Abstract
Background
Safety of endoscopic resection (ER) for early gastric cancers (EGC) with mixed histology predominantly of differentiated type has not been securely established, since those lesions tend to exhibit lymph node metastasis, compared to pure differentiated type. The purpose of this study was to evaluate clinicopathologic characteristics, therapeutic outcomes, and risk for lymph node metastasis in predominantly differentiated mixed EGC treated by ER.
Methods
A total of 1,016 patients with 1,039 EGCs underwent ER between January 2007 and June 2013. Enrolled lesions were divided into groups of either pure differentiated (n = 1,011) or predominantly differentiated mixed (n = 28), according to the presence of mixed histology predominantly of differentiated type in ER specimen.
Results
Mixed histology predominantly of differentiated type was diagnosed in 2.7 % of lesions. Larger size, mid-third location, and moderately differentiated histology on forceps biopsy were independent risk factors for the predominantly differentiated mixed histologic type of EGC in multivariate analysis. En bloc resection rate tended to be lower, and complete and curative resection rates were significantly lower in the predominantly differentiated mixed group. The rate of lymph node metastasis in the lesions with additional operation tended to be higher, in this mixed histology group.
Conclusions
Larger size, mid-third location, and moderately differentiated histology on forceps biopsy carry the significant risk for mixed histology predominantly of differentiated type. EGC with predominantly differentiated mixed histologic type affects therapeutic outcomes and consequent clinical course accompanied by possibly higher risk for lymph node metastasis. The safety of ER for predominantly differentiated mixed EGC should be validated by further prospective investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In eastern countries that have a high prevalence of gastric cancer [1, 2], endoscopic resection (ER), including endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), is a curative treatment option for some early gastric cancers (EGC) [3–6]. Recently, considerable data also have been reported from the Western world as ER is gaining wide acceptance [7]. The standard and expanded indications for ER of EGC primarily include differentiated histologic type [8, 9]. Because EGCs with undifferentiated histology carry an increased risk of lymph node metastasis [9–12], they are only indicated for ESD in extremely limited cases. Undifferentiated EGC must be intramucosal, with no evidence of ulceration, and ≤2 cm in diameter, to be amenable to treatment with ESD [13]. Thus, the precise histologic diagnosis of differentiated versus undifferentiated type is critical for deciding upon treatment: ER versus surgical resection.
However, some gastric tumors include a mixture of differentiated and undifferentiated components. Gastric cancers that include a mixture of differentiated and undifferentiated components are classified according to the predominant histologic type by the World Health Organization classification of gastrointestinal tumors [14]. Earlier surgical series revealed that EGCs with mixed histology predominantly of differentiated type tended to exhibit a higher incidence of nodal invasion than pure differentiated EGCs [15, 16]. Since a very low probability of lymph node metastasis is the most fundamental condition for endoscopic treatment of EGC, the safety of ER for EGC with mixed histology predominantly of differentiated type has not been securely established. Moreover, the feasibility of successful resection of predominantly differentiated mixed EGC by ER has not been proven definitively.
Despite the prognostic importance of mixed histology predominantly of differentiated type in ER for EGC, no studies have ever investigated outcomes of ER for predominantly differentiated mixed EGC, compared to those in pure differentiated EGC. Therefore, the purposes of this study were to evaluate the clinicopathologic characteristics predictive of predominantly differentiated mixed EGC and to assess the clinical implications of predominantly differentiated mixed histologic type in terms of therapeutic outcomes, clinical course after ER, and risk for lymph node metastasis.
Methods
Patients
A total of 1,016 consecutive patients with 1,039 differentiated lesions were enrolled between January 2007 and June 2013 at a high-volume tertiary referral center. All patients underwent ER for EGC, according to standard and expanded indications [13], and lesions were diagnosed as solely well-differentiated (n = 684) or solely moderately-differentiated (n = 327) or predominantly differentiated mixed adenocarcinomas (n = 28) in specimens obtained by ER. Enrolled lesions were categorized into the following two groups, according to the presence of mixed histology predominantly of differentiated type in specimen obtained by ER: pure differentiated (n = 1,011) or predominantly differentiated mixed (n = 28). The mixed histology predominantly of differentiated type consisted of a major differentiated component, and a minor undifferentiated component which was more than 0 % but less than 50 % in the lesion.
