release of these cytokines leads to expression of acute-phase reactants, such as highly sensitive C-reactive protein (HS-CRP). This biomolecular response has been seen to be quantitatively different in LS as compared to conventional open surgery [9]. While we know about the biomolecular insult in LS, the extent of it being contributed by surgical energy is not known. The component of biomolecular response attributable to energized dissection (ED) can be studied by application of surgical energy during an index LS, which can be done safely with or without ED. Laparoscopic cholecystectomy (LC) is an index LS [10]. Biomolecular footprint of LS is also uniquely impacted by the different energy forms apart from the devices used. Hence, a specific thermal component needs to be defined if any [11]. We have earlier published the clinical evidence of additional inflammatory response attributable to ED [12]. Based upon our experience of safe LC with or without ED [12–27], we undertook this study to evaluate the biomolecular inflammatory impact of ED in an index LS, i.e., LC.
Materials and methods
This triple-blind randomized study was conducted at a tertiary-level advanced laparoscopy center in collaboration with independent external collaborators from national medical colleges. [Clinical Trials Registry, India (REF/2014/06/007153)]. An integrated hospital information system (HIS) was used to maintain data. The HIS used in our institution (Trakcare version W650) auto locks the data once recorded. Iatrogenic gallbladder perforation (GbP) was taken as an index of potentially avoidable surgical inflammatory insult due to its higher incidence with ED [13]. The GbP incidence was 39 % in our most recent 100 consecutive LCs. Hence, a study sample size of 102 patients was found to be necessary to detect a fall of GbP rate from 39 to 10 % (39 % for ED and 10 % for non-ED) with an alpha error of 0.05 and power of 90 %. The study was approved by the institutional protocol committee and the ethics review board. All consecutive candidates of LC, i.e., symptomatic gallbladder disease patients who had been referred to us for cholecystectomy, were considered for the study. All these eligible LC candidates were explained about our results and practice of the two techniques of LC, i.e., with ED or non-ED (nED). They were detailed about the two techniques and the equivalence of results as well as their safe application by entire hierarchy of the surgical team from basic surgical trainees to consultants [12–27]. They were explained about the need of repeated blood sampling as required for the study along with the proposed study protocol. They were explained that investigations besides the standard ones will be done with blood sampling at three time schedules, i.e., 0 h just before first incision (H0), 4 h from the first incision (H4) and 24 h from first incision (H24). They were explained the investigations being generic to the inflammatory response to surgery. They were informed about the investigations to be done at H0, H4 and H24, i.e., as IL-6, TNF-α and HS-CRP. All the LC candidates who consented for the study were then recruited for the study with the following criteria:
Inclusion criteria
-
They were referred to our surgery for symptomatic gallstone disease.
-
They were able to understand the study protocol and provide the informed consent.
Exclusion criteria
-
Those having a concomitant common bile duct (CBD) stone or abnormal liver function test (LFT) or having had documented pancreatitis or any CBD intervention in preceding 6 weeks.
-
Suspicion of gallbladder carcinoma, i.e., a mass or eccentric gallbladder wall thickening on ultrasound.
-
Recommended cholecystectomy concomitantly with any other planned surgeries such as hysterectomy, appendectomy and hernia repairs etc.
-
Those on regular analgesics or self-medication or having any chronic anti-inflammatory drug usage or having neuropathies or autoimmune disorders, or taking any steroids/immunomodulator/cytotoxic medicines.
-
Those having known hypersensitivity to any of the drugs included in the study protocol.
-
Those unfit for general anesthesia (GA) due to un-optimized comorbidity.
