Abstract
The study aimed to evaluate the effects of traditional dysphagia therapy (TDT) and neuromuscular electrical stimulation (NMES) combined with TDT on functionality of oral intake, dysphagia symptom severity, swallowing- and voice-related quality of life, leakage, penetration–aspiration, and residue levels in patients with post-stroke dysphagia (PSD). Thirty-four patients with PSD were included in our prospective, randomized, controlled, and single-blind study. The patients were divided into two groups: (1) TDT only (control group, n = 17) and (2) TDT with NMES (experimental group, n = 17). TDT was applied to both groups for three consecutive weeks, 5 days a week, 45 min a day. Sensory NMES was applied to the experimental group for 45 min per session. Patients were evaluated by the functional oral intake scale (FOIS), the eating assessment tool (EAT-10), the swallowing quality of life questionnaire (SWAL-QOL), and the voice-related quality of life questionnaire (VRQOL) at baseline, immediately post-intervention, and at the 3rd month post-intervention. Fiberoptic endoscopic evaluation of swallowing (FEES) with liquid and semi-solid food was performed pre- and post-intervention. A significant post-intervention improvement was observed on all scales in both groups, and these improvements were maintained 3 months post-intervention. Leakage and penetration–aspiration levels with semi-solid food declined only in the experimental group. In conclusion, TDT is a non-invasive and inexpensive method that leads to improvement in many swallowing-related features in stroke patients; however, NMES as an adjunct therapy is costly but can provide additional benefits for improving features, such as penetration–aspiration and residue levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The most frequent cause of neurogenic dysphagia is post-stroke dysphagia (PSD), which is characterized by problems in the oropharyngeal stage [1]. PSD has been described as a difficulty in swallowing that affects most patients in the first few hours and days after a cerebrovascular accident, CVA [2]. The prevalence of PSD has been reported as up to 60%, and this rate can reach up to 100% when minor swallowing disorders are considered [3]. PSD can increase morbidity and mortality in stroke patients due to prolonged hospital stays, delays in the rehabilitation process, malnutrition, dehydration, aspiration pneumonia, increased risk of infection, and even death. Early evaluation of PSD and initiation of dysphagia therapy prevent PSD complications [4].
According to stroke rehabilitation guidelines, patients with dysphagia should receive swallowing rehabilitation 1 week after a CVA [5]. Swallowing rehabilitation comprises traditional dysphagia therapy (TDT) and other rehabilitative approaches, such as neuromuscular electrical stimulation (NMES). TDT includes patient education, oral hygiene, diet regulation, nutritional support, and compensatory and rehabilitative approaches [6, 7].
Since the first article investigating the use of NMES in PSD rehabilitation was published (2001), few trials have investigated the effects of NMES on PSD [8]. In studies investigating the application of NMES to swallowing muscles, sensory or motor stimulation was applied to the suprahyoid or infrahyoid regions [9,10,11,12]. Different protocols have been studied regarding stimulation features and session frequency. In previous studies, intervention durations were 1–8 weeks, 2–5 days a week, for 10 min to 1 h. The stimulation frequency was 30–80 Hz, and the pulse duration was 300 µs to 800 ms [9, 13,14,15,16,17]. The optimum NMES application has not been determined.
Previous studies have shown conflicting results regarding the use of NMES alone in PSD therapy. Although some show that the use of NMES along with TDT will provide additional benefits, others have the opposite opinion [9, 10, 14, 15, 18].
The consensus in PSD trials to date is that there is insufficient evidence for the NMES use alone in swallowing therapy and that NMES may contribute more to the improvement of swallowing than TDT alone [9, 10, 19]. Studies evaluating NMES use in PSD have provided inconsistent results; therefore, the efficacy and safety of this method remain controversial. To determine whether NMES is effective and in which patients NMES may be more effective, high-quality studies are needed to evaluate electrode placement, application methods, and the short- and long-term effects of NMES on swallowing function [14].
Using standardized scales and instrumental assessment methods, we investigated and compared the effects of TDT and the addition of NMES to TDT in PSD. To the best of our knowledge, our study is the first to compare the effects of TDT and TDT–NMES on the severity of dysphagia symptoms, the physiology of swallowing, and patients’ quality of life related to swallowing and voice, as well as the effects of these therapies on functional swallowing.
Methods
Our study was designed as a randomized, controlled, prospective, single-blind study. Thirty-eight stroke patients were eligible for our study, which was conducted from April 2020 to October 2020. The inclusion and exclusion criteria are presented in Table 1.
Participants were randomized into two groups: the TDT with NMES group (experimental) and the TDT group (control). Randomization was provided via a code generator program on the computer using the permuted block randomization method. The researcher (E.T.C.) who prepared the randomization list applied the treatment program to the patients. Another researcher (S.S.) was blinded to the intervention that measured the functional oral intake scale (FOIS), the eating assessment tool (EAT-10), the swallowing quality of life questionnaire (SWAL-QOL), and the voice-related quality of life questionnaire (VRQOL) pre-, post-, and in the 3rd month after the intervention. Pre- and post-intervention fiberoptic endoscopic evaluations of swallowing (FEES) measurements were recorded by an otorhinolaryngologist (C.S.) blinded to the treatment, and the records were evaluated by another otorhinolaryngologist (C.D.) who was also blinded to the treatment. All extracted data were analyzed by the data analyst who was blinded to the study.
