Abstract
Introduction
The results of cardiomyotomy in patients of achalasic megaesophagus with axis deviation are not satisfactory, and several authors have advocated an esophagectomy in these patients. We describe the technical details and outcomes of a novel technique of laparoscopic esophagogastroplasty for end-stage achalasia.
Methods
Patients with end-stage achalasia, characterized by tortuous megaesophagus were selected. The surgery was performed in supine position using five abdominal ports. The steps included mobilization of the gastroesophageal junction and lower intrathoracic esophagus, straightening and anchoring the pulled intrathoracic esophagus into the abdomen, and a side-side esophagogastroplasty.
Results
Four patients with megaesophagus due to end-stage achalasia underwent this procedure. The average duration of surgery was 177.5 (range, 120–240) min. All patients could be ambulated on the first postoperative day. Oral feeding was initiated by the third postoperative day, and all patients had significant improvements in their dysphagia scores. All patients had excellent cosmetic results and were discharged by the fifth postoperative day. An upper gastrointestinal contrast study done at 6 weeks after surgery did not show any hold up of contrast, and there was decrease in the convolutions and diameter of the esophagus. At a mean follow-up of 10.5 (range, 3–15) months, all patients are euphagic without significant symptoms of gastroesophageal reflux.
Conclusions
Laparoscopic esophagogastroplasty is an effective option for relieving dysphagia in megaesophagus due to achalasia with axis deviation and is a reasonable alternative before subjecting to a major and potentially morbid esophagectomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Achalasia is a motility disorder of the esophagus that is characterized by absence of esophageal peristalsis and incomplete relaxation of a frequently hypertensive lower esophageal sphincter to swallowing [1]. The pathological changes that occur in achalasia include inflammation and loss of ganglion cells with fibrosis of the myenteric nerves [2]. Significant reductions of nitric oxide and vasoactive intestinal polypeptide also occur [3, 4]. The exact etiology resulting in these changes is not known, and thus all treatment modalities are palliative and focus on relieving dysphagia by targeting the lower esophageal sphincter. Laparoscopic myotomy with partial fundoplication has been shown to provide superior and longer-lasting symptomatic relief compared with other treatment modalities and is considered the procedure of choice to treat early stages of achalasia. The outcomes of cardiomyotomy are less satisfactory for late stages of achalasia with megaesophagus (especially in the presence of axis deviation), and various authors have suggested performing esophagectomy in such cases [5–7]. The present article describes the technical details and outcomes of a novel minimally invasive technique of “laparoscopic esophagogastroplasty” in four patients with end-stage achalasia.
Methods
Surgical technique
The procedure is performed under general anesthesia. The patient is positioned supine with a leg split, and the surgeon stands to the right of the patient. The procedure is done using five ports as follows: a supraumbilical 10-mm port (for the laparoscope), a 12-mm port in the right midclavicular line 2 cm above the umbilicus, a 5-mm right subcostal port in the anterior axillary line, a 5-mm port in the left midclavicular line 2 cm above the umbilicus, and a 5-mm epigastric port for retraction of the left lobe of the liver.
Mobilization of the gastroesophageal junction and lower intrathoracic esophagus
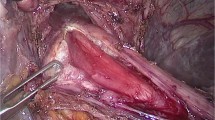
After inserting the laparoscope, the gastroesophageal junction is identified and the peritoneum over it is divided (Fig. 1A). The intra-abdominal esophagus is dissected and looped, carefully preserving the anterior and posterior vagal nerves (Fig. 1B). The esophageal hiatus is opened and lower intrathoracic esophagus is mobilized as done in a transhiatal esophagectomy (Fig. 1C). The purpose of this mobilization is to straighten the lower intrathoracic esophagus so that a length of 5–7.5 cm can be pulled into the abdomen below the level of the hiatus (Fig. 1D). The pulled intrathoracic esophagus is then fixed to the perimeter of the hiatus (esophagopexy) using interrupted sutures (Fig. 1E).