We reviewed the clinical, endoscopic, and histologic data which were prospectively collected. Written informed consents explaining possible procedure-related risks, complications, and alternative surgical options, were obtained from all patients before ER. This study was approved by the Institutional Review Board of Yonsei University College of Medicine, Korea.
Histologic diagnosis
A single pathologist in our hospital was involved in the histologic diagnosis in this study. After resection, ER specimens were only minimally stretched to avoid overextension, pinned on a styrofoam board, and immediately immersed in formalin fixative for 4 h. Fixed specimens were sectioned serially at 2-mm intervals, and entirely embedded in paraffin. Histologic diagnoses were performed based on the World Health Organization classification of gastrointestinal tumors [14, 17]. The histologic diagnoses were divided into two categories, based on the Japanese Classification of Gastric Carcinoma, as either differentiated or undifferentiated histologic type [18]. The differentiated histologic type included well-differentiated adenocarcinoma, moderately differentiated adenocarcinoma, and papillary adenocarcinoma. The undifferentiated histologic type consisted of poorly differentiated adenocarcinoma, signet-ring cell carcinoma, and mucinous adenocarcinoma. When a tumor consisted of more than one histologic type, a diagnosis of mixed histology was documented, with differentiation of major and minor components and their respective proportions.
Clinicopathologic characteristics
The size was based on pathologic size, with classification of no larger than 20 mm and larger than 20 mm. The location of lesions was divided into three sections by categorization of the longitudinal axis of the stomach (upper third containing the fundus, cardia, and upper body; mid-third containing the mid-body, lower body, and angle; and lower third containing the antrum and pylorus) and four sections by categorization of the cross-sectional circumference of the stomach (lesser curvature, posterior wall, greater curvature, and anterior wall). Macroscopic type was determined according to the macroscopic classification of EGC by the Japanese gastric cancer association [18]. In addition, macroscopic types were grouped as elevated, flat, or depressed types [19]. Submucosal invasion was evaluated by the endoscopic ultrasound (EUS) in a selected group of patients (572/1,039, 55.1 %) and was confirmed by histologic diagnosis.
Endoscopic resection
ER methods included EMR (including the injection-and-cut technique, EMR with the cap technique, and EMR by a snare after circumferential precutting with a knife) and ESD. The details of ER were described previously [19].
Therapeutic outcomes
The therapeutic outcomes of ER were classified as en bloc resection, complete resection, or curative resection. En bloc resection was defined as resection in a one-piece fashion, with no evidence of residual tumor by endoscope. Complete resection was defined as en bloc resection, with no evidence of cancer cells at any cut end and no lymphovascular invasion [20]. Incomplete resection was defined as resection that did not meet the complete resection criteria. Curative resection was defined as complete resection with no submucosal invasion deeper than 500 μm from the muscularis mucosa [21]. Non-curative resection was defined as resection that did not meet the curative resection criteria.
Statistical analysis
The χ 2 test or Fisher’s exact test was used to test categorical data. The Student’s t test or Mann–Whitney U test was used for non-categorical data. Multivariate logistic regression analysis was performed to assess the relationship of clinicopathological characteristics and mixed histology predominantly of differentiated type. Characteristics with a univariate significance of P < 0.05 were candidates for multivariate analysis. The exception was submucosal invasion of the tumor determined by EUS, because EUS was only performed in a subpopulation of patients. P < 0.05 was considered statistically significant. Statistical analysis was performed using the SAS program (version 9.2, SAS Institute, Cary, NC, USA).
