Abstract
Dysphagia or swallowing dysfunction is common in patients with acute or critical illness, and diverse methods of dysphagia rehabilitation are provided worldwide. We aimed to examine the efficacy of rehabilitation to treat dysphagia in patients with acute or critical illness. We searched PubMed, ICHUSHI, and Cochrane Central Register of Controlled Trials databases from inception to November 22, 2023 for relevant randomized controlled trials. We focused on dysphagic patients with acute or critical illness who were not orotracheally intubated. Our target intervention included conventional rehabilitation and nerve stimulation/neuromodulation techniques as dysphagia rehabilitation. Comparators were conventional or standard care or no dysphagia interventions. Primary outcomes included mortality, incidence of pneumonia during the study period, and health-related quality of life (HRQoL) scores within 90 days of hospital discharge. We pooled the data using a random-effects model, and classified the certainty of evidence based on the Grading of Recommendations, Assessment, Development, and Evaluation system. Nineteen randomized controlled trials involving 1,096 participants were included. Dysphagia rehabilitation was associated with a reduced incidence of pneumonia (risk ratio [RR], 0.66; 95% confidence interval [CI], 0.54–0.81; moderate certainty), but not with reduced mortality (RR, 0.92; 95% CI, 0.61–1.39; very low certainty) or improved HRQoL scores (mean difference, -0.20; 95% CI, -20.34 to 19.94; very low certainty). Based on the available moderate- or very low- quality evidence, while dysphagia rehabilitation had no impact on mortality or HRQoL, they might reduce the incidence of pneumonia in dysphagic patients with acute or critical illness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Dysphagia or swallowing dysfunction is common in acute or critical illness with a prevalence of 3–84% [1,2,3,4,5,6]. It is associated with short-term complications, such as aspiration, pneumonia, reintubation, and death [1, 2, 5, 6], prolonged length of stay, increased resource use and costs [5,6,7], as well as long-term consequences like malnutrition and decreased quality of life [3, 8]. The substantial individual and societal burdens necessitate awareness and treatment of dysphagia in acute and critical care settings.
Several mechanisms of dysphagia take place in acute or critical illnesses, which include direct nervous system damage, direct mucosal trauma and dysfunctional sensation in oropharyngeal and laryngeal, direct mucosal injury due to endotracheal or tracheostomy tubes, gastroesophageal reflux, and dyssynchronous breathing and swallowing [1, 2, 6]. Conventional rehabilitation includes postural changes, compensatory maneuvers, and therapeutic exercises, which are provided in addition to dietary texture modification [1, 2, 6]. Furthermore, there is a recent advance in nerve stimulation and neuromodulation techniques that target neuroplasticity [9], which include repetitive transcranial magnetic stimulation (rTMS), pharyngeal electrical stimulation (PES), and transcranial direct current stimulation (tDCS). These techniques are beginning to be used in conjunction with conventional physical rehabilitation [9]. A recent international survey, however, identified a diversity in dysphagia rehabilitation provided in intensive care settings worldwide [10]. To inform evidence-based dysphagia therapy in acute or critical settings, a comprehensive knowledge on the efficacy and safety of dysphagia rehabilitation is needed.
Hence, we conducted a systematic review and meta-analysis on the efficacy and safety of dysphagia rehabilitation in dysphagic patients with acute or critical illness. We focused on conventional rehabilitation and nerve stimulation/neuromodulation techniques as dysphagia rehabilitation.
Methods
The study protocol was registered at the PROSPERO (CRD42022302244). The study is reported in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement [11].
Eligibility Criteria
Type of Studies
We included randomized controlled trials that examined rehabilitation for dysphagia in adult patients with acute or critical illness. Quasi-randomized trials and observational or nonrandomized studies were excluded.