A Gastroesophageal junction is identified and peritoneum over it is divided. B Intra-abdominal esophagus is mobilized and looped. C Mobilization of the lower intrathoracic esophagus via the esophageal hiatus (dotted line), anterior dissection of the esophagus frees it from the pericardium (solid arrow depicts the pericardium). D Approximately 5–7.5 cm of intrathoracic esophagus mobilized and pulled into the abdomen. E Pulled intrathoracic esophagus is fixed to the perimeter of the esophageal hiatus using interrupted sutures (esophagopexy). F Gastric fundus is aligned along the esophagus with a suture (solid arrow), anterior gastrotomy is made for insertion of the stapler. G An endo-GIA stapler is introduced into the stomach via the gastrotomy such that one limb is in the esophageal lumen across the lower esophageal sphincter and the other limb is in the fundus of the stomach. H Two successive firings of the stapler results in a wide esophagogastric anastomosis (solid arrow depicts the staple line). I Completed esophagogastroplasty. J Gastrotomy closed with interrupted sutures. Eso esophagus, St stomach
Mobilization of the gastric fundus and performance of the esophagogastroplasty
The fundus of the stomach is mobilized and is fixed to the mobilized esophagus using one or two interrupted sutures (Fig. 1F). Then, an anterior gastrotomy is made on the cardia of the stomach using a harmonic scalpel (Fig. 1F). An endoscopic linear cutter and stapler are inserted into the stomach via the gastrotomy such that one limb of the stapler is inside the fundus and the other limb is inside the esophagus (Fig. 1G). With two successive firings, the stapler creates a wide esophagogastric anastomosis disrupting the lower esophageal sphincter. The camera is now introduced inside the stomach via the gastrostomy to inspect the esophagogastric anastomosis, and the scope is easily passed into the esophagus without any resistance (Fig. 1H). Figure 1I depicts the completed esophagogastroplasty. The anterior gastrostomy is closed in two layers using interrupted silk sutures (Fig. 1J).
Results
During the study period between January 2011 and March 2012, 20 patients of achalasia of the esophagus were managed surgically at our center. The patients were classified as per the classification proposed by Pechlivanides et al. [8]. Of these, 16 patients with stages I–III underwent a cardiomyotomy with or without a partial fundoplication alone. Four patients with stage IV were considered for the described procedure. All patients had symptoms of dysphagia; three patients in addition had symptoms of heart burn and chest pain. The mean age of the patients was 24 (range, 20–30) years and 3 were female. The median duration of symptoms was 2.5 (range, 1–4) years. All patients had failed endoscopic dilatation treatment. The patients underwent a barium swallow examination, which showed a markedly dilated and tortuous esophagus with axis deviation. An upper gastrointestinal endoscopy with esophageal manometry confirmed the diagnosis of achalasia in all the patients.
The mean duration of surgery was 177.5 (range, 120–240) min, and the intraoperative blood loss was minimal. None of the patients required any blood transfusions or postoperative ventilatory support. All patients could be ambulated on the first postoperative day. The oral feeding was initiated by the third postoperative day, and all patients had significant improvements in their dysphagia scores. All patients had excellent cosmetic results and were discharged by the fifth postoperative day. An upper gastrointestinal contrast study done at 6 weeks following surgery did not show any hold up of contrast at the lower end of the esophagus, and there was decrease in the convolutions and diameter of the esophagus in all the patients (Fig. 2). At a mean follow-up of 10.5 months, all patients were euphagic with good-excellent results: three patients have more than 90 % relief, and one patient has 70 % relief in dysphagia. One patient complained of mild symptoms of gastroesophageal reflux, which was successfully managed with medical treatment.
Discussion
Treatment of end-stage achalasia especially in the presence of gross esophageal dilatation and axis deviation is challenging. Cardiomyotomy with a partial fundoplication is considered the procedure of choice in patients with early achalasia [9] and can even be considered in patients with a dilated (>6 cm) achalasic esophagus. If the axis of the esophagus is straight, a well-performed esophagomyotomy provides good relief of dysphagia and allows the esophagus to empty by gravity [5]. However, esophagomyotomy is less effective in patients who have an associated supradiaphragmatic axis deviation, which interferes in emptying despite a good cardiomyotomy. Persistent dysphagia, ongoing retention esophagitis, reflux, recurrent chest infections due to aspiration and development of malignancy are the potential complications. The purpose of treatment in these patients is to provide adequate drainage of the esophagus, relieve dysphagia, prevent symptoms of aspiration, and improve the quality of life with a procedure that is minimally invasive and is associated with very low morbidity and mortality.
Because the reported outcomes with drainage procedures have been less satisfactory, different types of resectional operative procedures have been proposed. A limited distal esophageal resection with interposition of a short segment of colon was shown by Hsu et al. [10]. Other authors have advocated performing an open [5, 7], laparoscopic [6], and vagal sparing esophagectomy [11]. These authors believe that resection of the diseased esophagus leads to a marked functional improvement and the advanced decompensated stage of the esophagus is irreversible and not influenced by conservative and/or non-resectional surgical procedures [7]. Although effective, an esophagectomy is a major operation for a benign disease and has a risk for morbidity and mortality. In the results of esophagectomy for end-stage achalasia reported by Devaney et al. [5], the incidence of major complications was reported to be 30 %, which included anastomotic leaks (10 %), hoarseness of voice (5 %), bleeding (2 %), chylothorax (2 %), tracheal tear (1 %) etc. There was a 2 % mortality rate, and the average duration of hospitalization was 12.5 days. Fifty percent of patients required postoperative anastomotic dilatations. In addition, there are specific issues while performing an esophagectomy in patients with a megaesophagus. The hypertrophied esophageal muscle of achalasic esophagus is supplied by hypertrophied esophageal arteries, which are at risk of bleeding if avulsed during a transhiatal esophagectomy. The dilated esophagus often is deviated, and its mobilization may result in injury to the pleurae and the adjacent intrathoracic structures. Similarly in the neck, the mobilization of the dilated cervical esophagus may result in injury to the recurrent laryngeal nerve.