Results
Baseline characteristics of patients
A total of 1,016 patients with 1,039 EGCs (795 men and 244 women; mean age, 59.5 ± 9.8 years) were treated by ER. Specifically, 18 EMRs and 1,021 ESDs were performed. Mixed histology predominantly of differentiated type was diagnosed in 2.7 % (28/1,039) of lesions. The minor components in the predominantly differentiated mixed group included poorly differentiated adenocarcinomas (n = 7), signet-ring cell carcinomas (n = 15), and mucinous adenocarcinomas (n = 6).
The baseline characteristics of lesions are shown in Table 1. There were no significant differences in baseline characteristics between the pure differentiated group and the predominantly differentiated mixed group, including sex, alcohol, smoking, comorbidities, use of antiplatelets, and use of anticoagulation, with the exception of younger age in the predominantly differentiated mixed group.
Clinicopathological characteristics related to mixed histology predominantly of differentiated type
Univariate analysis of clinicopathological features between the two groups is listed in Table 2. Larger size (P < 0.001), location in the mid-third of the stomach (P = 0.013), submucosal invasion determined by EUS (P = 0.019), and moderately differentiated histology in specimens obtained by forceps biopsy (P = 0.001) were significantly associated with the predominantly differentiated mixed group, compared with the pure differentiated group.
In a multivariate analysis, larger size (OR = 3.96, 95 % CI 1.99–7.88, P < 0.001), mid-third location (OR = 2.64, 95 % CI 1.28–5.45, P = 0.009), and moderately differentiated histology on forceps biopsy (OR = 3.59, 95 % CI 1.77–7.26, P < 0.001) were independent risk factors for the mixed histology predominantly of differentiated type (Table 3).
We assessed characteristics of the endoscopic procedure, including procedure time during ER and complications of ER. The overall median ER time was 50 min (range 8–300 min). The overall complication rates for bleeding and perforation were 4.8 % (50/1,039) and 3.5 % (36/1,039), respectively. There were no significant differences between the two groups in terms of the ER procedure time and bleeding and perforation rates.
Therapeutic outcomes
The therapeutic outcomes in the two groups are summarized in Table 4. The overall en bloc resection, complete resection, and curative resection rates were 93.3 % (969/1,039), 85.8 % (891/1,039), and 82.2 % (854/1,039), respectively. The predominantly differentiated mixed group tended to have a lower en bloc resection rate (P = 0.114). Significantly lower rates of complete resection (P = 0.003) and curative resection (P = 0.009) were detected in the predominantly differentiated mixed group, compared with the pure differentiated group. Evidence of cancer invasion at the lateral cut end (P = 0.037) and lymphovascular invasion (P = 0.018) were also statistically higher in the predominantly differentiated mixed group, compared to the pure differentiated group.
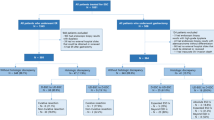
The overall clinical course of patients is shown in Fig. 1. Non-curative resection was performed on 17.8 % (185/1,039) of lesions and consisted of 174 lesions from the pure differentiated group and 11 lesions from the predominantly differentiated mixed group. Of the 185 lesions that had non-curative resection, 57.3 % of lesions (106/185) underwent additional operations after ER. The rates of additional operation due to non-curative resection were 9.8 % (99/1,011) and 25.0 % (7/28) in the two groups, respectively, which was significantly different (P = 0.018). Of the 106 lesions with additional operations due to non-curative resection, 7.5 % of lesions (8/106) exhibited lymph node metastasis. The rates of lymph node metastasis in the lesions with additional operation were 6.1 % (6/99) and 28.6 % (2/7) in the two groups, respectively. The predominantly differentiated mixed group tended to exhibit higher nodal invasion (P = 0.087).
Discussion
The present study focused on the clinicopathologic characteristics predictive of predominantly differentiated mixed histology, and the therapeutic outcomes and risk for lymph node metastasis of predominantly differentiated mixed EGC treated by ER.