Our study was approved by the Institutional Review Board and followed the principles of the Declaration of Helsinki. The study protocol was approved by the Istanbul University Istanbul Faculty of Medicine Clinical Research Ethics Committee (Approval Number 75346), and all methods were performed following the relevant guidelines and regulations. This study was also approved by the National Agency for Medicines and Medical Devices. Each patient signed an informed consent form, and the study was registered on clinicaltrials.gov (Registration Number NCT04421937).
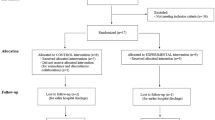
Figure 1 presents the Consolidated Standards of the Reporting Trials flow diagram.
Participant flow and study profile. TDT traditional dysphagia therapy, NMES neuromuscular electrical stimulation, FOIS functional oral intake scale, EAT-10 the eating assessment tool, SWAL-QOL the swallowing quality of life questionnaire, VRQOL the voice-related quality of life questionnaire, FEES fiberoptic endoscopic evaluation of swallowing
Intervention
TDT was used in both groups for three consecutive weeks at 5 days per week for 45 min per day. In addition to TDT, 45 min of NMES was applied to the experimental group.
TDT was a standardized program that included diet modification according to the patients’ oral intake levels, oral hygiene education, compensatory methods, and exercises. The TDT program was overseen by a physiatrist (E.T.C.). Compensatory methods comprised postural techniques, such as chin tuck and rotation lateral flexion of the head, and oral sensorimotor enhancement techniques, such as thermal-tactile stimulation (TTS). Oral motor control exercises consisting of jaw, tongue, and lip range of motion (ROM) exercises were performed for eight repetitions in each direction for three sets, minimum. The Masako maneuver, which is a pharyngeal ROM exercise, was performed with 10 repetitions for three sets, minimum. The chin tuck against resistance exercise, which provides suprahyoid muscle contraction and is also a pharyngeal ROM exercise, was performed at maximum patient toleration in three sets. The TDT program comprised exercises implemented in similar studies investigating PSD rehabilitation and recommended in current guidelines [5, 7, 14, 20]. During the exercise sessions, the patients were given short rest periods of less than 5 min in line with their requests.
NMES was applied via using a modified hand-held battery-powered electrical stimulator (VitalStim® Dual Channel Unit and Electrodes, Chattanooga Group, Hixson, TN, USA). The application protocols of NMES in PSD vary. Therefore, NMES session duration and frequency and the total number of sessions in this study were created by evaluating previous studies [9, 14,15,16,17, 21,22,23,24,25,26,27,28,29,30,31,32,33,34,35]. NMES was applied in 15 sessions for 3 weeks, 5 consecutive days per week, with each session lasting 45 min.
By reviewing previous studies, the NMES application method was determined as an 80 Hz pulse rate with a biphasic pulse duration of 700 μs and stimulation intensity not exceeding 25 mA [12, 14, 15, 19, 25, 36,37,38,39].
Proper electrode placement targeting the suprahyoid or infrahyoid muscles during electrotherapy of swallowing muscles is difficult due to the small and overlapping locations of these muscles. Given that the function of the suprahyoid muscles is to pull the hyoid upward and toward the mandible, the suprahyoid muscles are targeted during stimulation to prevent hyolaryngeal excursion in those with PSD. It is important for the thyrohyoid muscle to contract with sufficient force to protect the airway during swallowing; therefore, this muscle was also targeted in the stimulation [12, 40]. Based on these factors, the upper electrodes were placed on the mylohyoid muscle just above the hyoid bone, and the lower electrodes were placed horizontally on the thyrohyoid muscle (Fig. 2). The stimulation intensity level started at 2 mA, increased by 0.5 mA intervals, and stabilized when the patient felt vibration in their neck. During the application, the patient was observed by a physiatrist (E.T.C).
Evaluation Measurements
The patients’ clinical and demographic information was recorded at the beginning of the study. The patients were evaluated by outcome measures at baseline, immediately after the intervention, and at the 3rd month after the intervention. The FOIS was the primary outcome measure. The EAT-10, SWAL-QOL, and VRQOL were the secondary outcome measures. FEES was performed as a quantitative measure before and immediately after the intervention.
The FOIS is a seven-level scale that indicated oral intake level. This demonstrates a change in functional oral intake in stroke patients [41]. The EAT-10 is a 10-item scale that evaluates dysphagia symptom severity to monitor dysphagia treatment effectiveness [42]. An EAT-10 total score of ≥ 3 indicates the presence of oropharyngeal dysphagia [43], whereas ≥ 15 indicates the risk of aspiration [44]. The SWAL-QOL evaluates the quality of life of those who have swallowing disorders and comprises burden, communication, eating desire, eating duration, fatigue, fear, food selection, mental health, sleep, social functioning, and symptom frequency subsections [45,46,47]. The VRQOL assesses quality of life by questioning the emotional and functional effects of voice problems [48]. The outcome measures used in our study have national validity and reliability [45, 49, 50].
FEES was performed by an otorhinolaryngologist blinded to the study with boluses with different consistencies, including liquid (90 ml of a mixture of water and green food coloring) and semi-solid food (yogurt). The patients were first given 10 ml of fluid, and their oral capacity was evaluated, followed by a bolus of 90 ml of liquid using a straw. Subsequently, the patients were given one tablespoon of yogurt twice. A leakage assessment was performed during bolus intake. A score was given according to the localization where the bolus escaped: 0 = no leak, 1 = at the base of the tongue or vallecula, 2 = moves to lateral channels or the tip of the epiglottis, 3 = in the piriform sinuses or touches the laryngeal rim on the sides or back, and 4 = falls into the laryngeal vestibule or is aspirated before swallowing [51]. After swallowing, the patients were evaluated according to the eight-item penetration–aspiration scale, PAS [52]. A score was given according to the localization of the residue formed after swallowing: 0 = no residue, 1 = oropharyngeal, 2 = in the pyriform sinuses, and 3 = in the aditus larynx [53].