The current technique of esophagogastroplasty is a minimally invasive technique that has the potential to become the favored technique in patients of megaesophagus with axis deviation. The operative time is less, and the intraoperative blood loss is minimal. The essential components of the procedure include mobilization of the lower intrathoracic esophagus and fixing it to the perimeter of the esophageal hiatus, mobilizing the gastric fundus and fixing it to the esophagus, and performing a wide esophagogastric anastomosis. The mobilization and fixing of the lower intrathoracic esophagus not only straightens the deviated esophageal axis (which facilitates emptying by gravity) but also increases the length of the intra-abdominal esophagus, which is protective against gastroesophageal reflux. The wide esophagogastric anastomosis done in this procedure disrupts the lower esophageal sphincter and provides much better drainage compared with esophagomyotomy alone. In the present series, there was no significant postoperative morbidity in any of the patients, and all the patients could be discharged by the fifth postoperative day. There was good/excellent relief of dysphagia in all the four patients who underwent this procedure. None of the patient had any significant symptoms of gastroesophageal reflux. An upper gastrointestinal contrast study done at 6 weeks following surgery did not show any hold up of contrast and there was decrease in the convolutions and diameter of the esophagus (Fig. 2). In addition, there was an excellent cosmetic outcome in all the patients. At a mean follow-up of 10.5 months, all patients are euphagic to normal diet.
Thus, we feel that this new technique of “laparoscopic esophagogastroplasty” should be considered in the management of patients with end-stage achalasia with an axis deviation before subjecting them to a major and potentially more morbid procedure: esophagectomy.
References
Campos GM, Vittinghoff E, Rabl C, Takata M, Gadenstätter M, Lin F, Ciovica R (2009) Endoscopic and surgical treatments for achalasia: a systematic review and meta-analysis. Ann Surg 249(1):45–57
Goldblum JR, Rice TW, Richter JE (1996) Histopathologic features in esophagomyotomy specimens from patients with achalasia. Gastroenterology 111(3):648–654
Woltman TA, Pellegrini CA, Oelschlager BK (2005) Achalasia. Surg Clin North Am 85(3):483–493
Vaezi MF, Richter JE (1999) Diagnosis and management of achalasia. American College of Gastroenterology Practice Parameter Committee. Am J Gastroenterol 94(12):3406–3412
Devaney EJ, Lannettoni MD, Orringer MB, Marshall B (2001) Esophagectomy for achalasia: patient selection and clinical experience. Ann Thorac Surg 72(3):854–858
Palanivelu C, Rangarajan M, Jategaonkar PA, Maheshkumaar GS, Vijay Anand N (2008) Laparoscopic transhiatal esophagectomy for ‘sigmoid’ megaesophagus following failed cardiomyotomy: experience of 11 patients. Dig Dis Sci 53(6):1513–1518
Gockel I, Kneist W, Eckardt VF, Oberholzer K, Junginger T (2004) Subtotal esophageal resection in motility disorders of the esophagus. Dig Dis 22(4):396–401
Pechlivanides G, Chrysos E, Athanasakis E, Tsiaoussis J, Vassilakis JS, Xynos E (2001) Laparoscopic Heller cardiomyotomy and Dor fundoplication for esophageal achalasia: possible factors predicting outcome. Arch Surg 136(11):1240–1243
Stefanidis D, Richardson W, Farrell TM, Kohn GP, Augenstein V, Fanelli RD, Society of American Gastrointestinal and Endoscopic Surgeons (2012) SAGES guidelines for the surgical treatment of esophageal achalasia. Surg Endosc 26(2):296–311
Hsu HS, Wang CY, Hsieh CC, Huang MH (2003) Short-segment colon interposition for end-stage achalasia. Ann Thorac Surg 76(5):1706–1710
Banki F, Mason RJ, DeMeester SR, Hagen JA, Balaji NS, Crookes PF, Bremner CG, Peters JH, DeMeester TR (2002) Vagal-sparing esophagectomy: a more physiologic alternative. Ann Surg 236(3):324–335 discussion 335-336
Disclosures
Drs. Amit Javed and Anil K. Agarwal have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Agarwal, A.K., Javed, A. Laparoscopic esophagogastroplasty: a minimally invasive alternative to esophagectomy in the surgical management of megaesophagus with axis deviation. Surg Endosc 27, 2238–2242 (2013). https://doi.org/10.1007/s00464-012-2751-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-012-2751-0