Mixed histology predominantly of differentiated type in ER specimens was diagnosed in 2.7 % (28/1,039) of lesions in the study. In contrast, earlier studies using surgical specimens revealed that EGCs with predominantly differentiated mixed histology were 13.2 % of intramucosal cancers and 27.7 % of submucosal cancers [15, 16]. We attributed these discrepancies to different inclusion criteria between our study and previous surgical studies, because, to date, the indications for ER have only been applied to a limited subset of EGC patients. Moreover, the clinicopathologic features associated with mixed histology predominantly of differentiated type has not been proven definitively by these studies.
In the present study, larger size, mid-third location of tumor, and moderately differentiated histology on forceps biopsy were significantly associated with mixed histology predominantly of differentiated type in multivariate analysis. This was consistent with earlier studies which showed that larger size and a moderately differentiated major component correlated with mixed histology in EGC treated with surgery [15, 22, 23]. Nonetheless, these studies failed to distinguish the predominantly differentiated mixed versus predominantly undifferentiated mixed histologic types in EGCs with mixed histology [22, 23]. In the current study, the association of larger size with predominantly differentiated mixed histologic type may be due to an increased chance of developing a tumor with a heterogeneous composition of malignant cells in larger malignant lesions [24]. Furthermore, signet-ring cell carcinoma was the most common minor component (53.6 %, 15/28) in our study. This finding may contribute to the associations of younger age and mid-third location with the predominantly differentiated mixed group [25, 26].
To date, therapeutic outcomes of predominantly differentiated mixed EGC have not been evaluated. The en bloc resection, complete resection, and curative resection rates in the predominantly differentiated mixed group were 85.7 % (24/28), 64.3 % (18/28), and 60.7 % (17/28), respectively. These results were comparable to the reported therapeutic outcomes of ER for undifferentiated EGC of 83.1–100 % for en bloc resection, 55.0–90.7 % for complete resection, and 31.1–82.5 % for curative resection [19, 20, 27–32]. In addition, our data were also compatible with the treatment outcomes of ER for EGC with undifferentiated type in our center which ranged from 64.3 to 67.2 % in the earlier studies [19, 33]. However, outcomes in our study were much worse than the outcomes of ER for differentiated EGC of 86.1–97.0 % for en bloc resection, 88.9–93.4 % for complete resection, and 91.3–94.7 % for curative resection [21, 34–37]. The outcomes of the predominantly differentiated mixed group were also significantly lower than those of the pure differentiated group. We attributed these unsatisfactory outcomes in the predominantly differentiated mixed group to higher lateral cut end-positivity for cancer cells. This may be due to an ill-defined marginal delineation and intramucosal lateral spread originating from distinct biologic features of the undifferentiated type [38, 39]. Moreover, the limited success of outcomes also arose from higher lymphovascular invasion because this mixed histology group showed the larger size and the more frequent tumor cell extension to the lymphatic-rich submucosal layer [38, 40].
Consequently, 25.0 % of patients in the predominantly differentiated mixed group underwent additional operations after non-curative ER to treat residual tumor and lymph nodes, whereas only 9.8 % of patients with pure differentiated lesions had additional operations. Some part of patients with non-curative resection did not undergo surgery, because of advanced age, high comorbidities, and patients’ choice to refuse further surgical resection. Moreover, 28.6 % of predominantly differentiated mixed EGCs with additional operation exhibited lymph node metastasis, while only 6.1 % of pure differentiated lesions with additional operation had nodal invasion. This increasing tendency of nodal involvement in the predominantly differentiated mixed group is consistent with earlier studies [15, 16]. Thus, the safety of ER for EGC with mixed histology predominantly of differentiated type should be investigated further, based on large-scale prospective data on lymph node invasion and subsequent long-term outcomes, in addition to studies on enhancing the feasibility of complete and curative resections.
This study had some limitations. First, the number of patients with predominantly differentiated mixed EGC was relatively small. Second, the evaluation of lymph node metastasis from limited patients with additional operation after ER also can be a limitation to conclude the potential for lymph node metastasis in predominantly differentiated mixed EGC. Lastly, EUS was evaluated in selected patients only. Therefore, selection bias may have affected the study results.
