Abstract
This study investigated the relationship between tongue pressure during swallowing and dysphagia in patients with Parkinson’s disease (PD). A total of 24 patients with PD (12 men and 12 women, mean age 70.4 years) were studied. Their mean Hoehn and Yahr scale was 3.0 ± 1.3 (range 1–5). All participants underwent tongue pressure measurement and videofluorography during swallowing. Tongue pressure when swallowing 5 mL of barium on videofluorography was measured using a sensor sheet with five sensors. Based on the findings of videofluorography, the patients were divided into two groups: dysphagic PD group (n = 9) and non-dysphagic PD group (n = 15). The maximal magnitude (kPa), duration (s), time to peak pressure (s), and pressure gradient (kPa/s) of tongue pressure were analyzed for each part. For duration, time to peak pressure, and pressure gradient, similar values were calculated from the total waveform. There was no significant difference in maximal tongue pressure between the groups. The dysphagic PD group had prolonged duration of tongue pressure and time to peak pressure and a reduced pressure gradient compared with the non-dysphagic PD group. These results indicate that there is a clear difference in the temporal aspects of tongue pressure between the non-dysphagic and dysphagic PD patients. These differences provide the characteristics of tongue movement during swallowing in PD patients with dysphagia, which may be useful for the diagnosis and treatment of dysphagia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dysphagia is a common symptom in Parkinson’s disease (PD). It is seen in more than 70% of PD patients [1] and is the most important factor in death from aspiration pneumonia [2,3,4,5]. Various movement abnormalities occur in the swallowing organs in PD dysphagia, impairing food propulsion to the stomach. The most common characteristic is movement abnormalities in the oral phase [6,7,8,9,10]. In oral phase swallowing movements, tongue movement abnormalities in particular are a major factor increasing the risk of aspiration [8, 11].
There are many reports of tongue movement abnormalities in PD, but nearly all are subjective assessments from clinical observations or assessments based on videofluoroscopic swallowing study (VFSS) examinations [1, 10, 12,13,14,15,16]. VFSS makes it possible to see oral organ movement and the flow of food, but biomechanical movement in the oral phase cannot be quantitatively evaluated. With VFSS, there are also the problems of radiation exposure and patient burden. Considering the above, a non-invasive and objective assessment procedure of tongue movement during swallowing in PD patients should be established.
Several attempts have been made to objectively assess tongue movement in PD patients [15, 17]. According to a study that measured the maximum voluntary upward pressure of the tongue, the maximum tongue pressure in PD patients decreases as the disease stage progresses, and there is a decrease in endurance compared with healthy individuals of the same age group [15]. However, the method used in these measurements was not an assessment to reflect tongue-to-palate pressure during swallowing. In the oral phase of swallowing, the tongue pressure produced by contact of the tongue to the palate is a major driving force in propelling boluses from the oral cavity to the pharynx [18]. In recent years, using a tongue pressure measurement system with super-thin sensor sheets, associations have been reported between tongue pressure patterns during swallowing in healthy people [19, 20], changes with age [21], and dysphagia in stroke patients [22, 23]. To the best of our knowledge, no studies have measured tongue pressure during swallowing in PD patients and investigated its relationship with dysphagia. Objective assessments of tongue movement in PD patients may provide information that is useful in the diagnosis and treatment of dysphagia.
The purpose of this study was to examine the relationship between dysphagia and tongue pressure during swallowing in PD patients measured using a tongue pressure sensor system. We hypothesized that tongue pressure production during swallowing in PD patients with dysphagia would decrease. Measurement of tongue pressure during swallowing may be useful as a tool to detect tongue movement abnormalities in PD patients.