Type of Participants
We included patients who met the following criteria: (1) were aged ≥ 18 years; (2) had critical or acute illness determined below; (3) had newly developed dysphagia as determined by the original study authors, and (4) were not orotracheally intubated as of the study conduct and are able to undergo dysphagia rehabilitation. We operationally defined critical or acute illness as follows: (1) the patients were being treated at intensive care units (ICUs) or acute care units/wards; (2) for patients with newly diagnosed stroke, more than 50% had to be receiving acute stroke care, the average time from stroke onset to initiation of dysphagia rehabilitation was within 30 days [12], and/or were treated at stroke units. Patients who met at least one of the following criteria were excluded: (1) were not being treated at ICUs or acute care units/wards, (2) did not meet our operational criteria of acute or critical illness, (3) were orotracheally intubated or (4) dysphagia rehabilitation was prophylactically provided to patients regarding of whether they were dysphagic.
Type of Interventions
We focused on rehabilitation or approaches from the perspective of physical medicine for dysphagia, which was initiated in acute or critical care settings as determined above. We also considered nerve stimulation interventions or neuromodulation therapy, such as rTMS or tDCS, because they are conducted in conjunction with rehabilitation [9]. We excluded dysphagia interventions not considered as rehabilitation. Particularly, we did not consider acupuncture because it is alternative and complementary medicine and is not considered physical medicine therapy. No restrictions were placed on the dose or intensity of the rehabilitation used. The comparators included conventional or standard care and or no dysphagia rehabilitation. We considered the addition of dysphagia rehabilitation to the treatment given in the comparator group as the intervention.
Type of Outcome Measures
Our primary outcomes were mortality (during hospitalization or within 180 days of inclusion to the study), incidence of pneumonia during the study period, and health-related quality of life (HRQoL) scores within 90 days of hospital discharge. We accepted the definition of pneumonia used in each study.
Secondary outcomes included: (1) activities of daily living measured by the Functional Independence Measure, Barthel Index (BI), or Katz Index within 90 days of hospital discharge; (2) feeding status measured by the Food Intake Level Scale or Functional Oral Intake Scale (FOIS) for Dysphagia within 90 days of hospital discharge; (3) hospital length of stay; and (4) adverse events. We preferentially included adverse events related to the interventions; however, in cases where such outcomes were not reported, we included any serious adverse events.
Search Strategy
We searched PubMed, ICHUSHI (“Igaku CHUo zasSHI” meaning “Medical Central Journals” in Japanese), and Cochrane Central Register of Controlled Trials databases from inception to November 22, 2023. We also reviewed the reference lists of the selected publications to identify potentially relevant studies. We placed no restrictions on languages [13]. The search strategy is presented in Online Resource 1.
Study Selection
The first author (A.K.) and two other authors (S.W. and Y.K.) independently screened the titles and abstracts of the retrieved studies. They then retrieved and read the full text of potentially eligible articles and independently assessed their eligibility. Disagreement was resolved via consensus.
Data Extraction
The following data were extracted from each study: (1) patient characteristics (age, sex, and illness requiring acute or critical care); (2) study characteristics (country and settings where interventions were administered); (3) interventions (type and dose of interventions and comparators); and (4) outcomes of interest.
Risk of Bias Assessment
The first author (A.K.) and two other authors (S.W. and Y.K.) independently and in duplicate assessed the risk of bias for each outcome using the Cochrane risk of bias assessment tool [14, 15] and comparators; and 4) outcomes of interest. Any inconsistencies were resolved through discussion. In case of missing data, we did not contact the authors of the original studies.
Statistical Analysis
We calculated the risk ratio (RR) for dichotomous outcomes. When different studies used different scales to assess continuous outcomes, such as feeding status or HRQoL, we calculated the standardized mean difference; otherwise, we calculated the mean difference (MD). We pooled the data into a single arm when a study used several intervention groups [15]. We applied a continuity correction by adding 0.5 to each cell of the 2 × 2 table from the study if a study included zero events in either arm [16]. We pooled the data using the random-effects model [17]. Statistical heterogeneity was evaluated using Q and I2 statistics [18]. When a study provided no relevant outcome data, we narratively described its results. When more than 10 studies reported an outcome, we tested for publication bias using Egger’s method [19]. We used Review Manager 5.4 and Stata SE, version 17.0 (StataCorp, College Station, TX) for the analysis.