Despite these limitations, the present study has some merits. This is the first comparative study of the therapeutic outcomes and clinical courses of endoscopically resected EGCs with pure differentiated versus predominantly differentiated mixed histologic types. Moreover, this study suggests several clinicopathologic features predictive of mixed histology predominantly of differentiated type, which results in lower complete and curative resection rates of ER.
In conclusion, EGC with mixed histology predominantly of differentiated type affects therapeutic outcomes and consequent clinical course accompanied by possibly higher risk for lymph node metastasis. We should pay particular attention to the possibility of predominantly differentiated mixed histologic type if an EGC lesion exhibits larger size, mid-third location, and moderately differentiated histology on forceps biopsy. The further prospective studies on safety of ER for predominantly differentiated mixed EGC are warranted.
Abbreviations
- EGC:
-
Early gastric cancer
- ER:
-
Endoscopic resection
- EMR:
-
Endoscopic mucosal resection
- ESD:
-
Endoscopic submucosal dissection
- EUS:
-
Endoscopic ultrasound
References
Ahn YO, Park BJ, Yoo KY, Kim NK, Heo DS, Lee JK, Ahn HS, Kang DH, Kim H, Lee MS et al (1991) Incidence estimation of stomach cancer among Koreans. J Korean Med Sci 6(1):7–14
Nakamura K, Ueyama T, Yao T, Xuan ZX, Ambe K, Adachi Y, Yakeishi Y, Matsukuma A, Enjoji M (1992) Pathology and prognosis of gastric carcinoma. Findings in 10,000 patients who underwent primary gastrectomy. Cancer 70(5):1030–1037
Tada M, Murakami A, Karita M, Yanai H, Okita K (1993) Endoscopic resection of early gastric cancer. Endoscopy 25(7):445–450
Hirao M, Masuda K, Asanuma T, Naka H, Noda K, Matsuura K, Yamaguchi O, Ueda N (1988) Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline-epinephrine. Gastrointest Endosc 34(3):264–269
Gotoda T, Kondo H, Ono H, Saito Y, Yamaguchi H, Saito D, Yokota T (1999) A new endoscopic mucosal resection procedure using an insulation-tipped electrosurgical knife for rectal flat lesions: report of two cases. Gastrointest Endosc 50(4):560–563
Ohkuwa M, Hosokawa K, Boku N, Ohtu A, Tajiri H, Yoshida S (2001) New endoscopic treatment for intramucosal gastric tumors using an insulated-tip diathermic knife. Endoscopy 33(3):221–226
Probst A, Pommer B, Golger D, Anthuber M, Arnholdt H, Messmann H (2010) Endoscopic submucosal dissection in gastric neoplasia: experience from a European center. Endoscopy 42(12):1037–1044
Soetikno RM, Gotoda T, Nakanishi Y, Soehendra N (2003) Endoscopic mucosal resection. Gastrointest Endosc 57(4):567–579
Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y (2000) Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 3(4):219–225
Yamao T, Shirao K, Ono H, Kondo H, Saito D, Yamaguchi H, Sasako M, Sano T, Ochiai A, Yoshida S (1996) Risk factors for lymph node metastasis from intramucosal gastric carcinoma. Cancer 77(4):602–606
Balasubramanian SP (2001) Evaluation of the necessity for gastrectomy with lymph node dissection for patients with submucosal invasive gastric cancer (Br J Surg 2001; 88: 444–9). Br J Surg 88(8):1133–1134
Kurihara N, Kubota T, Otani Y, Ohgami M, Kumai K, Sugiura H, Kitajima M (1998) Lymph node metastasis of early gastric cancer with submucosal invasion. Br J Surg 85(6):835–839
Japanese Gastric Cancer Association (2011) Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 14(2):113–123