All included LC candidates underwent pre-anesthesia checkup (PAC) by an independent anesthetist regarding suitability of GA. The baseline hematology, biochemistry and other investigations were recorded in the HIS. All surgeries were undertaken under GA in the same operation theater with the same set of instruments/resources with the same non-surgical/nursing team. Surgeries were performed by surgeons equi-versatile in ED and nED technique of LC. Surgeries were done by surgeons (excluding the first & second authors) trained in both techniques of LC. Monopolar electrosurgery was used in the prospective ED group and was kept on standby as rescue in nED group, as per our standard operation protocol [17]. Computer-generated randomization was done by external research coordinators who were tele-communicated about the posting of a case for surgery. After induction of GA, the scrubbed team being ready and operation area draped, a non-surgical member was informed of the randomly allocated group, i.e., ED or nED, by the external research coordinator via telephone, and surgery undertaken accordingly. A standard universal perioperative “drugs-to-be-used protocol” was followed. A baseline blood sample was drawn in appropriate vacutainers from a pre-marked virgin venous site for baseline (H0) estimation of the biomolecular markers. Standard LC technique with 12–14 mm capanosufflation was used for ED group and our published technique for nED group [19]. The peroperative data were noted by the anesthetist and recorded in the study protocol. Subsequent blood samples for the biomolecular marker were drawn at 4 h (H4) and 24 h (H24).
The operating time was defined as the time from incision for first/primary port to separation of the gallbladder from the liver. The cholecystectomy specimens were extracted in an endobag. After the surgery, the patients were shifted to postoperative area to be observed by anesthetist and nurses not involved in surgery. Standard dischargeability protocol was followed by the staff in the postoperative ward as follows:
-
Complete and satisfactory recovery from GA without any hemodynamic variation or instability.
-
No demand for any pharmacological intervention beyond standard institutional protocol.
-
Ability to walk to washroom, having passed urine with ability to take care of her/his garments.
-
Having tolerated liquids after demanding and feeling hungry.
-
Self-expressed desire to go home and confidence to follow normal ABCDEF (A-Activity, B-Bath, C-Commitment, D-Diet, E-Exercise, F-Family life) [28].
The data till the dischargeability criteria were recorded in HIS by the staff in postoperative area. The blood samples drawn at H0, H4 and H24 were sent to the laboratory for estimation of biomolecular markers, i.e., IL-6, TNF-α and HS-CRP. The results were entered in HIS by the laboratory technicians. Any deviation from pharmacological protocol, need for additional perfusions, peroperative complications, i.e., bilio-visceral injury, failure to discharge within 24 h of surgery and rehospitalization within 3 weeks, were taken as a criteria for withdrawal from study analysis.
Endpoint for the study
The impact of energized dissection, on the quantitative levels of IL-6, TNF-α and HS-CRP at the defined time stations (H0, H4 and H24) and on their kinetic behavior in the postoperative period till 24 h, was the primary endpoint.
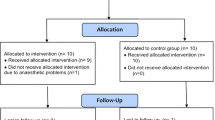
The data from the HIS were exported to a Microsoft Excel sheet for analysis by the external non-surgical members and analyzed in terms of the two study groups. The analysis was done using SPSS version 17 (Statistical Package for Social Sciences). Qualitative data were compared with Chi-square test and quantitative data with the “Student’s t test.” A p value of <0.05 was considered significant. The methodology flow chart of the study is shown in Fig. 1. After the analysis, the external coordinators gave the code of group I and group II. The group I was LC with ED, and group II was LC without ED, i.e., nED.
Results
The study with 102 consecutive consenting candidates was conducted from January 2014 to December 2014. The study population was randomized preoperatively (juxta-preoperative) to be allocated to group I (n = 51) and group II (n = 51). In group I, one patient had peroperatively detected BDI and one had prolonged hospitalization beyond 24 h, followed by rehospitalization in third postoperative week. She had biliary leak with peritonitis. These two patients were thus withdrawn from data analysis. The data were analyzed for the remaining 49 patients in group I and 51 patients in group II. The patients in group I and group II were well matched in terms of demographics (except for the age, p = 0.04), body mass index (BMI), socioeconomic class [29], clinical presentation, comorbidities, personal habits and PAC assessment grades (Table 1). The proinflammatory cytokines have been known to be related to the BMI and not the age of the patients.