Methods
Participants
The subjects were 24 PD patients (12 men, 12 women; mean age ± SD: 70.4 ± 7.9 years; age range 54–89 years) who were either inpatients or outpatients at the Hyogo College of Medicine Hospital and Hyogo College of Medicine Sasayama Medical Center. Based on neurological diagnosis, they were classified by the Hoehn and Yahr scale as follows: (1) n = 3; (2) n = 7; (3) n = 4; (4) n = 6; and (5) n = 4. Mean disease duration was 7.3 ± 5.5 years. All subjects were assessed with the Unified Parkinson’s disease rating scale (UPDRS) III and the Japanese version of the swallowing disturbance questionnaire (SDQ-J) [24]. Patients who ingested food orally, were able to open their mouth so that the sensor sheet could be attached within the oral cavity, and had the ability to follow the examiner’s instructions were eligible for inclusion in the study. Patients with stroke or other central nervous system diseases that could cause dysphagia, those who were not ingesting food orally at all at the time of the study, and those who could not follow instructions due to dementia or cognitive dysfunction were excluded. This study was approved by the Ethics Committee of Hyogo College of Medicine (No. 1809), and informed consent was obtained from all patients.
Equipment
Tongue Pressure Measurement
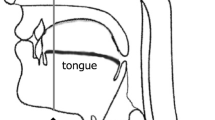
Tongue pressure during swallowing was measured using a tongue pressure sensor sheet (Swallow-SCAN, Nitta, Osaka, Japan) attached to the palate [20]. The sensor sheet (approximately 0.1 mm in thickness) had five pressure-sensitive parts, and measurements were made with channel 1 at the mid-anterior part, channel 2 at the mid-median part, channel 3 at the mid-posterior part, channel R at the right circumferential part, and channel L at the left circumferential part on the hard palate (Fig. 1). This sensor sheet is T-shaped to fit the curve of the palate. The sensor sheet comes in three sizes (small, medium, and large), so it could be selected to match the size of a subject’s palate. The sensor sheet was attached using a sheet-type denture adhesive (Touch Correct 2, Shionogi, Tokyo, Japan). To avoid occlusal interference, the sensor sheet wire came out of the oral cavity from the oral vestibule and was connected to a personal computer. The pressure measured by the sensor sheet was recorded at a sampling rate of 100 Hz and displayed on the personal computer in real time using dedicated analysis software.
VFSS
In this study, VFSS was recorded simultaneously with measurements of tongue pressure during swallowing in all patients. VFSS was performed from the side, visualizing a field of view that enabled assessment of the organs from the oral cavity to the upper esophagus and the flow of food. The tests were done with the subjects seated in a chair in an upright position. 5 mL of barium liquid (40% wt/volume%) was used as the test sample, and injected at the floor of the patient’s mouth with a syringe. The patient held this sample in his or her mouth until swallowing a total of three times at the direction of the examiner. Some patients were on anti-parkinsonian medications, and the tests were performed during a time when the PD symptoms were well-controlled. None of the patients showed signs of dyskinesias.
Data Analysis
Tongue Pressure During Swallowing
Maximal magnitude, duration, time to peak pressure, and gradient of tongue pressure were calculated from data recorded with the tongue pressure sensor sheet (Fig. 2a) [19]. The analysis was done with the following measures: maximal tongue pressure (kPa), which was the peak value on the tongue pressure waveform; duration, which was the time from tongue pressure onset to offset; time to peak pressure (s), which was the time to reach peak pressure from tongue pressure onset; and pressure gradient (kPa/s), which was the peak pressure divided by the time to peak pressure [25]. These measures were calculated for each channel in the analyses. Duration, time to peak pressure, and gradient pressure were also analyzed from the waveforms shown on all channels (Fig. 2b).
Swallowing Events on VFSS Images
Oral transit time (OTT) and pharyngeal transit time (PTT) were analyzed in a time phase analysis showing the flow of barium [26, 27]. OTT was defined at the time from the first frame in which a bolus moved posteriorly until the leading edge of the bolus passed the point where the base of the tongue and the lower border of the mandible intersect. PTT was defined as the time from the moment when the leading edge of the bolus passed the lower border of the mandible until the moment the trailing edge of the bolus passed the cricopharyngeal region. For OTT and PTT, the recorded VFSS videos were input to a personal computer and analyzed at 30 frames/s using Adobe Premiere Elements 11 (Adobe Japan, Tokyo, Japan).