Statistical Analysis
Taking into account the results of a previous study [54] and assuming an α error of 0.05% and a power of 80%, the required sample size was estimated to be 17 patients per group. Assuming a dropout rate of 10%, 19 participants in total would be needed in each group.
In the evaluation of the data, besides descriptive statistical methods (mean, standard deviation, median, interquartile range), the normality of the distribution was determined with the Shapiro–Wilk test. Independent sample t-test was used to compare the normally distributed variables in paired groups. Friedman Test was used for multiple time comparisons of non-normally distributed variables, Dunn's multiple comparison test was used for subgroup comparisons, and Wilcoxon test was used for time comparisons. For variables without normal distribution, the Mann–Whitney U test was used to compare paired groups and the χ2 test to compare qualitative data. The significance of the results was evaluated at the p < 0.05 level. All analyses were conducted using the NCSS 2021 Statistical Software (Number Cruncher Statistical System, Utah, USA).
Results
There was no significant difference between the two groups in terms of the patients’ initial demographic and clinical characteristics (Table 2). There was no significant difference at the baseline assessment between the two groups in terms of all outcome measures (Table 3). No adverse effects were observed during the interventions or 3 months after the interventions.
Statistically significant changes were observed pre- and post-intervention, and in the 3rd month after the intervention. FOIS levels and EAT-10, SWAL-QOL, and VRQOL scores in both groups are shown in Table 3. In all outcome measures, the post-intervention and 3rd month control evaluations were found to be statistically significantly improved compared to the pre-intervention evaluations in both groups. There was no significant change in the outcome measures, except SWAL-QOL, in the post-intervention and the 3rd month after the intervention in either group. In the TDT–NMES group, there was an improvement in SWAL-QOL in the 3rd month after the intervention compared to the post-intervention (p = 0.027), while there was no significant change in the TDT group (Table 4).
There was no significant change in the presence of oropharyngeal dysphagia (EAT-10 score ≥ 3) or aspiration risk (EAT-10 score ≥ 15) in either group. While not statistically significant, aspiration risk was detected in 78.6% and 88.2% of the patients in the pre-intervention TDT and TDT–NMES groups, respectively, and these rates decreased to 29.4% and 5.9%, respectively, after the intervention (Table 5).
There was no significant difference at the baseline assessment between the two groups in terms of FEES findings (Table 6). In the TDT group, there was significant improvement in all parameters in FEES with liquid, but improvement was observed only for post-swallowing residues with semi-solid food. Conversely, in the TDT–NMES group, significant improvement was achieved in all parameters in FEES with both liquid and semi-solid food. Leakage levels with semi-solid food were significantly lower in the TDT–NMES group than in the TDT group post-intervention.
Discussion
This study found improvement in swallowing function, dysphagia symptom severity, and quality of life in patients with PSD using both TDT and TDT–NMES, and these improvements were preserved at the 3rd month after the intervention. Additionally, improvements in PAS and leakage scores were better with semi-solid food in the group in which NMES was added to the therapy regimen.
Functionality of Oral Intake
FOIS, which was the primary outcome measure, improved in both groups. This improvement was maintained in both groups at the 3rd month after the intervention, and there was no significant difference between the groups.
Huang et al. [16] divided 29 patients with PSD who had suffered a stroke less than 3 months prior into 3 groups: TDT (n = 11), NMES (n = 8), and TDT–NMES (n = 10). NMES was applied for 10 sessions at 60 min per session for 3 sessions per week. The dual-channel electrodes were placed in one vertical line, with channel one above the thyroid notch and channel two below the thyroid notch. TDT comprised a combination of oral exercise, compensation methods, TTS, and swallowing maneuvers. The patients were evaluated using FOIS, PAS, and the functional dysphagia scale (FDS). They had hemispheric lesions and were at stage 4 or lower according to FOIS. Although there were differences in NMES application features and the clinical characteristics of the participants in this study, a similar significant improvement in FOIS was achieved with interventions in all three groups, and no significant difference was found among the groups in terms of results. However, PAS levels with dense foods were significantly better in the TDT–NMES group [16]. These results, similar to the results of our study, show that TDT and TDT–NMES both improve swallowing functionality, and that NMES added to TDT may further improve the physiology of swallowing dense foods.
Unlike our study, patients in studies showing greater improvement in the functionality of oral intake with the addition of NMES to TDT were in the acute [36, 37, 55], acute/subacute [25], or subacute phases [38]. These studies included patients who were tube-fed [36,37,38, 55], had aspiration [37], or could not tolerate all food consistencies without restriction [25]. Therefore, in acute or subacute patients with PSD and in patients with a FOIS of 5 or lower, NMES, in addition to TDT, may improve swallowing functionality more than TDT alone.
Dysphagia Symptom Severity
In our study, comprehensive TDT was effective in reducing the severity of dysphagia symptoms, and the addition of NMES did not provide additional benefits. In former studies, NMES as an adjunct therapy reduced dysphagia symptom severity by reducing the EAT-10 [34, 56]. To the best of our knowledge, no study has compared the effects of TDT and TDT–NMES on dysphagia symptom severity using the EAT-10. Our study showed that, although not statistically significant, according to the EAT-10 scores, patients at risk of aspiration had a reduced risk after dysphagia rehabilitation. To reveal statistically significant results, new studies with a larger sample sizes are needed.