The certainty of evidence was classified as high, moderate, low, or very low according to the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) system [20]. The level was downgraded based on the seriousness of limitations (risk of bias), inconsistency, indirectness of evidence, imprecision, and publication bias. We assessed the risk of bias for the evidence based on the framework proposed by Furukawa et al. [21]. We a priori anticipated that there would be substantial but acceptable clinical heterogeneity and focused on statistical heterogeneity to assess inconsistency. Indirectness of evidence, which refers to the generalizability of the findings, was assessed based on the relevance of the population, type of intervention, comparator, or outcomes in the included studies in relation to our research question. Imprecision was assessed based on the confidence intervals (CIs) of the pooled results and the sample size relative to the optimal information size (OIS). While we used a total size of 400 as an empiric OIS for continuous outcomes [22], we estimated the OIS for dichotomous outcomes with required information size (RIS) obtained via trial sequential analysis (TSA) [23]. We estimated RIS according to a relative risk reduction of 25%, a 5 risk of a type-one error, and a power of 80%, which is recommended by the GRADE working group [22]. We estimated the proportion of events in the control group from all trials included in TSA. We used TSA software, version 0.9 beta (Copenhagen Trial Unit, Copenhagen, Denmark) to conduct trial sequential analysis.
Results
Study Characteristics
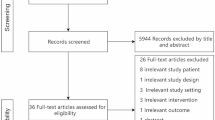
Our initial database search identified 2,556 titles and abstracts. After application of our inclusion and exclusion criteria, we included 19 randomized controlled studies involving 1,096 participants in the analysis [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41] (Fig. 1). All studies were reported in English.
The reported mean age of patients ranged from 49.5 to 74.9 years, with the proportion of women ranging from 27.2 to 70.4% (Table 1). The patient conditions included non-tracheotomized, acute stroke (14 studies) [24, 25, 27,28,29, 31, 33,34,35,36,37, 39,40,41,42], status post tracheostomy (two studies) [26, 30], and status after immediately extubation (two studies) [30, 32]. The median sample size was 40 (range, 14–306).
Dysphagia rehabilitation included repetitive transcranial magnetic stimulation (six studies) [25, 27, 28, 35, 39, 40], pharyngeal electrical stimulation (four studies) [26, 30, 31, 33], transcranial direct current stimulation (four studies) [29, 31, 36, 41], behavioral intervention [24], functional electrical stimulation [37], neuromuscular electrical stimulation [42], speech therapy [32], and swallowing stimulation [34] (one study each). Comparators included sham stimulation/therapy (14 studies) [25,26,27,28,29,30,31,32,33, 36, 38,39,40,41], followed by usual care or conventional therapy (five studies) [24, 34, 35, 37, 42]. One study compared two interventions using rTMS (high-frequency rTMS on the bilateral hemispheres and a combination of high-intensity rTMS on the affected hemisphere and low-intensity rTMS on the unaffected hemisphere) compared with sham stimulation in stroke patients [40]. Another study compared behavioral intervention of two intensities with usual care [24]. The intervention period and the longest period of outcome follow-up ranged from 3 days to 1 month and from 3 days to 6 months, respectively.
Risk of Bias
Fifteen (78.9%) of the 19 included studies performed adequate sequence generation [24, 26, 28,29,30,31,32,33,34, 36, 37, 39,40,41,42], whereas nine (47.4%) conducted adequate allocation concealment [24,25,26, 28, 30, 33, 36, 39, 41] (Fig. 2). All studies but one (94.7%) [34] had adequately blinded outcome assessors and were at a low risk of incomplete outcome reporting, respectively. Ten studies (52.6%) were deemed at a low risk of selective reporting bias [24, 30, 31, 33, 34, 36,37,38,39, 41].