Hamilton SR, Aaltonen AL (eds) (2000) Pathology and genetics of tumours of the digestive system. World Health Organization Classification of Tumours. IARC Press, Lyon
Takizawa K, Ono H, Kakushima N, Tanaka M, Hasuike N, Matsubayashi H, Yamagichi Y, Bando E, Terashima M, Kusafuka K, Nakajima T (2012) Risk of lymph node metastases from intramucosal gastric cancer in relation to histological types: how to manage the mixed histological type for endoscopic submucosal dissection. Gastric Cancer. doi:10.1007/s10120-012-0220-z
Hanaoka N, Tanabe S, Mikami T, Okayasu I, Saigenji K (2009) Mixed-histologic-type submucosal invasive gastric cancer as a risk factor for lymph node metastasis: feasibility of endoscopic submucosal dissection. Endoscopy 41(5):427–432
Kim WH, Park CK, Kim YB (2005) A standardized pathology report for gastric cancer. Korean J Pathol 39:106–113
Japanese Gastric Cancer Association (2011) Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 14(2):101–112
Kim JH, Lee YC, Kim H, Song KH, Lee SK, Cheon JH, Kim H, Hyung WJ, Noh SH, Kim CB, Chung JB (2009) Endoscopic resection for undifferentiated early gastric cancer. Gastrointest Endosc 69(4):e1–e9
Kang HY, Kim SG, Kim JS, Jung HC, Song IS (2010) Clinical outcomes of endoscopic submucosal dissection for undifferentiated early gastric cancer. Surg Endosc 24(3):509–516
Isomoto H, Shikuwa S, Yamaguchi N, Fukuda E, Ikeda K, Nishiyama H, Ohnita K, Mizuta Y, Shiozawa J, Kohno S (2009) Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut 58(3):331–336
Saito A, Shimoda T, Nakanishi Y, Ochiai A, Toda G (2001) Histologic heterogeneity and mucin phenotypic expression in early gastric cancer. Pathol Int 51(3):165–171
Huh CW, da Jung H, Kim JH, Lee YC, Kim H, Kim H, Yoon SO, Youn YH, Park H, Lee SI, Choi SH, Cheong JH, Noh SH (2013) Signet ring cell mixed histology may show more aggressive behavior than other histologies in early gastric cancer. J Surg Oncol 107(2):124–129
Zheng HC, Li XH, Hara T, Masuda S, Yang XH, Guan YF, Takano Y (2008) Mixed-type gastric carcinomas exhibit more aggressive features and indicate the histogenesis of carcinomas. Virchows Arch 452(5):525–534
Kunisaki C, Shimada H, Nomura M, Matsuda G, Otsuka Y, Akiyama H (2004) Therapeutic strategy for signet ring cell carcinoma of the stomach. Br J Surg 91(10):1319–1324
Kwon KJ, Shim KN, Song EM, Choi JY, Kim SE, Jung HK, Jung SA (2013) Clinicopathological characteristics and prognosis of signet ring cell carcinoma of the stomach. Gastric Cancer. doi:10.1007/s10120-013-0234-1
Okada K, Fujisaki J, Yoshida T, Ishikawa H, Suganuma T, Kasuga A, Omae M, Kubota M, Ishiyama A, Hirasawa T, Chino A, Inamori M, Yamamoto Y, Yamamoto N, Tsuchida T, Tamegai Y, Nakajima A, Hoshino E, Igarashi M (2012) Long-term outcomes of endoscopic submucosal dissection for undifferentiated-type early gastric cancer. Endoscopy 44(2):122–127
Kim YY, Jeon SW, Kim J, Park JC, Cho KB, Park KS, Kim E, Chung YJ, Kwon JG, Jung JT, Kim EY, Kim KO, Jang B, Lee SH, Yang CH (2013) Endoscopic submucosal dissection for early gastric cancer with undifferentiated histology: could we extend the criteria beyond? Surg Endosc. doi:10.1007/s00464-013-3099-9
Abe S, Oda I, Suzuki H, Nonaka S, Yoshinaga S, Odagaki T, Taniguchi H, Kushima R, Saito Y (2013) Short- and long-term outcomes of endoscopic submucosal dissection for undifferentiated early gastric cancer. Endoscopy 45(9):703–707
