Abstract
Dysphagia and aspiration are prevalent in amyotrophic lateral sclerosis (ALS) and contribute to malnutrition, aspiration pneumonia, and death. Early detection of at risk individuals is critical to ensure maintenance of safe oral intake and optimal pulmonary function. We therefore aimed to determine the discriminant ability of voluntary cough airflow measures in detecting penetration/aspiration status in ALS patients. Seventy individuals with ALS (El-Escorial criteria) completed voluntary cough spirometry testing and underwent a standardized videofluoroscopic swallowing evaluation (VFSE). A rater blinded to aspiration status derived six objective measures of voluntary cough airflow and evaluated airway safety using the penetration–aspiration scale (PAS). A between groups ANOVA (safe vs. unsafe swallowers) was conducted and sensitivity, specificity, area under the curve (AUC) and likelihood ratios were calculated. VFSE analysis revealed 24 penetrator/aspirators (PAS ≥3) and 46 non-penetrator/aspirators (PAS ≤2). Cough volume acceleration (CVA), peak expiratory flow rise time (PEFRT), and peak expiratory flow rate (PEFR) were significantly different between airway safety groups (p < 0.05) and demonstrated significant discriminant ability to detect the presence of penetration/aspiration with AUC values of: 0.85, 0.81, and 0.78, respectively. CVA <45.28 L/s/s, PEFR <3.97 L/s, and PEFRT >76 ms had sensitivities of 91.3, 82.6, and 73.9 %, respectively, and specificities of 82.2, 73.9, and 78.3 % for identifying ALS penetrator/aspirators. Voluntary cough airflow measures identified ALS patients at risk for penetration/aspiration and may be a valuable screening tool with high clinical utility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease causing concurrent upper and lower motor neuron injury, resulting in progressive weakness and spasticity of limb, respiratory and bulbar musculature. ALS patients are at increased risk for airway compromise due to dysphagia (leading to material entering the airway during swallowing) and dystussia (contributing to the inability to effectively eject tracheal aspirate). Sequelae of aspiration in ALS include malnutrition, compromised respiratory function and respiratory failure, pneumonia, increased risk of death and degraded quality of life and psychological health [1–5]. Early identification of at risk individuals is critical to ensure optimal medical management, safe oral intake, and preservation of pulmonary function.
Traditional clinical swallowing evaluations (CSE) assess the movement, integrity, and function of the oral motor structures and performance during liquid and solid food swallowing trials [6]. One crucial limitation of the CSE, however, is the inability to accurately detect silent aspiration [7]. A recent study in 107 hospitalized patients, for example, found that the CSE identified only 30 % of aspirators confirmed by the gold standard instrumental videofluoroscopic swallowing evaluation (VFSE) [8]. This specific limitation of the CSE is of particular relevance to the ALS patient population, who we have documented have a relatively high incidence of silent aspiration (55 %) [9] coupled with the fact that silent aspiration has been noted to confer a 13-fold increase in the likelihood of pneumonia in dysphagic individuals, likely due to the challenges of properly gaging and mitigating aspiration risk [10].
The co-occurrence of dysphagia and dystussia across a number of neurologic patient populations has focused attention on the potential utility of cough testing in assessing aspiration risk during the CSE [11]. In Parkinson’s disease (PD), voluntary cough airflow measures have been noted to differ in patients with and without dysphagia (defined by penetration–aspiration scale scores) [12, 13] and demonstrated to have high sensitivity and specificity in detecting penetration/aspiration confirmed by VFSE [14]. Additionally, decreased cough sensitivity and a blunted urge to cough were noted in dysphagic PD patients in a capsaicin challenge task [15]. In stroke patients, both voluntary cough spirometry airflow parameters [16] and reflexive cough testing [7] have been documented to show good discriminant ability to identify aspirators versus non-aspirators.
The potential role of voluntary cough airflow measures in detecting aspiration in individuals with ALS has not been previously investigated. The purpose of the current study was to determine the discriminant ability of voluntary cough airflow measures in detecting the presence of penetration/aspiration during swallowing in individuals with ALS. We hypothesized that voluntary cough airflow would differ between safe and unsafe ALS swallowers and demonstrate a high sensitivity and specificity for the identification of penetration and aspiration.