Quality of Life
Swallowing includes not only physiological food intake but also social, psychological, and cultural experiences. Given that most studies have investigated physiological swallowing function, more studies on the quality of life of patients with PSD are needed. In our study, the SWAL-QOL of all patients increased with dysphagia rehabilitation. The 3rd-month evaluations indicated that the SWAL-QOL continued to increase after the treatment was terminated in the group with NMES added to the treatment regimen. This may have been due to the cortical reorganization effects of NMES [12].
Zhang et al. [39] divided 82 patients with PSD who had a medullary stroke less than 1 month prior into 3 groups: traditional swallowing therapy (TT, n = 27), the sensory approach combined with TT (n = 28), and the motor approach combined with TT (n = 27). TT included postural adjustment, diet modification, TTS, oropharyngeal strengthening exercises, and swallowing maneuvers. Electrical stimulation sessions were conducted for 20 min, 2 times per day, 5 days per week over a 4-week period; two electrodes were placed parallel to the digastric muscle in the submental region. Zhang et al. revealed improvement in the SWAL-QOL in all groups, similar to our study, but the improvement was greater with NMES and TT than with TT alone, unlike our study. The greatest improvement in SWAL-QOL was in the group that applied the sensory approach [39], which was the method used in our study. Unlike our study, the fact that NMES provided additional benefits for SWAL-QOL may have been due to the clinical characteristics of the patients included, who were in the acute phase after medullary stroke.
Unlike our study, Xia et al. [28] reported that the SWAL-QOL in PSD improved more when NMES was added to the swallowing rehabilitation program [28]. Xia et al. defined the swallowing rehabilitation program they applied as basic training and direct food intake training, which was simpler than the TDT in our study and lacked many exercise types [28]. Accordingly, a well-designed swallowing rehabilitation program consisting of a sufficient number and variety of exercises can improve SWAL-QOL in patients with PSD.
Byeon [57] divided patients with PSD into three groups: the Mendelsohn maneuver and NMES (n = 15), the Mendelsohn maneuver alone (n = 15), and NMES alone (n = 13). Evaluations were conducted with FDS and SWAL-QOL both before and after treatment. The NMES with the Mendelsohn maneuver group had the highest FDS and SWAL-QOL scores, followed by the Mendelsohn maneuver alone group and the NMES alone group. The results of Byeon’s study showed that NMES may be more effective when combined with exercises than a single intervention method [57]. Byeon did not provide long-term results; however, in our study, in the long-term SWAL-QOL evaluation, similar to Byeon et al.’s study, there was a greater improvement in SWAL-QOL in the TDT–NMES group compared to the TDT group. This result suggests that NMES, as an adjunct therapy, provides further improvements in SWAL-QOL.
Swallowing and phonation share a common structure in the form of the respiratory–digestive system [58]. Therefore, according to the location and size of the lesion, voice changes that reduce the quality of life can be seen in patients with PSD [59]. According to our research, this study is the first to evaluate the effects of dysphagia rehabilitation programs on VRQOL. The results of this study showed that dysphagia therapy provides an increase in VRQOL, and this was maintained at the 3rd month post-intervention.
Fiberoptic Endoscopic Evaluation of Swallowing
FEES is a gold-standard test for the objective evaluation of PSD and is successful in demonstrating leakage, residue, penetration, and aspiration [60, 61]. We have demonstrated that FEES is an appropriate tool for dysphagia evaluation. Our FEES findings suggest that comprehensive swallowing therapy alone can reduce the levels of leakage, penetration, aspiration and residue in patients with PSD, and that NMES added to TDT may provide additional benefits in selected patient groups with worse functionality in oral intake.
Lim et al. [32] randomized 28 patients with PSD into 2 groups: TTS (n = 12) and TTS–NMES (n = 16). The intervention, defined as traditional therapy in their study, had weaker content than ours. Lim et al. administered TTS to the control group, while we applied TDT, which we created by combining many exercises used in dysphagia rehabilitation, to the control group. Unlike our study, Lim et al. revealed that NMES as an adjunct therapy, reduced PAS, but traditional therapy alone did not [32]. This difference may have been due to the different methods we applied to the control groups. Dysphagia rehabilitation, which comprises well-designed exercises in combination, is more beneficial than using a single type of exercise and improves PAS levels.
Simonelli et al. [37] divided their patients with PSD into two groups: TDT (n = 15) and TDT–NMES (n = 16). Similar to our study, they applied a comprehensive combination of various exercises (oral, tongue, and laryngeal motor exercises; laryngeal adduction elevation; TTS; tongue strengthening; effortful swallowing; the Mendelsohn and Masako maneuvers; and the Shaker exercise) to the TDT group and performed FEES before and after treatment. While our study included subacute and chronic stroke patients, Simonelli et al. included only patients with subacute strokes. In Simonelli et al., TDT–NMES was more effective at reducing PAS than TDT in patients with subacute PSD, all of whom were fed via a feeding tube [37]. This finding supports our theory that NMES, as an adjunct therapy, may provide more successful results in reducing PAS in patients with low functionality in oral intake.
Neuromuscular Electrical Stimulation Application
There is no standard for electrode placement or stimulation parameters for NMES in PSD. Electrode placement on the suprahyoid muscles and thyrohyoid provides hyolaryngeal excursion in dysphagic patients with reduced hyolaryngeal elevation and weak muscles [12, 40, 62]. We placed two sets of electrodes to target the mylohyoid and thyrohyoid muscles. Similarly, Terré and Mearin [38] used two sets of electrodes and targeted these muscles. Sensory NMES, in which the stimulation threshold depended on the lowest current level at which the patients felt tingling in their necks, was applied in our study. Considering the effects of sensory stimulation on the long-term reorganization of the human cortex, the use of sensory electrical stimulation is recommended to improve swallowing function [12]. Sensory NMES applied in patients with PSD resulted in significant improvement in various dysphagia parameters, such as delayed swallowing response time and aspiration prevalence [63]. Motor NMES selectively activates type II muscle fibers, while type I muscle fibers are activated in voluntary contractions [9, 12]. Although this difference is accepted as a way to strengthen the muscles and develop their functions, it may not be enough to carry the results into functional activities.