With reference to previous studies, residue of the bolus in the oral cavity and pharynx was assessed on a scale of 1–3 points [28]. The amount of residue was assessed as follows: (1) no residue or a very small amount of adhering residue; (2) moderate residue or less than half of the initial amount; and (3) much residue or more than half of the initial amount. Penetration and aspiration were assessed based on 8 levels using the penetration aspiration scale (P-A scale) [29]. A score of 1 showed no penetration, 2–5 showed penetration, and 6–8 showed aspiration. In accordance with these criteria, patients with a score ≥ 2 for residue in the oral cavity and/or pharynx or a score ≥ 6 on the P-A scale were classified into the dysphagic PD group, with the remainder in the non-dysphagic PD group.
Statistical Analysis
To examine the temporal relationship between the flow of barium from the oral cavity to the pharynx and the tongue pressure during swallowing, the relationships between the temporal items of tongue pressure and OTT and PTT were analyzed using Pearson’s correlation coefficient. The unpaired t test, Mann–Whitney U test, and Fisher’s exact test were used in 2-group comparisons between those with and without dysphagia. Statistical analysis was performed using SPSS 22.0J (IBM Japan, Tokyo, Japan), with the level of significance set at α = 0.05.
Results
The demographic and clinical characteristics of the two groups are shown in Table 1. There were 15 people in the non-dysphagic PD group and 9 people in the dysphagic PD group. No significant differences were seen between the groups in sex, age, Hoehn and Yahr (H&Y) scale, disease duration, or UPDRS III or SDQ-J scores.
Comparisons of maximal magnitude, duration, time to peak pressure, and pressure gradient of tongue pressure at each channel between the non-dysphagic PD group and the dysphagic PD group are provided in Figs. 3, 4, 5, and 6. The means and the standard error of each comparison are given in Tables 2, 3, 4, and 5.
The maximal magnitude of tongue pressure was higher in the circumferential part (channel R and channel L) than in the mid part in both groups. In the mid part, higher values were seen from the anterior part (channel 1) to the posterior part (channel 2, channel 3), in order. Comparing the two groups, the maximal magnitude of tongue pressure was not significantly different at all channels (Fig. 3; Table 2).
The duration of tongue pressure was longer in the dysphagic PD group than in the non-dysphagic PD group at all channels. In the circumferential part (channels R and L), tongue pressure duration was significantly longer in the dysphagic PD group than in the non-dysphagic PD group (Fig. 4; Table 3). Table 6 shows a significant positive correlation between total tongue pressure duration during swallowing and OTT (r = 0.716, p < 0.001). No significant correlation was seen between total tongue pressure duration during swallowing and PTT (r = − 0.268, p = 0.205).
The time to peak pressure was significantly longer in the dysphagic PD group than in the non-dysphagic PD group at all channels except for channel 2 and channel 3 (Fig. 5; Table 4). A significant positive correlation was found between total time to peak pressure duration and OTT (r = 0.761, p < 0.001) (Table 6). No significant correlation was seen between total tongue pressure duration during swallowing and PTT (r = − 0.002, p = 0.994).
The pressure gradient at all channels except for channel 2 was significantly greater in the non-dysphagic PD group than in the dysphagic PD group (Fig. 6; Table 5). No significant correlation was seen between the total pressure gradient and OTT (r = − 0.172, p = 0.421) and PTT (r = − 0.265, p = 0.21) (Table 6).
Discussion
This study was the first to measure tongue pressure during swallowing in PD patients and clarify the relationship between parameters of tongue pressure and dysphagia. The most interesting finding was that, while there was no difference between the groups in the maximal magnitude of tongue pressure during swallowing, the dysphagic PD group had a longer duration and time to peak pressure than the non-dysphagic PD group. A tongue pressure gradient, in which agility of the tongue movement decreased, was also identified in dysphagic PD patients. This suggests that it may be possible to predict dysphagia in PD patients using the temporal aspects of tongue pressure as an indicator, which would also be clinically meaningful.