Primary Outcomes
Mortality
Mortality data were available in 13 studies including 787 patients [24, 26,27,28,29,30,31,32,33,34,35,36, 42]. Dysphagia rehabilitation was not associated with reduced mortality (50/458 vs. 31/329; RR, 0.92; 95% CI, 0.61–1.39; P = 0.70; I2 = 0%; Fig. 3). The certainty of evidence for this outcome according to the GRADE system was very low (Table 2). There was no statistical evidence for publication bias using Egger’s test (P = 0.52).
Incidence of Pneumonia
Seven studies including 611 participants provided data on the incidence of pneumonia [24, 26, 31, 33, 34, 38, 42]. Dysphagia rehabilitation was associated with a reduced incidence of pneumonia (93/357 vs. 100/254; RR, 0.66; 95% CI, 0.54–0.81; P < 0.00001; I2 = 0%; Fig. 4). The certainty of evidence for this outcome was moderate (Table 2).
HRQoL Scores
One study including 27 participants examined the effects of dysphagia rehabilitation on the HRQoL score (Dysphagia Handicap Index; DHI) [34]. Dysphagia rehabilitation was not associated with an increased HRQoL score (MD, -0.20; 95% CI, -20.34 to 19.94; P = 0.98). Another study reported a significant improvement in the DHI score with the high-frequency rTMS on the bilateral hemispheres and with a combination of high-intensity rTMS on the affected hemisphere and low-intensity rTMS on the unaffected hemisphere compared with sham stimulation in acute stroke patients at one month after the intervention [40]. The certainty of evidence for this outcome was very low (Table 2).
Secondary Outcomes
Activity of Daily Living
One study including 27 participants examined the effects of dysphagia rehabilitation on the patients’ activities of daily living measued with BI [34]. Dysphagia rehabilitation was not associated with an increased activities of daily living (MD, -14.60; 95% CI, -37.58 to 8.38; P = 0.21). One study, each, reported that use of rTMS for 5 days were associated with an increased BI score compared with sham at 2 months [28] or 3 months after the intervention [25]. The certainty of evidence for this outcome was very low (Table 2).
Feeding Status
Seven studies assessed the feeding status using the FOIS in 313 patients [26, 31, 32, 36, 37, 41, 42]. Dysphagia rehabilitation was associated with improved feeding status (MD, 1.03; 95% CI, 0.43 to 1.62; P = 0.0007; I2 = 37%; Online Resource 2). Another study reported a significant improvement in the FOIS score with the high-frequency rTMS on the bilateral hemispheres compared with sham stimulation and a combination of high-intensity rTMS on the affected hemisphere and low-intensity rTMS on the unaffected hemisphere in acute stroke patients at one month after the intervention [40]. The certainty of evidence for this outcome was moderate (Table 2).
Hospital Length of Stay
Five studies including 482 patients reported data on the length of hospital stay [24, 30, 31, 34, 38]. Dysphagia rehabilitation was not associated with reduced hospital length of stay (MD, -0.35 days; 95% CI, -5.94 to 5.25; P = 0.90; I2 = 80%; Online Resource 3). The certainty of evidence for this outcome was very low (Table 2).
Adverse Events
Eight studies (646 participants) provided data on adverse events [24, 26, 29, 30, 36, 39, 41, 42]. We pooled any serious adverse events from these studies. Dysphagia rehabilitation was not associated with an increased incidence of adverse events (103/387 vs. 71/259; RR, 0.78; 95% CI, 0.45–1.36; P = 0.39; I2 = 29%; Online Resource 4). The certainty of evidence for this outcome was moderate (Table 2).
Subgroup Analyses by Patient Condition
Subgroup analyses by patient condition were possible for five of our outcomes; they suggested no significant differences in mortality (P = 0.18), incidence of pneumonia (P = 0.82), or feeding status (P = 0.30), length of hospital stay (P = 0.08), or adverse events (P = 0.14) between subgroups (Online Resource 5).
Discussion
Our study findings suggest that dysphagia rehabilitation reduced the incidence of pneumonia and feeding status in dysphagic patients with acute or critical illness, without other significant efficacy benefits or adverse events. The certainty of evidence assessed with the GRADE system for most outcomes was very low, except that for the incidence of pneumonia, feeding status, and adverse events (moderate, each).
