Abstract
In this study, the efficacy of two dysphagia interventions, the Chin Tuck against Resistance (CTAR) and Shaker exercises, were evaluated based on two principles in exercise science—muscle-specificity and training intensity. Both exercises were developed to strengthen the suprahyoid muscles, whose contractions facilitate the opening of the upper esophageal sphincter, thereby improving bolus transfer. Thirty-nine healthy adults performed two trials of both exercises in counter-balanced order. Surface electromyography (sEMG) recordings were simultaneously collected from suprahyoid muscle group and sternocleidomastoid muscle during the exercises. Converging results using sEMG amplitude analyses suggested that the CTAR was more specific in targeting the suprahyoid muscles than the Shaker exercise. Fatigue analyses on sEMG signals further indicated that the suprahyoid muscle group were equally or significantly fatigued (depending on metric), when participants carried out CTAR compared to the Shaker exercise. Importantly, unlike during Shaker exercise, the sternocleidomastoid muscles were significantly less activated and fatigued during CTAR. Lowering the chin against resistance is therefore sufficiently specific and intense to fatigue the suprahyoid muscles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Specificity and intensity (or overload) are two basic principles of exercise training perceived as applicable to swallowing rehabilitation [1]. Specificity refers to the importance of the activity to improve the strength of the target muscles. Intensity refers to the exercise’s ability to overload the target muscles. The increased demand due to overloading is known to cause muscle fatigue and trigger neuronal adaptations, including muscle strengthening [see 2]. In this paper, we evaluate the efficacy of two dysphagia therapy exercises, the Shaker exercise [3] and the Chin Tuck against Resistance (CTAR) exercise [4], based on the tenets of muscle specificity and intensity (operationalized in terms of muscle fatigue).
Muscle-Specificity and the Two Exercises
Both the Shaker and CTAR exercises were specifically designed to improve swallowing by strengthening the suprahyoid musclesFootnote 1 [3, 4]. The reason for targeting the suprahyoids is that during a normal swallow, adequate opening of the upper esophageal sphincter (UES) is important for a smooth bolus transit during the pharyngeal phase. Diminished UES opening appears to be a significant risk factor especially for the elderly population [5], although the true prevalence of dysphagia secondary to reduced UES opening is not yet known. Opening the UES depends on four main factors: (1) the relaxation of the tonically contracted sphincter muscle; (2) distensibility of the cricopharyngeus to stretch and accommodate an incoming bolus; (3) the bolus properties (e.g., size), which affects the bolus drive and hypopharyngeal pressure; and finally, (4) the traction generated by suprahyoid muscle contraction [6]. Out of these, only the suprahyoid contraction is accessible for direct intervention with current methodologies. The ideal therapy intervention to help patients with dysphagia due to reduced UES opening would thus be one that specifically maximizes recruitment of the suprahyoid muscles.
The suprahyoid muscles are a collective group made up of the digastricus, mylohyoid, geniohyoid, and the stylohyoid muscles. Structurally, each of these muscles has insertions on the hyoid bone. When the suprahyoid muscles contract, the hyoid bone is elevated upward and forward [7]. This superior–anterior hyoid movement triggers cascading biomechanical events (e.g., thyrohyoid shortening) that eventually pull open the UES [6]. Evidence supporting this direct relationship between the suprahyoid contraction, which results in hyoid displacement, and UES opening is well-documented (For evidence tracking the biomechanical correlates between the suprahyoid activity and UES opening: see [8] and [9]; for clinical reports on esophageal obstruction following suprahyoid fibrosis: see [10] or [11]; for an overview: see [12]). For this reason, specificity in targeting the suprahyoid muscles is likely to be important during therapeutic intervention.