Materials and Methods
Participants
Seventy individuals with a diagnosis of probable or definite ALS by the Revised El-Escorial Criteria [17] were evaluated. Mean age was 62.9 years (range 30–83, SD 9.9), 61.4 % were males (n = 43), mean ALSFRS-R score was 34.7 (range 16–47, SD 7.4), and mean disease duration from symptom onset was 17.2 months (range 2–72 months, SD 13.1). Diagnosis was confirmed by a neuromuscular neurologist at a university ALS clinic. Patients were enrolled if they had (1) preserved cognition as evidenced by a score of >24 points on the Mini Mental Status Exam [18]; (2) no allergies to barium; (3) no tracheotomy or mechanical invasive ventilation; (4) absence of diaphragmatic pacer; and (5) no significant concurrent respiratory disease. All patients who met these inclusion criteria were offered to participate in the study.
Procedures and Outcomes
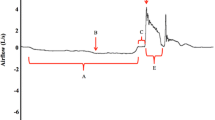
This study was approved by the local university Institutional Review Board (Protocol 00005321 and Protocol 00009178) and conducted in accordance to the Declaration of Helsinki. All patients meet the inclusion criteria and provided informed written consent to participate. Following consent, participants attended a single testing session that included voluntary cough spirometry testing and a standardized VFSE. Cough was assessed using an oral pneumotachograph (MLT 1000, ADInstruments, Inc; Colorado Springs, CO), connected to a spirometer filter (MQ 304 Spirometer Filter, Vacumed; Ventura, CA) during voluntary cough production. A 3-L syringe was used to calibrate airflow and volume. The participant was seated with a nasal clip in place to occlude nasal airflow during cough production. The spirometry filter was attached to the pneumotachograph, placed in the patient’s mouth by the examiner, and held in place throughout the duration of cough testing. The examiner placed their thumb and index finger around the upper and lower lip line, as needed, to ensure adequate seal around the mouthpiece of the filter and to minimize labial air leakage. Patients were instructed to complete three tidal breaths into the pneumotachograph, take a deep breath in and then “cough hard like something is stuck in your throat.” The examiner modeled three very strong coughs in succession to ensure that the patient understood the requirements of this task. Airflow signal was measured, low-pass filtered at 150 Hz, digitized at 1000 Hz and displayed on a portable laptop computer using Lab Chart Version 7 (Microsoft Corp; Redmond, WA). For the subsequent analysis the airflow waveforms were low-pass filtered at 60 Hz. Physiologic voluntary cough measures derived from the cough flow waveforms included: (1) Inspiratory phase duration (IPD): time from onset of inspiration following tidal volume breathing to the end of inspiration prior to the compression phase of cough; (2) Peak inspiratory flow rate (PIFR): peak inspiratory flow during the inspiratory phase preceding the cough; (3) Compression phase duration (CPD): time from the end of the inspiratory phase to the beginning of the expiratory phase; (4) Peak expiratory flow rise time (PEFRT): time from the beginning of expiratory phase to the peak expiratory flow; (5) Peak expiratory flow rate (PEFR): peak airflow during the expiratory phase of the cough; and (6) cough volume acceleration (CVA): Peak expiratory flow rate dividing by peak expiratory flow rise time (CVA = PEFR/PEFRT). Figure 1 provides an example of a cough flow waveform with these measures identified. Cough spirometry measures were performed on the three cough epochs produced in the test, and the average across the cough waveforms used for data analysis.