In previous studies, the NMES session durations were 10 [17], 20 [21,22,23], 30 [24,25,26,27,28,29], 40 [30], and 60 [16, 31,32,33,34] min. The number of sessions varied between 3 [17] and 30 [35] in total; there were frequently 10 [16, 26, 34], 12 [22], 15 [25, 30, 31], 20 [24], 24 [23], and 28 [29] sessions. The sessions were completed in 2–6 weeks, and the session frequency varied between 2 and 5 per week [9, 14, 15]. In some studies, sessions were conducted twice per day [24, 29]. Similar to our method Diéguez-Pérez et al. found that NMES was most effective at 60–80 Hz, with a 700 μs pulse duration. However, unlike our method, for Diéguez-Pérez et al. NMES was most effective at motor intensity threshold and stimulations of 20–30 min [11]. Although our NMES application method was well tolerated, the optimum administration procedure needs to be determined.
Strengths and Limitations
The strengths of this study are the 3-month follow-up period, all evaluations were done completely blind to the intervention, and standardized scales and methods were used as primary and secondary outcome measures for evaluating the effectiveness and the safety of swallowing. This is the first study to investigate the effects of dysphagia rehabilitation on VRQOL. FEES, allowing evaluation of the safety of swallowing, was repeated with different food consistencies. The standardized TDT, organized with the combination of various exercises, was applied to all patients. Another strength is that the distribution of demographic and clinical characteristics that may affect the results of our study and the pre-treatment values of treatment evaluation parameters were homogeneous between the two groups.
The main limitation is the relatively small sample size. The second limitation is the study population heterogeneity. Given that stroke patients with hemisphere or brain stem lesions were included in the study, swallowing functions may be affected differently in these patients. Third, since both subacute and chronic patients were included in the study, the natural effect of post-stroke recovery may have contributed to the recovery provided by the intervention. However, we did not include an untreated control group in the study because it would not have been ethically appropriate. Another limitation of our study is that it was not double-blind. The application of electrical stimulation may have a placebo effect, which should be considered when interpreting the results. Finally, the lack of consensus on optimum electrode placement and stimulation settings also limited the study.
Conclusion
The current study demonstrated that NMES as an adjunct therapy provides additional benefits in some swallow-related conditions. On the other hand, an inclusive dysphagia therapy combined with patient education, diet modification, and compensatory and rehabilitative techniques can improve all swallowing-related particularities and is an inexpensive, device-free, and non-invasive method compared to NMES. The NMES is more costly due to the need for a stimulation device and specific electrodes. There is a need for a standardized guideline for the treatment of PSD. To carry out a cost-effective treatment, patients who will benefit most from NMES should be determined.
In conclusion, additional benefits of NMES have been demonstrated by the limited data obtained with a small sample size in our study. There is a need for additional larger studies to confirm these findings.
References
Ickenstein GW, Stein J, Ambrosi D, Goldstein R, Horn M, Bogdahn U. Predictors of survival after severe dysphagic stroke. J Neurol. 2005;252(12):1510–6. https://doi.org/10.1007/s00415-005-0906-9.
Cohen DL, Roffe C, Beavan J, Blackett B, Fairfield CA, Hamdy S, et al. Post-stroke dysphagia: a review and design considerations for future trials. Int J Stroke. 2016;11(4):399–411. https://doi.org/10.1177/1747493016639057.
Smithard DG. Dysphagia management and stroke units. Curr Phys Med Rehabil Rep. 2016;4(4):287–94. https://doi.org/10.1007/s40141-016-0137-2.
Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005;36(12):2756–63. https://doi.org/10.1161/01.STR.0000190056.76543.eb.
Party ISW. National clinical guideline for stroke. 4th ed. London: Royal College of Physicians; 2012.
Dworzynski K, Ritchie G, Playford ED. Stroke rehabilitation: long-term rehabilitation after stroke. Clin Med. 2015;15(5):461. https://doi.org/10.7861/clinmedicine.15-5-461.
Umay E, Eyigor S, Ertekin C, Unlu Z, Selcuk B, Bahat G, et al. Best practice recommendations for stroke patients with dysphagia: a Delphi-based consensus study of experts in Turkey-Part II: rehabilitation. Dysphagia. 2021;36(5):800–20. https://doi.org/10.1007/s00455-020-10218-8.
Feed ML, Freed L, Chatburc RL, Christian M. Electrical stimulation for swallowing disorders caused by stroke. Respir Care. 2001;46(5):466–74.
Alamer A, Melese H, Nigussie F. Effectiveness of neuromuscular electrical stimulation on post-stroke dysphagia: a systematic review of randomized controlled trials. Clin Interv Aging. 2020;3(15):1521–31. https://doi.org/10.2147/CIA.S262596.