Past studies have reported factors such as decreased tongue elevation and decreased tongue propulsive force in PD patients [10, 30, 31]. The maximal magnitude of tongue pressure measured in this study is thought to be the propulsive force of the tongue needed to propel boluses. From the fact that no decrease was seen in the maximal magnitude of tongue pressure in the dysphagic group in this study, it is possible that using tongue propulsive force alone as an indicator may be not useful in understanding the progression of dysphagia in PD patients. Meanwhile, tongue pressure duration was significantly longer in the posterior periphery (channel R, channel L) in the dysphagic PD group than in the non-dysphagic PD group. These data may be able to explain the findings of tongue movement abnormalities reported in past studies, such as uncoordinated posterior tongue movement or longer tongue elevation [7]. In a study using tongue pressure sensor sheets, Tamine et al. [21] reported that healthy elderly people had a higher maximal magnitude of tongue pressure in the posterior periphery and longer tongue pressure duration than young people. This is thought to be due to increased posterior tongue pressure and regulation of swallowing pressure to extend duration in response to decreased anterior tongue pressure in elderly people. The results of the present study indicate that there may be compensatory movement in the dysphagic PD group by extending the posterior tongue-to-palate contact time when boluses are sent to the pharynx.
The time to peak pressure and the pressure gradient measured in this study may be considered indicators of tongue movement speed during swallowing. Umemoto et al. [15] measured the speed of tongue movement in PD patients with VFSS, and they reported a significant negative correlation with oropharyngeal transit time. In that study, no significant correlation was seen between maximum tongue pressure from voluntarily pushing the tongue upward and oropharyngeal transit time. They reasoned that, in PD patients, inhibition of food transit in the oral cavity and pharynx may be due more to tongue bradykinesia than to tongue muscle strength. In the present study as well, the total duration and time to peak pressure were significantly correlated with OTT. This shows that tongue movement speed affects the flow of boluses.
In the present study, tongue movement up to the generation of maximum tongue pressure during swallowing was delayed in PD patients with advanced dysphagia. It has been shown in previous studies that tongue movement is affected by bradykinesia and rigidity in PD patients [32,33,34]. Several reports suggest that the discharge patterns of motor units are irregular and intermittent despite motor units being recruited by voluntary effort in PD patients with bradykinesia [35,36,37]. This evidence may also help us to interpret the tongue movement abnormality observed as the delay of tongue movement during swallowing in the present study.
It has been reported that dysphagia in PD patients is a serious condition related to the severity of the disease [38], and that it tends to worsen with a longer disease course [39]. It has also been reported, however, that the level of dysphagia is not related to disease severity as determined by disability of the limbs or disease stage [9, 34]. In the present study, patients were divided into two groups by VFSS, but no significant differences were seen for the H&Y scale, disease duration, or UPDRS III, which are related to the severity of PD. This suggests that PD severity and the level of dysphagia are not necessarily related. In PD patients, it is possible for dysphagia to occur even when the disease stage is mild and there are no symptoms. It is characteristic of PD patients that, in many cases, subjective awareness of dysphagia is poor [40], and there is much silent aspiration. In the present study, all patients were interviewed with the SDQ-J, and there was no significant difference between the two groups. Since subjects respond subjectively regarding their own swallowing status on the SDQ-J, it is possible that there was an effect from a lack of subjective symptoms in the patients in this study.
The above indicates the importance of early screening for the risk of dysphagia in PD patients, regardless of disease severity or the presence or absence of symptoms. Dysphagia in PD presents diverse impairments from the oral cavity to the esophagus, but tongue movement abnormalities that occur from an early stage of PD are important as indicators that portend dysphagia [17]. The tongue pressure sensor sheets used in this study are limited to measurements of tongue-to-palate pressure in the oral cavity. Therefore, it is thought to be difficult to accurately diagnose dysphagia with only measurements of tongue pressure during swallowing. However, in PD patients, it may be possible to understand the degree to which dysphagia has progressed from changes in the oral phase. It was shown that a delay in tongue movement during swallowing was related to tongue movement abnormalities in PD patients. The above indicates that measurements of tongue pressure during swallowing may be useful for the assessment and treatment of dysphagia in PD patients. The present study demonstrated the difference in tongue pressure between the non-dysphagic and dysphagic PD groups. However, the present study sample was small, and further research with larger samples is necessary to analyze the parameters of tongue pressure in detail in PD patients.