To the best of our knowledge, only one systematic review has focused on dysphagia interventions in patients with acute or critical illness [43]. That review by Duncan et al. published in 2020 encompassed numerous dysphagia interventions, including acupuncture, and a variety of illness acuity/severity, including subacute illness [43]. In contrast, we focused on dysphagia rehabilitation from the physical medicine perspective in patients in the acute or critical phase of illness. While the target population and interventions are restricted in our review compared with those in Duncan et al’s, ours included a comparable number of studies with newly published ones since 2020. Further, both reviews yielded similar findings on the incidence of pneumonia and adverse events [43]. Thus, clinicians administering dysphagia interventions in such patients should be aware that dysphagia rehabilitation could reduce pneumonia without significant adverse events.
The included studies in our review represented three distinct status: non-intubated stroke, tracheostomized, and status immediately after extubation. The underlying mechanism of dysphagia differ across these conditions, which at least include cortical and/or subcortical damage for stroke and mechanical injury to the mucosal damage for status post tracheostomy or extubation. Although not statistically significant, the effects size differed between these entities, which might explain the statistical heterogeneity found in feeding status and length of hospital stay. Furthermore, most studies examined non-tracheomized stroke patients [24, 25, 27,28,29, 31, 33,34,35,36,37, 39,40,41,42]; studies on status post tracheostomy and immediately after extubation are limited. Thus, more studies are required to elucidate whether patients post tracheostomy and immediately after extubation have merits with dysphagia rehabilitation.
Duncan et al’s review [43] and ours poses three research implications. First, both reviews examined partly overlapping but different outcomes. Compared with the number of studies included in the entire reviews, the number of studies included for each analysis/outcome was small in both reviews. We suspect that one etiology for this inconsistency and variability in outcomes could have been a lack of consensus on the outcomes in this area. A core outcome set for dysphagia interventions should be established and examined in future studies. Second, while our study focused on interventions to abortively treat dysphagia, Duncan et al. also reviewed prophylactic interventions for dysphagia [43]. To date, only a few studies have examined the efficacy of prophylactic, dysphagia rehabilitation in acute or critical illness [44, 45]. More studies are needed to examine whether prophylactic dysphagia interventions are effective and which population get benefitted with which prophylactic dysphagia interventions. Third, while dysphagia could last long after acute phase of illness, many of the studies in our review reported short-term outcomes. A follow-up study of a randomized trial of primary care intervention in sepsis suggested that dysphagia symptoms lasted over 24 months after ICU discharge [46]; however, the longest follow-up of outcomes in most of the studies in our review was within 3 months [27,28,29,30,31,32, 35,36,37,38,39,40,41,42]. Studies to examine the long-term efficacy of dysphagia interventions are warranted.
Our study has several strengths. First, our comprehensive literature search located 19 studies. With a large number of studies, we were able to review an overall efficacy and safety of dysphagia rehabilitation from physical medicine perspective in patients with acute or critical illness. Second, we complied with the Cochrane methodology and assessed the certainty of evidence using the GRADE system.
Nonetheless, this study also has limitations. First, despite our comprehensive search, the number of included studies for some outcomes were small, which could have led to lack of statistical power, resulting in non-significant findings for such outcomes as well as the certainty of evidence evaluated with the GRADE system. Second, some of the rehabilitation conducted worldwide were not examined in the original studies (e.g., respiratory exercises) [10], for which we cannot make any conclusions. Third, the dysphagia interventions reported in the included studies were diverse but the number of studies for each intervention were small. This precludes investigations into the optimal type of dysphagia intervention for patients with acute or critical illness. Furthermore, only a few studies have attempted to compare the efficacy of multiple dysphagia rehabilitation options [47,48,49]. More studies on dysphagia rehabilitation in patients with acute or critical illness are warranted.