Example of voluntary cough spirometry waveform depicting derived objective measures. Four temporal measures that include inspiratory phase duration, compression phase duration, expiratory phase duration, and peak expiratory flow rise time (PEFRT) are shown. In addition, measures of peak inspiratory flow rate (PIFR) and peak expiratory flow rate (PEFR) are identified. Cough volume acceleration (CVA) is not depicted but is calculated by dividing PEFRT by PEFR (i.e., CVA = PEFR/PEFRT)
In the VFSE, patients were seated upright in a lateral viewing plane using a properly collimated Phillips BV Endura fluoroscopic C-arm unit (GE OEC 8800 Digital Mobile C-Arm system type 718074). A Swallowing Signals Lab unit (Kay Pentax, Lincoln Park, NJ) digitally recorded the fluoroscopic images at 29.97 frames per second using a scan converter. A video counter was used to imprint a time code and the participants’ identification code on each examination to aid subsequent analysis. In accordance with a standard protocol, a penny (U.S. currency, 1.9 cm) was taped to the chin or neck of the patient for measurement calibration. Lateral views were obtained while the patient, seated in an examination chair, was administered liquid barium measured with a syringe or graduated medicine cup. A standardized bolus presentation protocol was used and consisted of two 1-cc boluses of thin liquid contrast agent (Varibar Thin, EZ-EM, Inc., Westbury, NY), one 3-cc of thin liquid contrast, one 3-cc of pudding (EZ-pudding, EZ-EM, Inc.), one 20-cc bolus of thin liquid contrast and sequential bolus swallowing of a 90-cc thin liquid contrast. Airway safety was evaluated using the validated penetration–aspiration scale (PAS) [19] an eight-point ordinal scale of airway safety describing the degree of airway invasion, the participant’s response, and whether the invasive material is successfully ejected from the airway. Two raters independently performed the PAS analysis in a blinded fashion on every bolus trial. For statistical analysis, the worst PAS score was utilized as has been previously performed [12]. When agreement was not reached on a PAS score, a third expert rater determined the final PAS score. This discordance occurred only in one subject.
Statistical Analysis
ALS patients were divided into two airway safety groups using the conventionally accepted method. Safe swallowers or non-penetrator/aspirators included all patients with a PAS ≤2. Unsafe swallowers or penetrator/aspirators scored a PAS ≥3. A between groups analysis of variance (ANOVA) was performed to determine if differences in voluntary cough airflow measures existed across airway safety groups. Sensitivity, specificity, receiver operator characteristics, and likelihood ratios were then calculated to determine the discriminative ability of voluntary cough airflow measures to identify safe versus unsafe swallowers. SPSS (Version 17) was used for all statistical tests and alpha was set at 0.05.
Results
Voluntary Cough Profiles for Safe vs. Unsafe ALS Swallowers
VFSE analysis identified 24 penetrator/aspirators and 46 non-penetrator/aspirators. Percentage of safe swallowing (PAS ≤ 2) for each bolus trial was as follows: 95.5 % on 1 cc thin, 89.4 % on 3 cc thin, 98.5 % on 3 cc paste, 83.1 % on 20 cc thin and 72.2 % on 90 cc thin. Table 1 summarizes ANOVA results comparing voluntary cough airflow measure means (SEMS and 95 % confidence intervals provided) across swallow airway safety groups. Significant differences between safe versus unsafe swallowers were observed for CVA [F(1,68) = 22.67, p < 0.0001], PEFRT [F(1,68) = 18.67, p < 0.0001], and PEFR [F(1,68) = 9.80, p < 0.004] (Fig. 2).
Videofluoroscopic image and voluntary cough airflow waveform examples from an ALS patient who demonstrated safe swallowing (a) and an ALS patient who aspirated without a cough response (b). The aspirator demonstrated a smaller peak inspiratory flow rate (PIFR), a longer compression phase duration (CPD), and a smaller peak expiratory flow rate (PEFR). Asp aspiration
Discriminant Ability of Voluntary Cough Airflow Measures for Detecting Aspiration
Three voluntary cough airflow measures were significant for the detection of penetration/aspiration (PAS ≥3). These measures, in rank order of significance, included: 1: CVA (AUC = 0.83, p < 0.001); 2: PEFRT (AUC = 0.82, p < 0.001); and 3: PEFR (AUC = 0.78, p < 0.001). Table 2 summarizes ROC analysis data and Fig. 3 displays ROC graphs for CVA, PEFRT, and PEFR. Sensitivity values were 91.3, 82.6, and 73.9 for CVA, PEFRT, and PEFR, respectively. Specificity values were 82.2, 73.9, and 78.3 for CVA, PEFRT, and PEFR, respectively. Cut points and the associated likelihood ratios are provided in Table 2.