Park J, Choi KD, Kim MY, Lee JH, Song HJ, Lee GH, Jung HY, Kim JH (2012) Is endoscopic resection an acceptable treatment for undifferentiated EGC? Hepatogastroenterology 59(114):607–611
Kamada K, Tomatsuri N, Yoshida N (2012) Endoscopic submucosal dissection for undifferentiated early gastric cancer as the expanded indication lesion. Digestion 85(2):111–115
Yamamoto Y, Fujisaki J, Hirasawa T, Ishiyama A, Yoshimoto K, Ueki N, Chino A, Tsuchida T, Hoshino E, Hiki N, Fukunaga T, Sano T, Yamaguchi T, Takahashi H, Miyata S, Yamamoto N, Kato Y, Igarashi M (2010) Therapeutic outcomes of endoscopic submucosal dissection of undifferentiated-type intramucosal gastric cancer without ulceration and preoperatively diagnosed as 20 millimetres or less in diameter. Dig Endosc 22(2):112–118
Kim MN, Kim HK, Shim CN, Lee HJ, Lee H, Park JC, Shin SK, Lee SK, Lee YC (2014) Tumour size is related to the curability of signet ring cell early gastric cancer with endoscopic submucosal dissection: a retrospective single centre study. Dig Liver Dis. doi:10.1016/j.dld.2014.05.019
Lee H, Yun WK, Min BH, Lee JH, Rhee PL, Kim KM, Rhee JC, Kim JJ (2011) A feasibility study on the expanded indication for endoscopic submucosal dissection of early gastric cancer. Surg Endosc 25(6):1985–1993
Ahn JY, Jung HY, Choi KD, Choi JY, Kim MY, Lee JH, Choi KS, Kim do H, Song HJ, Lee GH, Kim JH, Park YS (2011) Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc 74(3):485–493
Yamaguchi N, Isomoto H, Fukuda E, Ikeda K, Nishiyama H, Akiyama M, Ozawa E, Ohnita K, Hayashi T, Nakao K, Kohno S, Shikuwa S (2009) Clinical outcomes of endoscopic submucosal dissection for early gastric cancer by indication criteria. Digestion 80(3):173–181
Goto O, Fujishiro M, Kodashima S, Ono S, Omata M (2009) Outcomes of endoscopic submucosal dissection for early gastric cancer with special reference to validation for curability criteria. Endoscopy 41(2):118–122
Nagahama T, Yao K, Maki S, Yasaka M, Takaki Y, Matsui T, Tanabe H, Iwashita A, Ota A (2011) Usefulness of magnifying endoscopy with narrow-band imaging for determining the horizontal extent of early gastric cancer when there is an unclear margin by chromoendoscopy (with video). Gastrointest Endosc 74(6):1259–1267
Sawada S, Fujisaki J, Yamamoto N, Kato Y, Ishiyama A, Ueki N, Hirasawa T, Yamamoto Y, Tsuchida T, Tatewaki M, Hoshino E, Igarashi M, Takahashi H, Fujita R (2010) Expansion of indications for endoscopic treatment of undifferentiated mucosal gastric cancer: analysis of intramucosal spread in resected specimens. Dig Dis Sci 55(5):1376–1380
Sako A, Kitayama J, Ishikawa M, Yamashita H, Nagawa H (2006) Impact of immunohistochemically identified lymphatic invasion on nodal metastasis in early gastric cancer. Gastric Cancer 9(4):295–302
Conflict of interest
There are no financial or other conflicts of interest to disclose.
Disclosures
Choong Nam Shim, Hyunsoo Chung, Jun Chul Park, Hyuk Lee, Sung Kwan Shin, Sang Kil Lee, Yong Chan Lee have disclosed no financial relationships relevant to this publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shim, C.N., Chung, H., Park, J.C. et al. Early gastric cancer with mixed histology predominantly of differentiated type is a distinct subtype with different therapeutic outcomes of endoscopic resection. Surg Endosc 29, 1787–1794 (2015). https://doi.org/10.1007/s00464-014-3861-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-014-3861-7