In these patients, there were no technical difficulties, any need to deviate from the protocol, any peroperative complication or need for conversion. The peroperative parameters, i.e., inflammation status, were also similar in the two groups. Operative time was relatively shorter in group II with a mean time of 24 min (19–30) versus 39 min (27–46) in group I. This difference in the time in terms of ED and nED is consistent with our earlier published results [24]. There was no difference in the time for recovery from GA. However, the patients in group II had a significantly faster fulfillment of discharge criteria both as per nursing and surgical assessment. The peroperative and postoperative parameters are given in Table 2. The two groups had statistically similar baseline levels of IL-6, TNF-α and HS-CRP. All these biomarkers were raised in both the groups at H0, H4 and H24 with respect to the baseline levels (Table 3). The IL-6 rise was significantly higher in group I and continued to rise even after H4 of surgery, while it was significantly lower in group II at H4 and fell at H24. The elevation in HS-CRP in the two groups was similar at H4 without any significant difference. The H4 to H24 rise in the HS-CRP levels was significantly higher with energized dissection (group I). The H0 to H4 behavior of TNF-α was similar to the IL-6 response. The H4 to H24 behavior of TNF-α was different in the two groups. In group I (energized dissection), TNF-α had a significant rise in levels, while in the group II (without energized dissection) the rising behavior of TNF-α had abated without any further significant change. These kinetic patterns of progressive cytokine response seen exclusively in group I (ED) and the absence of such progression in group II (nED) with a stable acute-phase reactant (HS-CRP) are shown in Fig. 2 and Table 3.
Trends of IL-6, TNF-α and HS-CRP at 0 (filled blue square), 4 (filled green square) and 24 h (filled yellow square) of surgery in the two groups. There was a significant increase in all the three cytokine levels from 0 to 4 h in both the groups (p values <0.001). From 4 to 24 h, all three molecules showed significant increase in group I (p < 0.05), whereas in group II, IL-6 showed significant fall by 24 h (p = 0.004), TNF-α showed no significant change (p = 0.063) and HS-CRP continued to rise (p = 0.000) (Color figure online)
Discussion
Laparoscopic surgery has captured the imagination of community and profession in equal measure. Beginning with application of LS to well-defined conditions, surgeons are reporting successful application of LS to ever-increasing spectrum of complex procedures. These advances have been possible due to well matched and paralleled technological advances. Given the importance of unhindered endovision and seamless surgical navigation, the surgical energy-based technology is an indispensable part of LS armamentarium. The concerns about the invisible collateral damage from ED and our gap in the knowledge for its safe application are well recognized. These concerns have been articulated as a much needed bridge to overcome the perceived gap in patient safety [3]. This “gap in patient safety” has led to many structured bridge-building guidelines [4–6]. Apart from the knowledge gap and technological failure, the “man–machine interface” is also a challenge [1]. LC is the commonest LS which is performed at all hierarchical levels of healthcare facilities. It has become an index for advances in LS as well as their assessment [10–12]. Surgery has its attendant inflammatory response that impacts the clinical outcomes as well as PROs [12]. Avoidance of ED in LC avoids the many cited complications of ED [2]. It has also been shown to be associated with better PROs [12]. Realization and appreciation of catastrophic ED-related issues [2], specific implications in index adverse events such as CBD injury [30], emphasis on bridging patient safety gap [3], development of enhanced recovery “SMART” programs [31], in this era of informed “informed consent” [32], have necessitated judicious incorporation of surgical technologies in practice. Evaluation of innovations, with uncommon but serious outcome differences with seemingly similar but scientifically distinguishable interventions, remains a surgical challenge [12]. The common-sensically feared additional inflammation from invisible collateral damage needs a measurable objective assessment. The inflammatory response of invisible collateral insult has a molecular footprint. Biomolecular inflammatory response as evinced by alterations in IL-6, TNF-α and HS-CRP is a valuable metrics for visualizing such invisible collateral inflammatory insult. These molecules have been accepted as quantitative markers of intraperitoneal insult with an intervention proportionate response [7–9]. Apart from their rise being related to the extent of trauma, interventions to moderate their response have been associated with improved PROs [15, 33]. The published cytokine kinetics have shown that these biomarkers have a precise and rapid response to inflammatory stimulus, peaking in less than 6 h and having a short half-life, e.g., <20 min for TNF-α [34, 35]. These biomarkers have been shown to be related to intraperitoneal insult irrespective of nature of surgery and have been found to be both, i.e., more sensitive and specific than catecholamine and cortisol [36]. These cytokines have pleiotropic adverse, quantitative as well as qualitative effects such as cellular dysfunction, immune dysfunction, cascading of acute-phase reactants (HS-CRP), sleep disturbances, fatigue and memory consolidations and have also been related to subsequent development of postoperative complications [37–39]. In the present study, all the three inflammatory biomarkers showed a significantly pronounced rise in LS with energized dissection as compared to LS without energized dissection (Table 3). The additional bio-inflammatory response seen with ED seemed to be worse, as indicated by a slower return of the biomarker levels toward baseline in comparison with the nED group (Table 3). Short half-life cytokines reflecting acute surgical insult, i.e., IL-6 and TNF-α, were found to be either falling or not rising significantly after 4 h in patients undergone surgery without energized dissection. This indicates that there was no continuing inflammatory stimulus apart from the surgical trauma. For them to continue rising after 4 h, some continuing inflammatory stimulus would be required. Failure of continuing proinflammatory cytokine response in the absence of energized dissection indicates termination of the surgically induced inflammatory cascade. The inflammatory stimulus required for continuing cytokine response beyond 4 h was evident in the presence of energized dissection. The continuing proinflammatory cytokine response seen exclusively with energized dissection indicates that termination of inflammation cascade failed to happen in patients of LS with ED. This substantiates that energized dissection negated the natural ablation of inflammatory cascade after 4 h.