Study Limitations
The results of this study must be interpreted in the light of a number of limitations. This study did not have a healthy control group, and differences in tongue pressures with the PD group remain unknown. Additionally, in this study, only a liquid consistency sample was used in measurements of tongue pressure during swallowing. Previous studies have reported that the modulations of tongue-palate pressure vary when swallowing different liquid consistencies [41,42,43]. Steele et al. reported that the amplitudes of tongue-palate pressure increased when swallowing thickened liquid stimuli by comparing swallows of water, honey-thick liquid, and two nectar-thick liquids [44]. Troche et al. [45] conducted an investigation with VFSS in PD patients and reported that the number of tongue pumps increased with samples having a pudding-thick consistency compared with liquids, and that oral transit time also increased. Liquids with greater viscosity cause larger amplitude and duration of tongue movement, which may be because of the time needed to adjust the flow properties of the bolus [17]. Moreover, tongue movement in the oral phase may change depending on the volume of liquid and food texture. This study used a single liquid volume, but it is possible that the tongue-to-palate contact during swallowing also changes with various volumes of liquid or food texture accompanying chewing motions. In future measurements of tongue pressure during swallowing in PD patients, various consistencies, volumes of liquid, and food textures will need to be investigated.
The number of patients in the present study was also small, and the relationship between PD severity and tongue pressure during swallowing could not be adequately investigated. Additionally, in the present patients, the condition of PD on/off was not strictly controlled. Detailed investigation will also be needed of changes in tongue pressure during swallowing with differences in disease severity, physical function, and PD symptoms.
Conclusions
This study measured tongue pressure during swallowing in PD patients and made comparisons between dysphagic and non-dysphagic groups classified based on videofluorography. In summary, the results showed no difference in the maximal magnitude of tongue pressure between the groups, but there were significant differences in tongue pressure duration, time to peak pressure, and pressure gradient. These findings suggest that measurement of the temporal aspects of tongue pressure during swallowing may be useful for the assessment of PD patients.
References
Edwards LL, Quigley EM, Harned RK, Hofman R, Pfeiffer RF. Characterization of swallowing and defecation in Parkinson’s disease. Am J Gastronterol. 1994;89(1):15–25.
Hely MA, Morris JG, Traficante R, Reid WG, O’Sullivan DJ, Williamson PM. The Sydney multicentre study of Parkinson’s disease: progression and mortality at 10 years. J Neurol Neurosurg Psychiatr. 1999;67:300–7.
Morgante L, Salemi G, Meneghini F, Di Rosa AE, Epifanio A, Grigoletto F, Ragonese P, Patti F, Reggio A, Di Perri R, Savettieri G. Parkinson disease survival: a population-based study. Arch Neurol. 2000;57:507–12.
Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344:665–71. https://doi.org/10.1056/nejm200103013440908.
Fernandez HH, Lapane KL. Predictors of mortality among nursing home residents with a diagnosis of Parkinson’s disease. Med Sci Monit. 2002;8:241–6.
Logemann J, Blonsky ER, Boshes B. Lingual control in Parkinson’s disease. Trans Am Neurol Assoc. 1973;98:276–8.
Blonsky ER, Logemann JA, Boshes B, Fisher HB. Comparison of speech and swallowing function in patients with tremor disorders and in normal geriatric patients: a cinefluorographic study. J Gerontol. 1975;30:299–303.
Robbins JA, Logemann JA, Kirshner HS. Swallowing and speech production in Parkinson’s disease. Ann Neurol. 1986;19:283–7. https://doi.org/10.1002/ana.410190310.
Ali GN, Wallace KL, Schwartz R, DeCarle DJ, Zagami AS, Cook IJ. Mechanisms of oral-pharyngeal dysphagia in patients with Parkinson’s disease. Gastroenterology. 1996;110:383–92.
Leopold NA, Kagel MC. Prepharyngeal dysphagia in Parkinson’s disease. Dysphagia. 1996;11:14–22.
Nakayama Y, Washio M, Mori M. Oral health conditions in patients with Parkinson’s disease. J Epidemiol. 2004;14:143–50.