Discussion
In this first examination of cough airflow patterns in ALS, we report that patients with unsafe swallowing demonstrate different expiratory airflow characteristics during voluntary cough as compared to ALS patients with safe swallowing. Specifically, CVA, a measure of cough effectiveness, was on average three times lower in ALS patients who penetrated/aspirated. ALS penetrator/aspirators PEFR’s were 50 % lower and the time to reach this maximal expiratory flow value during voluntary cough was two times longer. Further, CVA, PEFR, and PEFRT were identified as significant predictors of penetration/aspiration status in this group of ALS patients with lower and slower cough expulsion parameters predictive of poor airway safety during swallowing. ALS patients with a CVA less than 45.3 L/s/s were 5.6 times more likely to penetrate/aspirate. This value accurately identified 90.5 % of penetrator/aspirators and 82.2 % of non-penetrator/aspirators. PEFR’s lower than 4.0L correctly identified 73.9 % of unsafe swallowers. An ALS patient was 3.6 times more likely to penetrate/aspirate if his/her PEFR was lower than this cut-off. ALS patients whose time to reach the peak expiratory flow rate (PEFRT) was greater than 80 ms were 3.2 times more likely to penetrate/aspirate. This threshold identified 82.6 % of unsafe swallowers and 73.9 % of safe swallowers.
In this group of ALS patients, those with impaired airway protection during swallowing demonstrated lower peak expiratory flow rates, a longer time to generate maximum expiratory flow during voluntary cough and thus a less efficient, effective or expulsive voluntary cough (as indexed by a lower CVA). Recent animal work has highlighted the shared neuroanatomical configurations of both cough and swallow respiratory brainstem centers [11, 20, 21]. Thus, it is not surprising that ALS patients with dysphagia also demonstrate dystussia or aberrant voluntary cough. Furthermore, there is a strong association between bulbar dysfunction in ALS and the onset of respiratory weakness, making aspiration even more likely and more dangerous in this sub-population.
The noted impairments in the expiratory phase of voluntary cough in dysphagic ALS patients are likely related to the degeneration of the upper aerodigestive tract, respiratory, and laryngeal muscles that might compromise the ballistic force of the cough. The expiratory phase is initiated when the glottis opens (via vocal fold abduction) and the expiratory muscles (rectus abdominis, transversus abdominis, internal obliques, external obliques) contract to forcibly expel air from the lungs. Reductions in the ability to generate adequate expiratory airflow and the ability to reach peak airflow velocities in an efficient and timely manner in ALS are likely related to concomitant spasticity and atrophic weakness. Spasticity of the upper aerodigestive tract leads to muscle stiffness, slowness, and decreased contraction velocity. Atrophy contributes to flaccidity, weakness, and loss of laryngeal and expiratory forces required for a normal expiratory phase of cough. These factors may account for the reduced expiratory ballistic force seen in ALS patients who penetrate/aspirate. Weakness of the diaphragm, inspiratory, and laryngeal adductory muscles may further prevent the inhalation of adequate lung volume and complete glottic closure to adequately build subglottic pressure. Although both inspiratory and expiratory muscle strength are important for cough production, a recent investigation examining relationships between peak cough flow generation and inspiratory vs. expiratory muscle strength across different patient populations demonstrated that in ALS patients who had profound inspiratory and expiratory weakness, only maximum expiratory pressure was related to peak cough flow generation [22]. These authors concluded that since ALS patients have difficulties obtaining sufficient pre-cough lung volumes, their cough might depend predominantly on expiratory muscle strength [22].
These data documenting the discriminant ability of voluntary cough for predicting airway safety during swallowing add to a small but growing dataset highlighting the potential utility of cough testing to aide in the CSE. In stroke patients, the identical expiratory cough airflow measures (CVA, PEFR and PEFRT) were identified as being significant predictors of penetration/aspiration status. Similarly, in PD the same three expiratory airflow measures have been identified as showing discriminant ability to detect penetrator/aspirators. In this particular patient population, CPD was also identified as a predictor for unsafe swallowing and had the highest sensitivity and specificity for accurately identifying PD penetrator/aspirators. This finding is likely related to the bradykinesia that represents a hallmark characteristic in PD.