Conclusions
Based on the available moderate- or very low- quality evidence, while dysphagia rehabilitation had no impact on mortality or HRQoL, they might reduce the incidence of pneumonia in dysphagic patients with acute or critical illness. Our study also highlights the scarcity of studies on each dysphagia rehabilitation and patient conditions except for patients with non-tracheotomized stroke, as well as a need to establish a core outcome set to consistently assess the efficacy of dysphagia rehabilitation in future studies.
Data Availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
Abbreviations
- DHI:
-
Dysphagia Handicap Index
- FOIS:
-
Functional Oral Intake Scale
- GRADE:
-
Grading of Recommendations, Assessment, Development, and Evaluations
- HRQoL:
-
Health-related quality of life
- ICU:
-
Intensive care unit
- MD:
-
Mean difference
- OIS:
-
Optimal information size
- PES:
-
Pharyngeal electrical stimulation
- RIS:
-
Required information size
- RR:
-
Risk ratio
- rTMS:
-
Repetitive transcranial magnetic stimulation
- tDCS:
-
Transcranial direct current stimulation
- TSA:
-
Trial sequential analysis
References
Zuercher P, Moret CS, Dziewas R, Schefold JC. Dysphagia in the intensive care unit: epidemiology, mechanisms, and clinical management. Crit Care (London England). 2019;23(1):103.
Macht M, Wimbish T, Bodine C, Moss M. ICU-acquired swallowing disorders. Crit Care Med. 2013;41(10):2396–405.
Skoretz SA, Flowers HL, Martino R. The incidence of dysphagia following endotracheal intubation: a systematic review. Chest. 2010;137(3):665–73.
McIntyre M, Doeltgen S, Dalton N, Koppa M, Chimunda T. Post-extubation dysphagia incidence in critically ill patients: a systematic review and meta-analysis. Australian Crit care: Official J Confederation Australian Crit Care Nurses. 2021;34(1):67–75.
Schefold JC, Berger D, Zurcher P, Lensch M, Perren A, Jakob SM, et al. Dysphagia in mechanically ventilated ICU patients (DYnAMICS): a prospective observational trial. Crit Care Med. 2017;45(12):2061–9.
Macht M, Wimbish T, Clark BJ, Benson AB, Burnham EL, Williams A, et al. Postextubation dysphagia is persistent and associated with poor outcomes in survivors of critical illness. Crit Care (London England). 2011;15(5):R231.
Altman KW, Yu GP, Schaefer SD. Consequence of dysphagia in the hospitalized patient: impact on prognosis and hospital resources. Archives otolaryngology–head neck Surg. 2010;136(8):784–9.
Ekberg O, Hamdy S, Woisard V, Wuttge-Hannig A, Ortega P. Social and psychological burden of dysphagia: its impact on diagnosis and treatment. Dysphagia. 2002;17(2):139–46.
Evancho A, Tyler WJ, McGregor K. A review of combined neuromodulation and physical therapy interventions for enhanced neurorehabilitation. Front Hum Neurosci. 2023;17:1151218.
Spronk PE, Spronk LEJ, Egerod I, McGaughey J, McRae J, Rose L, et al. Dysphagia in Intensive Care evaluation (DICE): an International Cross-sectional Survey. Dysphagia. 2022;37(6):1451–60.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical Res ed). 2021;372:n71.
Canadian Stroke Best Practices. Definitions. 2022 Update. 2022 [Available from: https://www.strokebestpractices.ca/recommendations/acute-stroke-management/definitions.
Jackson JL, Kuriyama A, Anton A, Choi A, Fournier JP, Geier AK, et al. The Accuracy of Google Translate for Abstracting Data from Non-english-language trials for systematic reviews. Ann Intern Med. 2019;171(9):677–9.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clinical Res ed). 2011;343:d5928.
Higgins JP, Green. S. editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester (UK): Wiley; 2008.
Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med. 2004;23(9):1351–75.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical Res ed). 2003;327(7414):557–60.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Res ed). 1997;315(7109):629–34.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clinical Res ed). 2008;336(7650):924–6.