HS-CRP, an acute-phase reactant with a longer half-life continued to rise in both the groups. However, the rise in HS-CRP was significantly higher in patients having surgery with energized dissection. The muted HS-CRP response associated with avoidance of ED shows that the overall postoperative systemic inflammatory response was significantly lower as compared to surgery with ED.
This observation supports our hypothesis about the additional inflammatory postoperative insult due to energized dissection as a stand-alone feature of ED. Given the above-mentioned role of cytokine-induced pleiotropic adverse effects on various PROs as well subsequent complications, this study supports the earlier findings of better PROs following after LS done without ED. This study provides an objective support to potentially improved surgical inflammation-related PROs and faster convalescence in the patients undergoing LC without energized dissection as reported earlier [12]. This is the first study to have evaluated the “stand-alone” biomolecular inflammatory component of energized dissection in LS. The design of the study is a practical model for evaluating newer surgical energy devices and comparing different devices in an objective manner. This study is a continuation of our earlier work. Our earlier work provided with the needed laparoscopic procedure which is conventionally done with ED and could be done safely without ED. It also needed a surgical team versatile in both techniques but outside of the study participants. The staged pursuit of our current hypothesis is shown in Fig. 3, providing further strength to the study along with best possible blinding and randomization being done closest to the intervention and being tele-communicated. We are conducting further studies to see the objective quantitative association, if any, of the biomolecular surgical inflammatory response, with the PROs and overall postoperative convalescence in many other surgical procedures.
References
Agarwal BB, Agarwal S (2007) The man–machine interface, a paradox of technology. Is the black box (BB) concept an angel or a demon? Surg Endosc 21:1680
Sankaranarayanan G, Resapu RR, Jones DB, Schwaitzberg S, De S (2013) Common uses and cited complications of energy in surgery. Surg Endosc 27:3056–3072
Fuchshuber PR, Robinson TN, Feldman LS, Jones DB, Schwaitzberg SD (2014) The SAGES FUSE program: bridging a patient safety gap. Bull Am Coll Surg 99:18–27
Feldman LS, Fuchshuber P, Jones DB, Mischna J, Schwaitzberg SD, Force FT (2012) Surgeons don’t know what they don’t know about the safe use of energy in surgery. Surg Endosc 26:2735–2739
Madani A, Jones DB, Fuchshuber P, Robinson TN, Feldman LS (2014) Fundamental use of surgical energy™ (FUSE): a curriculum on surgical energy-based devices. Surg Endosc 28:2509–2512
Madani A, Watanabe Y, Vassiliou MC, Fuchshuber P, Jones DB, Schwaitzberg SD, Fried GM, Feldman LS (2014) Impact of a hands-on component on learning in the fundamental use of surgical energy™ (FUSE) curriculum: a randomized-controlled trial in surgical trainees. Surg Endosc 28:2772–2782
Chegini N (2002) Peritoneal molecular environment, adhesion formation and clinical implication. Front Biosci 1(7):e91–e115
Hildebrand F, Pape HC, Krettek C (2005) The importance of cytokines in the posttraumatic inflammatory reaction. Unfallchirurg 108(10):793–794, 796–803
Grande M, Tucci GF, Adorisio O, Barini A, Rulli F, Neri A, Franchi F, Farinon AM (2002) Systemic acute-phase response after laparoscopic and open cholecystectomy. Surg Endosc 16:313–316
