Abstract
Introduction
While the penetrance of robotic surgery into field of urology and gynecology has been significant, general surgeons have been slower adopters. We sought to compare laparoscopy and RAS among five different general surgical procedures with various penetrance of MIS.
Methods
Following IRB approval, the New York Statewide Planning and Research Cooperative System administrative data were used to identify five common laparoscopic general surgery procedures: cholecystectomy, colectomy, esophageal fundoplication (EF), Roux-en-Y gastric bypass (RYGB), and sleeve gastrectomy (SG) between 2008 and 2012. ICD-9 codes were used to select laparoscopic versus robotic procedures. Procedures were compared based on any complication and hospital length of stay (HLOS). Following descriptive analysis, propensity score analysis was used to estimate the population average differences between patients who underwent robotic-assisted and laparoscopic procedures.
Results
There were 1458 patients who had undergone robotic-assisted surgery and 166,790 patients who had undergone laparoscopic surgery among the five procedures between 2008 and 2012. Of the 1458 robotic cases, 186 were cholecystectomy, 307 were RYGB, 118 were SG, 288 were EF, and 559 were colectomy. Initial univariate analysis showed a significantly higher rate of overall complications and HLOS in the laparoscopic group compared to the robotic-assisted group. Laparoscopic colectomy had a significantly higher rate of complications and longer length of stay compared to robotic approaches. No difference in complications or HLOS was seen in the cholecystectomy group. Following propensity score analysis, patients who had undergone robotic-assisted colectomy had significantly lower rate of complications compared to those who underwent conventional laparoscopic procedure (p value = 0.0022). In addition, patients who underwent robotic-assisted SG had on average 1.22 days longer HLOS (p value = 0.0037).
Conclusion
Robotic approaches may facilitate safer adoption of minimally invasive approaches in areas where penetrance of conventional laparoscopy is low, such as in colorectal surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Since the introduction of laparoscopic cholecystectomy in late 1980s [1], laparoscopic surgery has gained significant popularity in many surgical specialties. Proponents of minimally invasive approaches cite the ability to provide advanced operative care while significantly enhancing patient recovery [2]. While advancement of minimally invasive surgery has increased significantly in certain areas, such as bariatric surgery [3] and general surgery [4, 5], its penetration in other specialties remains lower [6–8].
The advent of laparoscopic robotic-assisted surgery (RAS) has added a new dimension to the area of minimally invasive surgery. Additional benefits of robotics include three-dimensional (3D) binocular vision, improved dexterity due to the wristed instrumentation and elimination of natural tremor from computer adjustment [9]. Due to such advantages, it has been proposed that the robotic surgery platform is enabling surgeons who are not comfortable with standard laparoscopy to perform minimally invasive surgery. While the penetrance of RAS into the fields of urology and gynecology has been significant, general surgeons have been slower adopters, citing higher intraoperative cost and considerable learning curves with low perceived benefits compared to conventional laparoscopy. The purpose of our study was to compare outcomes of laparoscopy and RAS among five common general surgical procedures with various penetrance of minimally invasive techniques.
Methods
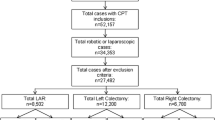
Following approval by our Institutional Review Board (IRB) and the New York Department of Health (DOH), the New York Statewide Planning and Research Cooperative System (SPARCS) administrative database was used to identify our study population. SPARCS is a longitudinal comprehensive data reporting system which collects patient level data on patient characteristics, diagnoses and treatments, services, and charges for every hospital discharge, ambulatory surgery patient, and emergency department admission in New York State. Using International Classification of Diseases, Ninth Revision (ICD-9) primary diagnosis codes (Table 1), 172,532 adult inpatients records (Age > 18 years of age) of patients who have undergone cholecystectomy, Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy (SG), esophageal fundoplication, and colectomy between 2008 and 2012 were identified. Following exclusion of duplicate records for same admission (n = 92), conversion to open surgery (n = 1453), multiple laparoscopic procedures (n = 1803), and multiple records over time (n = 2393), 166,790 patients with laparoscopic procedures were identified. Of these patients, 1458 of them (0.87 %) underwent RAS.
Preoperative, intraoperative and postoperative variables were recorded. Preoperative variables consisted of patient demographics, health system variables including insurance type, and comorbid conditions as defined by the Elixhauser classification [10]. Obesity and weight loss were excluded as comorbidities as they defined the condition under investigation for two procedures. Postoperative variables included length of stay and postoperative complications, which were identified using ICD-9 codes (Table 2).