Furukawa TA, Miura T, Chaimani A, Leucht S, Cipriani A, Noma H, et al. Using the contribution matrix to evaluate complex study limitations in a network meta-analysis: a case study of bipolar maintenance pharmacotherapy review. BMC Res Notes. 2016;9:218.
Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence–imprecision. J Clin Epidemiol. 2011;64(12):1283–93.
Wetterslev J, Jakobsen JC, Gluud C. Trial Sequential Analysis in systematic reviews with meta-analysis. BMC Med Res Methodol. 2017;17(1):39.
Carnaby G, Hankey GJ, Pizzi J. Behavioural intervention for dysphagia in acute stroke: a randomised controlled trial. Lancet Neurol. 2006;5(1):31–7.
Du J, Yang F, Liu L, Hu J, Cai B, Liu W, et al. Repetitive transcranial magnetic stimulation for rehabilitation of poststroke dysphagia: a randomized, double-blind clinical trial. Clin Neurophysiology: Official J Int Federation Clin Neurophysiol. 2016;127(3):1907–13.
Dziewas R, Stellato R, van der Tweel I, Walther E, Werner CJ, Braun T, et al. Pharyngeal electrical stimulation for early decannulation in tracheotomised patients with neurogenic dysphagia after stroke (PHAST-TRAC): a prospective, single-blinded, randomised trial. Lancet Neurol. 2018;17(10):849–59.
Khedr EM, Abo-Elfetoh N. Therapeutic role of rTMS on recovery of dysphagia in patients with lateral medullary syndrome and brainstem infarction. J Neurol Neurosurg Psychiatry. 2010;81(5):495–9.
Khedr EM, Abo-Elfetoh N, Rothwell JC. Treatment of post-stroke dysphagia with repetitive transcranial magnetic stimulation. Acta Neurol Scand. 2009;119(3):155–61.
Kumar S, Wagner CW, Frayne C, Zhu L, Selim M, Feng W, et al. Noninvasive brain stimulation may improve stroke-related dysphagia: a pilot study. Stroke. 2011;42(4):1035–40.
Suntrup S, Marian T, Schroder JB, Suttrup I, Muhle P, Oelenberg S, et al. Electrical pharyngeal stimulation for dysphagia treatment in tracheotomized stroke patients: a randomized controlled trial. Intensive Care Med. 2015;41(9):1629–37.
Suntrup-Krueger S, Ringmaier C, Muhle P, Wollbrink A, Kemmling A, Hanning U, et al. Randomized trial of transcranial direct current stimulation for poststroke dysphagia. Ann Neurol. 2018;83(2):328–40.
Turra GS, Schwartz IVD, Almeida ST, Martinez CC, Bridi M, Barreto SSM. Efficacy of speech therapy in post-intubation patients with oropharyngeal dysphagia: a randomized controlled trial. CoDAS. 2021;33(2):e20190246.
Vasant DH, Michou E, O’Leary N, Vail A, Mistry S, Hamdy S, et al. Pharyngeal electrical stimulation in Dysphagia Poststroke: a prospective, randomized single-blinded interventional study. Neurorehabilit Neural Repair. 2016;30(9):866–75.
Benfield JK, Hedstrom A, Everton LF, Bath PM, England TJ. Randomized controlled feasibility trial of swallow strength and skill training with surface electromyographic biofeedback in acute stroke patients with dysphagia. J Oral Rehabil. 2023;50(6):440–51.
Jiao Y, Peng W, Yang J, Li C. Effect of Repetitive Transcranial Magnetic Stimulation on the Nutritional Status and neurological function of patients with Postischemic Stroke Dysphagia. Neurologist. 2023;28(2):69–72.
Kumar S, Marchina S, Langmore S, Massaro J, Palmisano J, Wang N, et al. Fostering eating after stroke (FEASt) trial for improving post-stroke dysphagia with non-invasive brain stimulation. Sci Rep. 2022;12(1):9607.