Although the voluntary cough airflow testing included in the current investigation could be performed at the patients’ bedside; equipment costs, software and analysis requirements represent prohibitive factors in its implementation. Silverman et al. [23] recently documented that inexpensive hand-held peak cough flow meters capable of immediate digital read outs demonstrate a high concordance with the gold standard cough spirometry utilized in this investigation and therefore may be a useful alternative for use at the bedside without the need for expensive equipment, software or timely analysis.
Several limitations of the current investigation need to be highlighted. First, since the specific intent was to determine the discriminant ability of voluntary cough airflow on penetration/aspiration status, we utilized the PAS score as the only swallowing outcome. Although this suited the goals of this work, it must be acknowledged that the PAS score represents only one aspect of the disordered swallow and is therefore not a comprehensive measure of swallowing ability. Second, it must be highlighted that voluntary cough elicitation such as that utilized in the current study is different from a reflexive cough response elicited in response to tracheal aspiration. Although there have been reports that both voluntary and reflexive cough share similar motor output patterns [12, 15, 24], there are clear differences regarding the cortical input of these two distinct types of cough events. Future studies examining the utility of cough screening in the CSE of ALS patients, (recently performed in stroke [7] and PD [15] patient populations) will focus on induced or reflexive cough testing using irritant stimuli (e.g., fog, capsaicin, or citric acid). Finally, these results indicate that voluntary cough was able to detect the presence of penetration or aspiration during swallowing and do not necessarily predict short or long-term health outcomes.
Conclusions
This study is the first to illustrate the predictive relationship between objective measures of voluntary cough airflow and airway safety in individuals with ALS. Our results indicate that measures of reduced CVA, and PEFR, in addition to longer PEFRT’s, have discriminating ability to detect penetration/aspiration in ALS. These findings may be related to a reduced motor drive in both respiratory and deglutitive musculature in this patient population. Expiratory airflow measures of voluntary cough during the CSE may be clinically useful for assessing aspiration risk in individuals with ALS. Future studies will evaluate the utility of reflexive cough testing in this vulnerable patient population.
References
Chen A, Garrett CG. Otolaryngologic presentations of amyotrophic lateralsclerosis. Otolaryngol Head Neck Surg. 2005;132(3):500–4. doi:10.1016/j.otohns.2004.09.092.
Chio A, Logroscino G, Hardiman O, Swingler R, Mitchell D, Beghi E, Traynor BG, Eurals C. Prognostic factors in ALS: a critical review. Amyotroph Lateral Scler. 2009;10(5–6):310–23. doi:10.3109/17482960802566824.
Kuhnlein P, Gdynia HJ, Sperfeld AD, Lindner-Pfleghar B, Ludolph AC, Prosiegel M, Riecker A. Diagnosis and treatment of bulbar symptoms in amyotrophic lateral sclerosis. Nat Clin Pract Neurol. 2008;4(7):366–74. doi:10.1038/ncpneuro0853.
Paris G, Martinaud O, Petit A, Cuvelier A, Hannequin D, Roppeneck P, Verin E. Oropharyngeal dysphagia in amyotrophic lateral sclerosis alters quality of life. J Oral Rehabil. 2013;40(3):199–204. doi:10.1111/joor.12019.
Yang R, Huang R, Chen D, Song W, Zeng Y, Zhao B, Zhou D, Shang HF. Causes and places of death of patients with amyotrophic lateral sclerosis in south-west China. Amyotroph Lateral Scler. 2011;12(3):206–9. doi:10.3109/17482968.2011.572979.
Leonard R, Kendall K. Dysphagia assessment and treatment planning. 3rd ed. San Diego: Plural Publishing; 2013.
Miles A, Zeng IS, McLauchlan H, Huckabee ML. Cough reflex testing in Dysphagia following stroke: a randomized controlled trial. J Clin Med Res. 2013;5(3):222–33. doi:10.4021/jocmr1340w.
Splaingard ML, Hutchins B, Sulton LD, Chaudhuri G. Aspiration in rehabilitation patients: videofluoroscopy vs bedside clinical assessment. Arch Phys Med Rehabil. 1988;69(8):637–40.