Desimone L, Lovis J, M.C.S.S. Candidates. Critical Review: Is Neuromuscular Electrical Stimulation (NMES) a beneficial supplement to existing dysphagia interventions for post-stroke patients? 2021. https://www.uwo.ca/fhs/lwm/teaching/EBP/2020_21/DesimoneLovis.pdf. Accessed 16 Feb 2022
Diéguez-Pérez I, Leirós-Rodríguez R. Effectiveness of different application parameters of neuromuscular electrical stimulation for the treatment of dysphagia after a stroke: a systematic review. J Clin Med. 2020;9(8):2618. https://doi.org/10.3390/jcm9082618.
Poorjavad M, Talebian Moghadam S, Nakhostin Ansari N, Daemi M. Surface electrical stimulation for treating swallowing disorders after stroke: a review of the stimulation intensity levels and the electrode placements. Stroke Res Treat. 2014;2014: 918057. https://doi.org/10.1155/2014/918057.
Speyer R, Sutt A-L, Bergström L, Hamdy S, Heijnen BJ, Remijn L, et al. Neurostimulation in people with oropharyngeal dysphagia: a systematic review and meta-analyses of randomised controlled trials—Part I: pharyngeal and neuromuscular electrical stimulation. J Clin Med. 2022;11(3):776. https://doi.org/10.3390/jcm11030776.
Bath PM, Lee HS, Everton LF. Swallowing therapy for dysphagia in acute and subacute stroke. Cochrane Database Syst Rev. 2018;10(10):CD000323. https://doi.org/10.1002/14651858.CD000323.pub3.
Chen Y-W, Chang K-H, Chen H-C, Liang W-M, Wang Y-H, Lin Y-N. The effects of surface neuromuscular electrical stimulation on post-stroke dysphagia: a systemic review and meta-analysis. Clin Rehabil. 2016;30(1):24–35. https://doi.org/10.1177/0269215515571681.
Huang K-L, Liu T-Y, Huang Y-C, Leong C-P, Lin W-C, Pong Y-P. Functional outcome in acute stroke patients with oropharyngeal dysphagia after swallowing therapy. J Stroke Cerebrovasc Dis. 2014;23(10):2547–53. https://doi.org/10.1016/j.jstrokecerebrovasdis.2014.05.031.
Vasant DH, Michou E, O’Leary N, Vail A, Mistry S, Hamdy S, et al. Pharyngeal electrical stimulation in dysphagia poststroke: a prospective, randomized single-blinded interventional study. Neurorehabil Neural Repair. 2016;30(9):866–75. https://doi.org/10.1177/1545968316639129.
Chiang C-F, Lin M-T, Hsiao M-Y, Yeh Y-C, Liang Y-C, Wang T-G. Comparative efficacy of noninvasive neurostimulation therapies for acute and subacute poststroke dysphagia: a systematic review and network meta-analysis. Arch Phys Med Rehabil. 2019;100(4):739-750.e4. https://doi.org/10.1016/j.apmr.2018.09.117.
Sproson L, Pownall S, Enderby P, Freeman J. Combined electrical stimulation and exercise for swallow rehabilitation post-stroke: a pilot randomized control trial. Int J Lang Commun Disord. 2018;53(2):405–17. https://doi.org/10.1111/1460-6984.12359.
Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016;47(6):e98–169. https://doi.org/10.1161/STR.0000000000000098.
Konecny P, Elfmark M. Electrical stimulation of hyoid muscles in post-stroke dysphagia. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2018;162(1):40–2. https://doi.org/10.5507/bp.2017.043.
Park J-W, Kim Y, Oh J-C, Lee H-J. Effortful swallowing training combined with electrical stimulation in post-stroke dysphagia: a randomized controlled study. Dysphagia. 2012;27(4):521–7. https://doi.org/10.1007/s00455-012-9403-3.
Zeng Y, Yip J, Cui H, Guan L, Zhu H, Zhang W, et al. Efficacy of neuromuscular electrical stimulation in improving the negative psychological state in patients with cerebral infarction and dysphagia. Neurol Res. 2018;40(6):473–9. https://doi.org/10.1080/01616412.2018.1451015.
Lee KW, Kim SB, Lee JH, Lee SJ, Park JG, Jang KW. Effects of neuromuscular electrical stimulation for Masseter muscle on oral dysfunction after stroke. Ann Rehabil Med. 2019;43(1):11. https://doi.org/10.5535/arm.2019.43.1.11.
Lee KW, Kim SB, Lee JH, Lee SJ, Ri JW, Park JG. The effect of early neuromuscular electrical stimulation therapy in acute/subacute ischemic stroke patients with dysphagia. Ann Rehabil Med. 2014;38(2):153. https://doi.org/10.5535/arm.2014.38.2.153.
Lim K-B, Lee H-J, Yoo J, Kwon Y-G. Effect of low-frequency rTMS and NMES on subacute unilateral hemispheric stroke with dysphagia. Ann Rehabil Med. 2014;38(5):592. https://doi.org/10.5535/arm.2014.38.5.592.
Park JS, Oh DH, Hwang NK, Lee JH. Effects of neuromuscular electrical stimulation combined with effortful swallowing on post-stroke oropharyngeal dysphagia: a randomised controlled trial. J Oral Rehabil. 2016;43(6):426–34. https://doi.org/10.1111/joor.12390.
Xia W, Zheng C, Lei Q, Tang Z, Hua Q, Zhang Y, et al. Treatment of post-stroke dysphagia by VitalStim therapy coupled with conventional swallowing training. J Huazhong Univ Sci Technol (Med Sci). 2011;31(1):73–6. https://doi.org/10.1007/s11596-011-0153-5.
Zhao J-W, Wang Z-Y, Cao W-Z, Zhang Y-W, Song S-C, Kang W-G, et al. Therapeutic efficacy of swallowing neuromuscular electrical stimulation combined with acupuncture for post-stroke dysphagia. World J Acupunct Moxibustion. 2015;25(1):19–23. https://doi.org/10.1016/S1003-5257(15)30004-0.