Matos KC, de Oliveira VF, de Oliveira PLC, Carvalho FA, de Mesquita MRM, da Silva Queiroz CG, et al. Combined conventional speech therapy and functional electrical stimulation in acute stroke patients with dyphagia: a randomized controlled trial. BMC Neurol. 2022;22(1):231.
Suntrup-Krueger S, Labeit B, Marian T, Schröder J, Claus I, Ahring S, et al. Pharyngeal electrical stimulation for postextubation dysphagia in acute stroke: a randomized controlled pilot trial. Crit Care (London England). 2023;27(1):383.
Zhong L, Wen X, Liu Z, Li F, Ma X, Liu H, et al. Effects of bilateral cerebellar repetitive transcranial magnetic stimulation in poststroke dysphagia: a randomized sham-controlled trial. NeuroRehabilitation. 2023;52(2):227–34.
Zou F, Chen X, Niu L, Wang Y, Chen J, Li C, et al. Effect of Repetitive Transcranial Magnetic Stimulation on post-stroke Dysphagia in Acute Stage. Dysphagia. 2023;38(4):1117–27.
Farpour S, Asadi-Shekaari M, Borhani Haghighi A, Farpour HR. Improving swallowing function and ability in Post Stroke Dysphagia: a Randomized Clinical Trial. Dysphagia. 2023;38(1):330–9.
Lee KW, Kim SB, Lee JH, Lee SJ, Ri JW, Park JG. The effect of early neuromuscular electrical stimulation therapy in acute/subacute ischemic stroke patients with Dysphagia. Ann Rehabil Med. 2014;38(2):153–9.
Duncan S, McAuley DF, Walshe M, McGaughey J, Anand R, Fallis R, et al. Interventions for oropharyngeal dysphagia in acute and critical care: a systematic review and meta-analysis. Intensive Care Med. 2020;46(7):1326–38.
Hwang CH, Choi KH, Ko YS, Leem CM. Pre-emptive swallowing stimulation in long-term intubated patients. Clin Rehabil. 2007;21(1):41–6.
Siao SF, Ku SC, Tseng WH, Wei YC, Chang YC, Hsiao TY, et al. Effects of a swallowing and oral-care program on resuming oral feeding and reducing pneumonia in patients following endotracheal extubation: a randomized, open-label, controlled trial. Crit Care (London England). 2023;27(1):283.
Kosilek RP, Schmidt K, Baumeister SE, Gensichen J. Frequency and risk factors of post-intensive care syndrome components in a multicenter randomized controlled trial of German sepsis survivors. J Crit Care. 2021;65:268–73.
Howard MM, Block ES, Mishreki D, Kim T, Rosario ER. The effect of sensory level Versus Motor Level Electrical Stimulation of pharyngeal muscles in Acute Stroke patients with Dysphagia: a Randomized Trial. Dysphagia. 2023;38(3):943–53.
Li H, Zhao L, Yuan X, Zhang Q, Pang Y, Li H. Effect of transcranial direct current stimulation combined with respiratory training on dysphagia in post-stroke patients. Technol Health Care. 2023;31(1):11–9.
Bengisu S, Demir N, Krespi Y. Effectiveness of Conventional Dysphagia Therapy (CDT), Neuromuscular Electrical Stimulation (NMES), and Transcranial Direct Current Stimulation (tDCS) in Acute Post-stroke Dysphagia: a comparative evaluation. Dysphagia. 2024;39(1):77–91.
Acknowledgements
The authors would like to sincerely thank Dr. Rao Sun for retrieving Chinese articles.
Funding
There was no funding source for this manuscript.
Author information
Authors and Affiliations
Contributions
All authors participated in the conception of the study design, interpretation of the data, and critical revision of the manuscript. AK, SW, and YK participated in the acquisition of the data. AK analyzed the data and wrote the draft. All authors approved the submission of the current manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kuriyama, A., Watanabe, S., Katayama, Y. et al. Dysphagia Rehabilitation in Dysphagic Patients with Acute or Critical Illness: A Systematic Review and Meta-Analysis. Dysphagia (2024). https://doi.org/10.1007/s00455-024-10700-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00455-024-10700-7