Leopold NA, Kagel MC. Pharyngo-esophageal dysphagia in Parkinson’s disease. Dysphagia. 1997;12:11–8; discussion 19–20.
Nakashima K, Maeda M, Tabata M, Adachi Y, Kusumi M, Ohshiro H. Prognosis of Parkinson’s disease in Japan. Tottori University Parkinson’s Disease Epidemiology (TUPDE) Study Group. Eur Neurol. 1997;38(Suppl 2):60–3.
Nagaya M, Kachi T, Yamada T, Igata A. Videofluorographic study of swallowing in Parkinson’s disease. Dysphagia. 1998;13:95–100. https://doi.org/10.1007/pl00009562.
Umemoto G, Tsuboi Y, Kitashima A, Furuya H, Kikuta T. Impaired food transportation in Parkinson’s disease related to lingual bradykinesia. Dysphagia. 2011;26:250–5. https://doi.org/10.1007/s00455-010-9296-y.
Lin CW, Chang YC, Chen WS, Chang K, Chang HY, Wang TG. Prolonged swallowing time in dysphagic Parkinsonism patients with aspiration pneumonia. Arch Phys Med Rehabil. 2012;93:2080–4. https://doi.org/10.1016/j.apmr.2012.07.010.
Van Lieshout PH, Steele CM, Lang AE. Tongue control for swallowing in Parkinson’s disease: effects of age, rate, and stimulus consistency. Mov Disord. 2011;26:1725–9. https://doi.org/10.1002/mds.23690.
Shaker R, Cook IJ, Dodds WJ, Hogan WJ. Pressure-flow dynamics of the oral phase of swallowing. Dysphagia. 1988;3:79–84.
Ono T, Hori K, Nokubi T. Pattern of tongue pressure on hard palate during swallowing. Dysphagia. 2004;19:259–64.
Hori K, Ono T, Tamine K, Kondo J, Hamanaka S, Maeda Y, Dong J, Hatsuda M. Newly developed sensor sheet for measuring tongue pressure during swallowing. J Prosthodont Res. 2009;53:28–32. https://doi.org/10.1016/j.jpor.2008.08.008.
Tamine K, Ono T, Hori K, Kondoh J, Hamanaka S, Maeda Y. Age-related changes in tongue pressure during swallowing. J Dent Res. 2010;89:1097–101. https://doi.org/10.1177/0022034510370801.
Hirota N, Konaka K, Ono T, Tamine K, Kondo J, Hori K, Yoshimuta Y, Maeda Y, Sakoda S, Naritomi H. Reduced tongue pressure against the hard palate on the paralyzed side during swallowing predicts Dysphagia in patients with acute stroke. Stroke. 2010;41:2982–4. https://doi.org/10.1161/STROKEAHA.110.594960.
Konaka K, Kondo J, Hirota N, Tamine K, Hori K, Ono T, Maeda Y, Sakoda S, Naritomi H. Relationship between tongue pressure and dysphagia in stroke patients. Eur Neurol. 2010;64:101–7. https://doi.org/10.1159/000315140.
Yamamoto T, Ikeda K, Usui H, Miyamoto M, Murata M. Validation of the Japanese translation of the swallowing disturbance questionnaire in Parkinson’s disease patients. Qual Life Res. 2012;21:1299–303. https://doi.org/10.1007/s11136-011-0041-2.
Rogus-Pulia N, Churness K, Hind J, Gangnon R, Banaszynski K, Robbins J. Comparison of maximal lingual pressure generation during isometric gross and fine sensorimotor tasks in healthy adults. Arch Phys Med Rehabil. 2015;96:1785–94. https://doi.org/10.1016/j.apmr.2015.04.024.
Rademaker AW, Pauloski BR, Colangelo LA, Logemann JA. Age and volume effects on liquid swallowing function in normal women. J Speech Lang Hear Res. 1998;41:275–84.
Lof GL, Robbins J. Test–retest variability in normal swallowing. Dysphagia. 1990;4:236–42.