Guillén-Solà A, Messagi Sartor M, Bofill Soler N, Duarte E, Barrera MC, Marco E. Respiratory muscle strength training and neuromuscular electrical stimulation in subacute dysphagic stroke patients: a randomized controlled trial. Clin Rehabil. 2017;31(6):761–71. https://doi.org/10.1177/0269215516652446.
Bülow M, Speyer R, Baijens L, Woisard V, Ekberg O. Neuromuscular electrical stimulation (NMES) in stroke patients with oral and pharyngeal dysfunction. Dysphagia. 2008;23(3):302–9. https://doi.org/10.1007/s00455-007-9145-9.
Lim K-B, Lee H-J, Lim S-S, Choi Y-I. Neuromuscular electrical and thermal-tactile stimulation for dysphagia caused by stroke: a randomized controlled trial. J Rehabil Med. 2009;41(3):174–8. https://doi.org/10.2340/16501977-0317.
Permsirivanich W, Tipchatyotin S, Wongchai M, Leelamanit V, Setthawatcharawanich S, Sathirapanya P, et al. Comparing the effects of rehabilitation swallowing therapy vs. neuromuscular electrical stimulation therapy among stroke patients with persistent pharyngeal dysphagia: a randomized controlled study. Med J Med Assoc Thail. 2009;92(2):259.
Rofes L, Arreola V, López I, Martin A, Sebastian M, Ciurana A, et al. Effect of surface sensory and motor electrical stimulation on chronic poststroke oropharyngeal dysfunction. Neurogastroenterol Motil. 2013;25(11):888-e701. https://doi.org/10.1111/nmo.12211.
Park J, Oh D, Chang M, Kim K. Effects of expiratory muscle strength training on oropharyngeal dysphagia in subacute stroke patients: a randomised controlled trial. J Oral Rehabil. 2016;43(5):364–72. https://doi.org/10.1111/joor.12382.
Kushner DS, Peters K, Eroglu ST, Perless-Carroll M, Johnson-Greene D. Neuromuscular electrical stimulation efficacy in acute stroke feeding tube-dependent dysphagia during inpatient rehabilitation. Am J Phys Med Rehabil. 2013;92(6):486–95. https://doi.org/10.1097/PHM.0b013e31828762ec.
Simonelli M, Ruoppolo G, Iosa M, Morone G, Fusco A, Grasso MG, et al. A stimulus for eating. The use of neuromuscular transcutaneous electrical stimulation in patients affected by severe dysphagia after subacute stroke: a pilot randomized controlled trial. NeuroRehabilitation. 2019;44(1):103–10. https://doi.org/10.3233/NRE-182526.
Terré R, Mearin F. A randomized controlled study of neuromuscular electrical stimulation in oropharyngeal dysphagia secondary to acquired brain injury. Eur J Neurol. 2015;22(4):687-e44. https://doi.org/10.1111/ene.12631.
Zhang M, Tao T, Zhang Z-B, Zhu X, Fan W-G, Pu L-J, et al. Effectiveness of neuromuscular electrical stimulation on patients with dysphagia with medullary infarction. Arch Phys Med Rehabil. 2016;97(3):355–62. https://doi.org/10.1016/j.apmr.2015.10.104.
Sun Y, Chen X, Qiao J, Song G, Xu Y, Zhang Y, et al. Effects of transcutaneous neuromuscular electrical stimulation on swallowing disorders: a systematic review and meta-analysis. Am J Phys Med Rehabil. 2020;99(8):701. https://doi.org/10.1097/PHM.0000000000001397.
Crary MA, Mann GDC, Groher ME. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch Phys Med Rehabil. 2005;86(8):1516–20. https://doi.org/10.1016/j.apmr.2004.11.049.
Rofes L, Arreola V, Mukherjee R, Clavé P. Sensitivity and specificity of the Eating Assessment Tool and the Volume-Viscosity Swallow Test for clinical evaluation of oropharyngeal dysphagia. Neurogastroenterol Motil. 2014;26(9):1256–65. https://doi.org/10.1111/nmo.12382.
Belafsky PC, Mouadeb DA, Rees CJ, Pryor JC, Postma GN, Allen J, et al. Validity and reliability of the Eating Assessment Tool (EAT-10). Ann Otol Rhinol Laryngol. 2008;117(12):919–24. https://doi.org/10.1177/000348940811701210.
Cheney DM, Siddiqui MT, Litts JK, Kuhn MA, Belafsky PC. The ability of the 10-item eating assessment tool (EAT-10) to predict aspiration risk in persons with dysphagia. Ann Otol Rhinol Laryngol. 2015;124(5):351–4. https://doi.org/10.1177/0003489414558107.
Demir N, Arslan SS, İnal Ö, Ünlüer NÖ, Karaduman AA. Reliability and validity of the Turkish version of the swallow quality of life questionnaire. Fizyoterapi Rehabil. 2016;27(1):19–24. https://doi.org/10.21653/tfrd.271061.
Leow LP, Huckabee M-L, Anderson T, Beckert L. The impact of dysphagia on quality of life in ageing and Parkinson’s disease as measured by the swallowing quality of life (SWAL-QOL) questionnaire. Dysphagia. 2010;25(3):216–20. https://doi.org/10.1007/s00455-009-9245-9.
McHorney CA, Martin-Harris B, Robbins J, Rosenbek J. Clinical validity of the SWAL-QOL and SWAL-CARE outcome tools with respect to bolus flow measures. Dysphagia. 2006;21(3):141–8. https://doi.org/10.1007/s00455-005-0026-9.