Statistical analysis
Student’s t tests were used to compare patients’ age and HLOS between groups. Fisher’s exact tests and Chi-square tests with exact p value based on Monte Carlo simulation were used to compare categorical variables between groups, when applicable. Propensity score (PS) analysis was used to estimate the marginal (population average) differences between patients who underwent robotic-assisted and laparoscopic procedures [11–13]. For comparing complications (any complication), all the patient characteristics, comorbidities, surgery year, and region were used to estimate PS according to a logistic regression model and having robot-assisted procedure (yes/no) was the response variable. For comparing hospital length of stay (HLOS), all the patient characteristics, comorbidities, surgery year, region, and complications were used to estimate PS according to a similar logistic regression model with having robot-assisted procedure (yes/no) as the response variable. Propensity-based matching was used to create samples of patients who were similar in terms of propensity score, i.e., in terms of probability of getting the robotic-assisted surgery. Unmatched observations were discarded, thus leading to possibly non-representative samples of the original database. However, because the patients analyzed were matched on many confounders simultaneously, such analyses are likely to provide a more valid estimate of the treatment effect. In our study, almost all patients who underwent the robotic-assisted surgery had a matched patient who underwent a laparoscopic procedure. A 1:1 matching algorithm without replacement was used, where all treated patients were matched to the closest control within a range of 0.20 standard deviations of the logit of the estimated propensity score [14]. The success of the propensity score matching was assessed by checking standardized differences between groups before and after matching, i.e., the absolute difference in sample means divided by an estimate of the pooled standard deviation of the variable, expressed as a percentage [12]. If the standardized differences were <20 %, the difference in matched samples was considered as minimal. McNemar’s test was carried out for any complication, and paired t test was carried out for comparing LOS using PS-matched pairs. Sensitivity analysis for PS matching was carried out to determine the potential impact of unmeasured confounding variables on the significance of the observed treatment effect. The range of significant levels were in the context of McNemar’s test and permutational t test [11, 15, 16].
To analyze any complication, there were 186 successfully matched pairs for cholecystectomy, 307 successfully matched pairs for RYGB, 117 successfully matched pairs for SG, 288 successfully matched pairs for esophageal fundoplication, and 559 successfully matched pairs for colectomy. To analyze patients’ HLOS, there were 186 successfully matched pairs for cholecystectomy, 306 successfully matched pairs for RYGB, 117 successfully matched pairs for SG, 285 successfully matched pairs for esophageal fundoplication, and 559 successfully matched pairs for colectomy. In each matched pair, two patients shared similar characteristics. The standardized differences between groups before and after matching were <20 % for all procedures. That is, the difference in matched samples was considered as minimal. p value <0.05 was considered statistically significant, and analysis was performed using SAS 9.3 (SAS Institute Inc., Cary, NC) and R (R Foundation for Statistical Computing, Vienna, Austria).
Results
Of the 166,790 patients, 1458 (0.87 %) underwent robotic-assisted surgery and 165,332 (99.13 %) underwent laparoscopic approaches to the five procedures during years 2008–2012. Of the 1458 robotic cases, 186 (12.8 %) were cholecystectomy, 307 were RYGB (21.1 %), 118 (8.1 %) were SG, 288 (19.8 %) were esophageal fundoplication, and 559 (38.3 %) were colectomy. The descriptive results of patients’ characteristics, such as patients’ gender, race, source of payment, region, year of surgery, by procedure types are reported in Table 3. Patients’ comorbidities by procedure types are reported in Table 4. Initial univariate analysis showed a significantly higher rate of overall complications and HLOS in the laparoscopic group compared to the robotic-assisted group (19.28 vs 16.32 %, p value = 0.0041 and 5.18 vs 3.92 days, p value <0.0001). Upon further analysis comparing within procedures, laparoscopic colectomy had a significantly higher rate of complication and longer HLOS compared to the robotic approach (32.26 vs 22.9 % p value <0.0001 and 6.76 vs 5.11 days, p value <0.0001). No difference in complications or HLOS was seen in the cholecystectomy group (20.59 vs 20.43 %, p value = 1 and 4.92 vs 5.7 days, p value = 0.2371); RYGB group (6.32 vs 4.23 %, p value = 0.1557 and 2.49 vs 2.5 days, p value = 0.9197); SG group (4.97 vs 4.24 %, p value = 1 and 2.33 vs 2.37 days, p value = 0.64); EF group (14.87 vs 18.75 %, p value = 0.0887 and 3.35 vs 3.13 days, p value = 0.3737) (Table 5).