Gaziano J, Tabor L, Richter J, Plowman EK Prevalence, timing and source of aspiration in individuals with ALS. In: Dysphagia Research Society. Chicago; 2015.
Pikus L, Levine MS, Yang YX, Rubesin SE, Katzka DA, Laufer I, Gefter WB. Videofluoroscopic studies of swallowing dysfunction and the relative risk of pneumonia. AJR Am J Roentgenol. 2003;180(6):1613–6. doi:10.2214/ajr.180.6.1801613.
Troche MS, Brandimore AE, Godoy J, Hegland KW. A framework for understanding shared substrates of airway protection. J Appl Oral Sci. 2014;22(4):251–60.
Hegland KW, Okun MS, Troche MS. Sequential voluntary cough and aspiration or aspiration risk in Parkinson’s disease. Lung. 2014;192(4):601–8. doi:10.1007/s00408-014-9584-7.
Pitts T, Bolser D, Rosenbek J, Troche M, Sapienza C. Voluntary cough production and swallow dysfunction in Parkinson’s disease. Dysphagia. 2008;23(3):297–301. doi:10.1007/s00455-007-9144-x.
Pitts T, Troche M, Mann G, Rosenbek J, Okun MS, Sapienza C. Using voluntary cough to detect penetration and aspiration during oropharyngeal swallowing in patients with Parkinson disease. Chest. 2010;138(6):1426–31. doi:10.1378/chest.10-0342.
Troche MS, Brandimore AE, Okun MS, Davenport PW, Hegland KW. Decreased cough sensitivity and aspiration in Parkinson disease. Chest. 2014;146(5):1294–9. doi:10.1378/chest.14-0066.
Smith Hammond CA, Goldstein LB, Horner RD, Ying J, Gray L, Gonzalez-Rothi L, Bolser DC. Predicting aspiration in patients with ischemic stroke: comparison of clinical signs and aerodynamic measures of voluntary cough. Chest. 2009;135(3):769–77. doi:10.1378/chest.08-1122.
Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(5):293–9.
Reisberg B, Ferris SH, de Leon MJ, Crook T. The global deterioration scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139(9):1136–9.
Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11(2):93–8.
Bolser DC, Gestreau C, Morris KF, Davenport PW, Pitts TE. Central neural circuits for coordination of swallowing, breathing, and coughing: predictions from computational modeling and simulation. Otolaryngol Clin North Am. 2013;46(6):957–64. doi:10.1016/j.otc.2013.09.013.
Pitts T, Rose MJ, Mortensen AN, Poliacek I, Sapienza CM, Lindsey BG, Morris KF, Davenport PW, Bolser DC. Coordination of cough and swallow: a meta-behavioral response to aspiration. Respir Physiol Neurobiol. 2013;189(3):543–51. doi:10.1016/j.resp.2013.08.009.
Park JH, Kang SW, Lee SC, Choi WA, Kim DH. How respiratory muscle strength correlates with cough capacity in patients with respiratory muscle weakness. Yonsei Med J. 2010;51(3):392–7. doi:10.3349/ymj.2010.51.3.392.
Silverman EP, Carnaby-Mann G, Pitts T, Davenport P, Okun MS, Sapienza C. Concordance and discriminatory power of cough measurement devices for individuals with Parkinson disease. Chest. 2014;145(5):1089–96.
Wheeler Hegland K, Troche MS, Brandimore AE, Davenport PW, Okun MS. Comparison of voluntary and reflex cough effectiveness in Parkinson’s disease. Parkinsonism Relat Disord. 2014;20(11):1226–30. doi:10.1016/j.parkreldis.2014.09.010.
Acknowledgments
This study was funded, in part, by Grant R21HD075327 from the National Institute of Child Health and Development.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Plowman, E.K., Watts, S.A., Robison, R. et al. Voluntary Cough Airflow Differentiates Safe Versus Unsafe Swallowing in Amyotrophic Lateral Sclerosis. Dysphagia 31, 383–390 (2016). https://doi.org/10.1007/s00455-015-9687-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-015-9687-1