Agarwal BB, Chintamani C (2011) Reminder of the metrics of endosurgical innovation. Arch Surg 146:1108
Brokelman WJ, Lensvelt M, Borel Rinkes IH, Klinkenbijl JH, Reijnen MM (2011) Peritoneal changes due to laparoscopic surgery. Surg Endosc 25:1–9
Agarwal BB, Agarwal N, Agarwal KA, Goyal K, Nanvati JD, Manish K, Pandey H, Sharma S, Ali K, Mustafa ST, Gupta MK, Saluja S, Agarwal S (2014) Outcomes of laparoscopic cholecystectomy done with surgical energy versus done without surgical energy: a prospective-randomized control study. Surg Endosc 28:3059–3067
Agarwal BB (2008) Outcomes in laparoscopic cholecystectomy (LS) done with or without using energy sources (ES): results of a prospective randomized controlled study. 2008 Scientific Session of the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) Philadelphia, Pennsylvania, USA, 9–12 April 2008. Surg Endosc 22:S224
Agarwal BB, Mahajan KC (2010) Laparoscopic biliary tract injury prevention: zero tolerance, error free performance. Surg Endosc 24:728–729
Agarwal KA, Tripathi CD, Agarwal BB, Saluja S (2011) Efficacy of turmeric (curcumin) in pain and postoperative fatigue after laparoscopic cholecystectomy: a double-blind, randomized placebo-controlled study. Surg Endosc 25:3805–3810
Agarwal BB (2010) Energized dissection, energized hemostasis. Arch Surg 145:1021
Agarwal BB (2007) Are energy sources required in laparoscopic cholecystectomy? Or should they be standby? Surg Endosc 21:1042
Agarwal BB, Gupta M, Agarwal S, Mahajan K (2007) Anatomical footprint for safe laparoscopic cholecystectomy without using any energy source: a modified technique. Surg Endosc 21:2154–2158
Agarwal BB, Gupta M, Agarwal S, Mahajan KC (2007) Laparoscopic cholecystectomy without using any energy source. J Laparoendosc Adv Surg Tech A 17:296–301
Agarwal BB (2010) Results of laparoscopic cholecystectomy without energized dissection: a prospective study. Int J Surg 8:167–172
Agarwal BB, Sinha B, Mahajan KC (2010) Double blind randomized control study-outcomes of laparoscopic cholecystectomy (without using energy source) performed by a trainee or a consultant. 17th International Congress of the European association for endoscopic surgery (EAES) Prague, Czech Republic, 17–20 June 2009. Surg Endosc 24:S37
Agarwal BB, Jayaraman L, Mishra A, Sarangi R, Mahajan KC (2010) Clinical outcomes of laparoscopic cholecystectomy (without energised dissection) performed by a basic surgical trainee or a consultant-double blind randomized control study. 2010 Scientific session of the society of American gastrointestinal and endoscopic surgeons (SAGES) National Harbor, Maryland, USA, 14–17 April 2010. Surg Endosc 24:S580
Agarwal BB, Gupta MK, Agarwal S, Mahajan KC (2007) Avoiding any energy source for a safe and better laparoscopic cholecystectomy. 2007 Scientific session of the society of American gastrointestinal and endoscopic surgeons (SAGES) Las Vegas, Nevada, USA, 18–22 April 2007. Surg Endosc 21:S443
Agarwal BB, Agarwal S, Gupta MK, Agarwal N, Agarwal D, Goyal K, Saluja S, Mahajan KC, Agarwal KA, Pandey H (2013) Evaluation of avascular ‘‘holy plane’’ based cold dissection technique in consecutive unselected laparoscopic cholecystectomies: results of 7 year experience. 2013 Scientific session of the society of American gastrointestinal and endoscopic surgeons (SAGES) Baltimore, Maryland, USA, 17–20 April 2013. Surg Endosc 27:S396
Agarwal BB (2008) Journey of the carbon-literate and climate conscious endosurgeon having a head, heart, hands, and holistic sense of responsibility. Surg Endosc 22:2539–2540