Following 1:1 matched pairs, among the 559 matched pairs of colectomy patients, 86 patients who underwent robotic-assisted surgery had at least one complication while their matched patients did not, while 132 patients who underwent robotic-assisted surgery did not get complications while their matched patients did. Thus, according to McNemar’s test, robotic-assisted colectomy had a significantly lower rate of complications compared to those who underwent conventional laparoscopic procedures (p value = 0.0022, Table 6). Sensitivity analysis for PS matching was carried out to determine the potential impact of unmeasured confounding variables (hidden bias) on the significance of the observed difference in complications after colectomy: If there was an unmeasured binary variable that increased the odds of treatment by 15 % and this variable was almost perfectly associated with complication, the p value would be at most 0.042. However, if there was an unmeasured binary variable that increased the odds of treatment by 25 %, then p value could be as large as 0.156. No statistical significance was seen in terms of HLOS and complications for cholecystectomy (p value = 0.3409 and 0.9144, respectively), RYGB (p value = 0.5495 and 0.2005, respectively), EF (p value = 0.3742 and 0.3332, respectively). SG did not show any significant increase in complications between laparoscopic and RAS groups (p value = 0.7744); however, RAS SG had a significantly higher HLOS compared to laparoscopic SG (estimated difference is 0.33 day, p value = 0.0037). For this difference in SG, if there was an unmeasured that increased the odds of treatment by 25 % and this variable was almost perfectly associated with HLOS, the p value would be at most 0.0399. However, if there was an unmeasured binary variable that increased the odds of treatment by 30 %, then p value could be as large as 0.0555.
Discussion
Robotic surgery represents an exciting innovation in the area of minimally invasive surgery. Our study compares laparoscopic and RAS among five different general surgical procedures. Between 2008 and 2012, through the use of the SPARCS database, we were able to identify all patients undergoing five common surgical procedures: RYGB, SG, EF, colectomy, and cholecystectomy. When comparing procedures performed through laparoscopic approach to those performed with the assistance of a robot, initial univariate analysis showed a significantly higher rate of overall complications and HLOS in the laparoscopic group compared to the robotic group. However, additional analysis comparing separate procedures did not show any significant difference for cholecystectomy, RYGB, SG, and EF, in terms of complications. Interestingly, laparoscopic colectomy had significantly higher rate of complications and higher HLOS (p value <0.001 for both). Following propensity score analysis, the rate of complications remained significant for colectomy (p value = 0.0022), although there was no effect on the HLOS (p value = 0.3378). In addition, following PS analysis RAS performed in cholecystectomy, RYGB, SG, and EF were not associated with higher rate of complication. Robotic SG had a longer HLOS (p value = 0.0037).
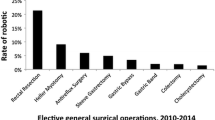
Penetrance of laparoscopy in colorectal surgery has been significantly lower than other general surgical fields and could possibly explain our findings. A study examining clinical data between 2007 and 2009 from the University Health System Consortium database reported overall utilization of laparoscopy in patients undergoing colon and rectal resections to be only 14.8 % [6]. Another study reported a rate of 11.8 and 8.9 % in 2005–2007 for benign disease and colon cancer, respectively [7]. In 2011, laparoscopic utilization had significantly increased to 42.2 % of cases in one report. However, there was a 15.8 % conversion rate to an open procedure [8]. Most recently, data from 2008 to 2012 from academic centers reported a 52.4 and 18.3 % rate of laparoscopic use for colectomy and rectal resection, respectively [17]. This rate of laparoscopic use was compared to other fields of surgery, as the authors reported 94.0 % for bariatric use, 83.7 % for antireflux surgery, 79.2 % for appendectomy, and 77.1 % for cholecystectomy. In addition, there was a high rate of conversion from laparoscopic to open surgery for colorectal resection [17]. Thus, although there is a significant increase in the penetrance of laparoscopy, the number of surgeries performed laparoscopically in the colorectal field is still relatively low. Surgeon’s laparoscopic experience and learning curve may have an effect on outcomes. This may contribute to our findings of higher complications following laparoscopic colorectal surgery. In addition, the technical aspects of colorectal surgery require concise movements in a very small space. The use of the robot may provide a better platform for these tight anatomic situations, thus leading to decreased complication rates. This may also correlate with the success of robotic platforms for gynecologic and urologic surgery.
Similar to our study, others have compared common laparoscopic, general, and bariatric procedures to their RAS counterpart. Only one study, to our knowledge, similarly examined several procedures. Villamere et al. [18] examined the University Health System Consortium to identify and compare laparoscopic versus robotic techniques for common elective procedures: gastric bypass, sleeve gastrectomy, gastric band, antireflux surgery, Heller myotomy, cholecystectomy, colectomy, and rectal resection. The authors did not observe any clinical benefits associated with the robotic approach.
There are several limitations of our study. The main limitation is inherent to the use of an administrative database, as there is a potential for coding errors. In addition, the data obtained are not clinically rich. The complications we have examined are within the immediate postoperative period; thus, any postoperative complications or returns to the emergency department and readmissions are not included. Finally, we could not compare costs of laparoscopic versus RAS surgery, as the SPARCS dataset reports charges and not costs. However, there has been a significant amount of literature showing that RAS is associated with higher costs compared to laparoscopy. The strengths of this study include the large sample size and uniqueness of the study, as it compares several general surgery and bariatric procedures in the state of New York.