Hogikyan ND, Sethuraman G. Validation of an instrument to measure voice-related quality of life (V-RQOL). J Voice. 1999;13(4):557–69. https://doi.org/10.1016/s0892-1997(99)80010-1.
Demir N, Arslan SS, İnal Ö, Karaduman AA. Reliability and validity of the Turkish eating assessment tool (T-EAT-10). Dysphagia. 2016;31(5):644–9. https://doi.org/10.1007/s00455-016-9723-9.
Tezcaner ZÇ, Aksoy S. Reliability and validity of the Turkish version of the voice-related quality of life measure. J Voice. 2017;31(2):262.e7-262.e11.
Langmore SE, Olney RK, Lomen-Hoerth C, Miller BL. Dysphagia in patients with frontotemporal lobar dementia. Arch Neurol. 2007;64(1):58–62. https://doi.org/10.1001/archneur.64.1.58.
Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration–aspiration scale. Dysphagia. 1996;11(2):93–8. https://doi.org/10.1007/BF00417897.
Neubauer PD, Hersey DP, Leder SB. Pharyngeal residue severity rating scales based on fiberoptic endoscopic evaluation of swallowing: a systematic review. Dysphagia. 2016;31(3):352–9. https://doi.org/10.1007/s00455-015-9682-6.
Frost J, Robinson HF, Hibberd J. A comparison of neuromuscular electrical stimulation and traditional therapy, versus traditional therapy in patients with longstanding dysphagia. Curr Opin Otolaryngol Head Neck Surg. 2018;26(3):167–73. https://doi.org/10.1097/MOO.0000000000000454.
Kushner DS, Johnson-Greene D, Cordero MK, Thomashaw SA, Rodriguez J. Swallowing outcomes and discharge destinations in acute-stroke tube-feeding-dependent dysphagia patients treated with neuromuscular-electrical-stimulation during inpatient rehabilitation. Am J Phys Med Rehabil. 2020;99(6):487–94. https://doi.org/10.1097/PHM.0000000000001353.
Güleç A, Albayrak I, Erdur Ö, Öztürk K, Levendoglu F. Effect of swallowing rehabilitation using traditional therapy, kinesiology taping and neuromuscular electrical stimulation on dysphagia in post-stroke patients: a randomized clinical trial. Clin Neurol Neurosurg. 2021;211: 107020. https://doi.org/10.1016/j.clineuro.2021.107020.
Byeon H. Combined effects of NMES and Mendelsohn maneuver on the swallowing function and swallowing-quality of life of patients with stroke-induced sub-acute swallowing disorders. Biomedicines. 2020;8(1):12. https://doi.org/10.3390/biomedicines8010012.
Ko KR, Park HJ, Hyun JK, Seo I-H, Kim TU. Effect of laryngopharyngeal neuromuscular electrical stimulation on dysphonia accompanied by dysphagia in post-stroke and traumatic brain injury patients: a pilot study. Ann Rehabil Med. 2016;40(4):600. https://doi.org/10.5535/arm.2016.40.4.600.
Altman KW, Schaefer SD, Yu G-P, Hertegard S, Lundy DS, Blumin JH. The voice and laryngeal dysfunction in stroke: a Report from the Neurolaryngology Subcommittee of the American Academy of Otolaryngology-Head and Neck Surgery. Otolaryngol Head Neck Surg. 2007;136(6):873–81. https://doi.org/10.1016/j.otohns.2007.02.032.
Giraldo-Cadavid LF, Leal-Leaño LR, Leon-Basantes GA, Bastidas AR, Garcia R, Ovalle S, et al. Accuracy of endoscopic and videofluoroscopic evaluations of swallowing for oropharyngeal dysphagia. Laryngoscope. 2017;127(9):2002–10. https://doi.org/10.1002/lary.26419.
González-Fernández M, Ottenstein L, Atanelov L, Christian AB. Dysphagia after stroke: an overview. Curr Phys Med Rehabil Rep. 2013;1(3):187–96. https://doi.org/10.1007/s40141-013-0017-y.
Ludlow CL, Humbert I, Saxon K, Poletto C, Sonies B, Crujido L. Effects of surface electrical stimulation both at rest and during swallowing in chronic pharyngeal dysphagia. Dysphagia. 2007;22(1):1–10. https://doi.org/10.1007/s00455-006-9029-4.
Cabib C, Ortega O, Kumru H, Palomeras E, Vilardell N, Alvarez-Berdugo D, et al. Neurorehabilitation strategies for poststroke oropharyngeal dysphagia: from compensation to the recovery of swallowing function. Ann NY Acad Sci. 2016;1380(1):121–38. https://doi.org/10.1111/nyas.13135.
Acknowledgements
The authors would like to thank clinical pharmacist Metin Sinan Elmalioglu for his assistance in editing the English version of this article and Rana Konyalioglu for her contributions in statistical evaluations. The authors are also grateful to Istanbul University Scientific Research Projects Coordination Unit for funding the study (Project Number 37068).
Funding
This study was funded by Istanbul University Scientific Research Projects Coordination Unit (Project Number 37068).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have a conflict of interest to report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tarihci Cakmak, E., Sen, E.I., Doruk, C. et al. The Effects of Neuromuscular Electrical Stimulation on Swallowing Functions in Post-stroke Dysphagia: A Randomized Controlled Trial. Dysphagia 38, 874–885 (2023). https://doi.org/10.1007/s00455-022-10512-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-022-10512-7