Agarwal BB, Chintamani AK, Goyal K, Mahajan KC (2012) Innovations in Endosurgery: journey into the past of the future. To ride the sils bandwagon or not? Indian J Surg 74:234–241
Agarwal BB (2009) Patient safety in laparoscopic cholecystectomy. Arch Surg 144:979
Agarwal BB, Agarwal S, Gupta M, Mahajan K (2008) Transaxillary endoscopic excision of benign breast lumps: a new technique. Surg Endosc 22:407–410
Park K (2013) Park’s textbook of preventive and social medicine, 22nd edn. M/s Banarsidas Bhanot Publishers, Jabalpur
Fingerhut A, Dziri C, Garden OJ, Gouma D, Millat B, Neugebauer E, Paganini A, Targarona E (2013) ATOM, the all-inclusive, nominal EAES classification of bile duct injuries during cholecystectomy. Surg Endosc 27:4608–4619
Feldman LS, Delaney CP (2014) Laparoscopy plus enhanced recovery: optimizing the benefits of MIS through SAGES ‘SMART’ program. Surg Endosc 28:1403–1406
Agarwal BB (2009) Informed consent-‘da Vinci code’ for our safety in empowered patient’s safety. Surg Endosc 23:1158–1160
Agarwal BB (2011) Do dietary spices impair the patient-reported outcomes for stapled hemorrhoidopexy? A randomized controlled study. Surg Endosc 25:1535–1540
Mascher B, Schlenke P, Seyfarth M (1999) Expression and kinetics of cytokines determined by intracellular staining using flow cytometry. J Immunol Methods 223:115–121
Oliver JC, Bland LA, Oettinger CW, Arduino MJ, McAllister SK, Aguero SM, Favero MS (1993) Cytokine kinetics in an in vitro whole blood model following an endotoxin challenge. Lymphokine Cytokine Res 12:115–120
Sendt W, Amberg R, Schöffel U, Hassan A, von Specht BU, Farthmann EH (1999) Local inflammatory peritoneal response to operative trauma: studies on cell activity, cytokine expression, and adhesion molecules. Eur J Surg 165:1024–1030
Rohleder N, Aringer M, Boentert M (2012) Role of interleukin-6 in stress, sleep, and fatigue. Ann N Y Acad Sci 1261:88–96
Choileain NN, Redmond HP (2006) Cell response to surgery. Arch Surg 141:1132–1140
Benedict C, Scheller J, Rose-John S, Born J, Marshall L (2009) Enhancing influence of intranasal interleukin-6 on slow-wave activity and memory consolidation during sleep. FASEB J 23:3629–3636
Agarwal KA, Agarwal BB, Mahajan KC (2010) Carbon footprint of laparoscopic cholecystectomy performed with or without energized dissection: a case controlled study. 2010 Scientific Session of the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) National Harbor, Maryland, USA, 14–17 April 2010. Surg Endosc 24:S590
Acknowledgments
This work was a continuation of our earlier studies. This started with blessings and guidance of Prof. Krishan C Mahajan. He was the founder chairman of our institution and the discipline of surgery in North India after Indian independence. He was very keen to see the conclusion of this study but left us at age 92 in January 2015 after continuously monitoring the present study till his last days. We dedicate this work to his memory. We thank Dr. Manoj Modi, a neonatologist in our institution for helping us with statistics. We are grateful to Ms Pooja and Ms Ramneek for preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Brij B. Agarwal, Juhil D. Nanavati, Nayan Agarwal, Naveen Sharma, Krishna A. Agarwal, Kumar Manish, Satish Saluja and Sneh Agarwal have no conflict of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Agarwal, B.B., Nanavati, J.D., Agarwal, N. et al. Biomolecular inflammatory response to surgical energy usage in laparoscopic surgery: results of a randomized study. Surg Endosc 30, 1733–1741 (2016). https://doi.org/10.1007/s00464-015-4408-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-015-4408-2