Conclusion
Our study compared complications and HLOS between five common general surgeries in terms of laparoscopic versus RAS methods. RAS exhibited non-superiority to laparoscopy in many common laparoscopic procedures in terms of complications and HLOS. Our results, however, support a possible benefit of robotics over conventional laparoscopy in colorectal surgery, where penetrance of conventional laparoscopy is low and complication rates are relatively high. Our study supports the notion that robotic approaches may facilitate safer adoption of minimally invasive approaches in these areas.
References
Mühe E (1991) Laparoscopic cholecystectomy—late results. Langenbecks Arch Chir Suppl Kongressbd. http://www.ncbi.nlm.nih.gov/pubmed/1838946
Wei HB, Wei B, Qi CL et al (2011) Laparoscopic versus open gastrectomy with D2 lymph node dissection for gastric cancer: a meta-analysis. Surg Laparosc Endosc Percutan Tech 21(6):283–390
Nguyen NT, Masoomi H, Magno CP, Nguyen XM, Laugenour K, Lane J (2011) Trends in use of bariatric surgery, 2003–2008. J Am Coll Surg 213(2):261–266
Bliss LA, Yang CJ, Kent TS, Ng SC, Critchlow JF, Tseng JF (2014) Appendicitis in the modern era: universal problem and variable treatment. Surg Endosc 29(7):1897–1902
Csujesz N, Ricciardi R, Tseng JF, Shah SA (2008) Current status of surgical management of acute cholecystitis in the United States. World J Surg 32:2230–2236
Carmichael JS, Masoomi H, Mills S, Stamos MJ, Nguyen MD (2011) Utilization of laparoscopy in colorectal surgery fro cancer at academic medical centers: does site of surgery affect rate of laparoscopy? Am Surg 77(10):1300–1304
Rea JD, Cone MM, Diggs BS, Deveney KE, Lu KC, Herzig DO (2011) Utilization of laparoscopic colectomy in the United States before and after the clinical outcomes of surgical therapy study group trial. Ann Surg 254(2):281–288
Simorov A, Shaligram A, Shostrom V, Boilesen E, Thompson J, Oleynikoc D (2012) Laparoscopic colon resection trends in utilization and rate of conversion to open procedure. Ann Surg 256(3):462–468
Cirocchi R, Partelli S, Trastulli S et al (2013) A systematic review on robotic pancreaticoduodenectomy. Surg Oncol 22:238–246
Elixhauser A, Syeiner C, Harris D (1998) Co-morbidity measures for use with administrative data. Med Care 36:8–27
Rosenbaum PR, Rubin DB (1983) The central role of propensity score in observational studies for causal effects. Biometrika 70:41–55
Faries DE, Leon AC, Haro JM, Obenchain RL (2010) Analysis of observational health care data using SAS. SAS Institute Inc., Cary, NC
Austin PC, Grootendorst P, Anderson GM (2007) A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med 26:734–753
Austin PC (2009) Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations. Biom J 51:171–184
Rosenbaum PR (1995) Observational studies. Springer, New York
Rosenbaum PR (2007) Sensitivity analysis for m-estimates, tests and confidence intervals in matched observational studies. Biometrics 63:456–464
Nguyen NT, Nguyen B, Shih A, Smith B, Hofmann S (2013) Use of laparoscopy in general surgical operations at academic centers. Surg Obes Relat Dis 9:15–20
Villamere J, Gebhard A, Vu S, Nguyen NT (2014) Utilization and outcome of laparoscopic versus robotic general and bariatric surgical procedures at Academic Medical Centers. Surg Endosc 29(7):1729–1736
Acknowledgments
We would like to thank the Foundation for Surgical Fellows for their support of our fellowship program. We also acknowledge the biostatistical consultation and support from the Biostatistical Consulting Core at the School of Medicine, Stony Brook University.
Disclosures
Dr. Pryor has Ownership interest in Transenterics, speaker for Gore and Novadaq, and consults for Freehold Medical and Intuitive. Dr. Telem is a consultant for Ethicon, speaker for Gore, and receives research funding from Cook. Dr. Altieri, Dr. Talamini, Dr. Yang, Dr. Halbert, Ms. Zhu have no conflict of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Altieri, M.S., Yang, J., Telem, D.A. et al. Robotic approaches may offer benefit in colorectal procedures, more controversial in other areas: a review of 168,248 cases. Surg Endosc 30, 925–933 (2016). https://doi.org/10.1007/s00464-015-4327-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-015-4327-2