Daniels SK, Schroeder MF, McClain M, Corey DM, Rosenbek JC, Foundas AL. Dysphagia in stroke: development of a standard method to examine swallowing recovery. J Rehabil Res Dev. 2006;43:347–56.
Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11:93–8.
Volonte MA, Porta M, Comi G. Clinical assessment of dysphagia in early phases of Parkinson’s disease. Neurol Sci. 2002;23(Suppl 2):S121–2. https://doi.org/10.1007/s100720200099.
Bird MR, Woodward MC, Gibson EM, Phyland DJ, Fonda D. Asymptomatic swallowing disorders in elderly patients with Parkinson’s disease: a description of findings on clinical examination and videofluoroscopy in 16 patients. Age Ageing. 1994;2:251–4.
Bushmann M, Dobmeyer SM, Leeker L, Perlmutter JS. Swallowing abnormalities and their response to treatment in Parkinson’s disease. Neurology. 1989;39(10):1309–14.
Edwards LL, Quigley EM, Pfeiffer RF. Gastrointestinal dysfunction in Parkinson’s disease: frequency and pathophysiology. Neurology. 1992;42(4):726–32.
Nilsson H, Ekberg O, Olsson R, Hindfelt B. Quantitative assessment of oral and pharyngeal function in Parkinson’s disease. Dysphagia. 1996;11:144–50.
Petajan JH, Jarcho LW. Motor unit control in Parkinson’s disease and the influence of levodopa. Neurology. 1975;25:866–9.
Milner-Brown HS, Fisher MA, Weiner WJ. Electrical properties of motor units in Parkinsonism and a possible relationship with bradykinesia. J Neurol Neurosurg Psychiatr. 1979;42(1):35–41.
Glendinning DS, Enoka RM. Motor unit behavior in Parkinson’s disease. Phys Ther. 1994;74(1):61–70.
Leopold NA, Kagel MC. Laryngeal deglutition movement in Parkinson’s disease. Neurology. 1997;48:373–6.
Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23:837–44. https://doi.org/10.1002/mds.21956.
Noyce AJ, Silveira-Moriyama L, Gilpin P, Ling H, Howard R, Lees AJ. Severe dysphagia as a presentation of Parkinson’s disease. Mov Disord. 2012;27:457–8. https://doi.org/10.1002/mds.24006.
Pouderoux P, Kahrilas PJ. Deglutitive tongue force modulation by volition, volume, and viscosity in humans. Gastroenterology. 1995;108(5):1418–26.
Nicosia MA, Hind JA, Roecker EB, Carnes M, Doyle J, Dengel GA, Robbins J. Age effects on the temporal evolution of isometric and swallowing pressure. J Gerontol Ser A: Biol Sci Med Sci. 2000;55(11):M634–40.
Steele CM, Bailey GL, Molfenter SM. Tongue pressure modulation during swallowing: water versus nectar-thick liquids. J Speech Lang Hear Res. 2010;53(2):273–83. https://doi.org/10.1044/1092-4388(2009/09-0076).
Steele CM, Molfenter SM, Peladeau-Pigeon M, Polacco RC, Yee C. Variations in tongue-palate swallowing pressures when swallowing xanthan gum-thickened liquids. Dysphagia. 2014;29(6):678–84. https://doi.org/10.1007/s00455-014-9561-6.
Troche MS, Sapienza CM, Rosenbek JC. Effects of bolus consistency on timing and safety of swallow in patients with Parkinson’s disease. Dysphagia. 2008;23:26–32. https://doi.org/10.1007/s00455-007-9090-7.
Acknowledgements
The authors gratefully acknowledge the members of the division of Neurology, Department of Internal Medicine, Hyogo College of Medicine for their assistance and suggestions.
Funding
This research was supported by JSPS KAKENHI Grant-in-Aid for Encouragement of Scientists (Number JP15H00635).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any commercial or financial involvement in connection with this study that represent or appear to represent any conflicts of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Fukuoka, T., Ono, T., Hori, K. et al. Tongue Pressure Measurement and Videofluoroscopic Study of Swallowing in Patients with Parkinson’s Disease. Dysphagia 34, 80–88 (2019). https://doi.org/10.1007/s00455-018-9916-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-018-9916-5