The Shaker exercise has two component tasks that target the isometric and isokinetic muscle contractions respectively. Patients first lie supine on a flat surface. They then perform three sustained head lifts for 60-s (with a 60-s rest between each lift; this is the isometric task), followed by thirty consecutive head lifts (the isokinetic task). Both isometric and isokinetic tasks should be performed three times per day for 6 weeks [3]. Autunes and Lunet [13] provided a comprehensive review of the research corroborating the treatment efficacy of the Shaker exercise (alternatively, see Easterling [14]). The strongest piece of evidence is provided by Shaker et al. [15]—after 6 weeks of performing the Shaker exercise, eleven older adults who were tube-fed and diagnosed with dysphagia secondary to abnormal UES opening were able to resume oral feeding. Importantly, the same pattern of results was replicated for a separate group of patients (N = 7) who crossed over from the placebo exercise to complete the 6-week Shaker exercise regime. Comparison of videofluoroscopy images taken before and after the Shaker regime also revealed significant increase in the anteroposterior diameter of the UES opening and the maximum anterior laryngeal excursion post-exercise. Follow-up research [16] was conducted on another relatively small group of adult patients (N = 11) to compare the effects of Shaker exercise against other swallowing therapies, such as the Mendelsohn manoeuver and tongue base exercises. Greater reduction in post-swallow aspiration was observed in patients performing the Shaker exercise (N = 5) versus those undergoing other swallowing therapies (N = 6), thus providing further clinical validation for the Shaker exercise’s usefulness.
Still, in terms of muscle-specificity, a primary limitation of the Shaker exercise was its early fatiguing of auxiliary muscles, most notably the sternocleidomastoid muscles, at the expense of the target suprahyoid muscles [17]. The Shaker exercise’s activation of non-targeted muscles is a consequence of its exercise dynamics. Performing head lifts from a supine position involves lifting one’s head against gravity, and this movement incorporates elements from both upper and lower cervical flexion. This means the principal muscles involved include the anterior vertebral flexors, assisted by the suprahyoids, infrahyoids, anterior scalene, and the sternocleidomastoid muscles [18]. In particular, the activation of the sternocleidomastoid muscles was identified as a crucial limiting factor based on comparisons of surface electromyography (sEMG) recordings taken across different muscle types, while participants performed the Shaker exercise [17, 19]. A consequence of exhausting auxiliary muscles, such as the sternocleidomastoid muscles, is likely to be reduced motivation, which results in poor patient compliance. Easterling, Grande, Kern, Sears, and Shaker [20] reported that only 50 % of the non-dysphagic older adults (aged 66–93 years) followed through with the isometric head lift across the stipulated 6 weeks. Within this pool of participants, there were also complaints of neck soreness and muscle discomfort. The authors thus stated, “evidence of early muscle fatigue indicated that the sternocleidomastoid muscle might limit the performance of the exercise and attainment of the exercise goals” (p. 135).
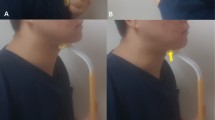
CTAR, on the other hand, is performed by tucking the chin down towards the manubrium sterni, with the individual seated upright on a chair. An inflatable rubber ball, placed between the chin and manubrium sterni, is squeezed in the process, providing resistance needed in the exercise (Fig. 1). The principal muscles activated by this upper cervical flexion are the suprahyoid and infrahyoid muscles, whose activations maintain airway patency and hyoid position respectively [21]. Recruitment of the suprahyoid muscle group is particularly prominent during chin tuck [22, p. 165], which suggests that in theory, CTAR adheres to the muscle-specificity principle. Preliminary evidence for CTAR, based on 40 healthy adults (aged 21–38 years), also revealed that significantly larger maximum sEMG values were recorded on the suprahyoid muscle group during the execution of CTAR than the equivalent Shaker exercises [4]. This means that the target muscles were recruited to a significantly greater extent during CTAR than the Shaker exercise. Subjective feedback based on a binary choice also showed that 80 % of the participants felt that CTAR was less strenuous than the Shaker exercise.
Overall, the results for CTAR are encouraging, but the study was limited in two ways. First, Yoon et al.’s [4] participants only performed condensed versions of the original CTAR and Shaker exercises. Full duration of the isometric tasks for the two exercises, for instance, should be 60-s, instead of the 10-s required of their participants. It is unclear at this stage, how the shortened durations might have influenced muscle recruitment (be it activation or fatigue), and thus the potential for treatment efficacy.
Second, Yoon et al. [4] did not evaluate the extent to which outstanding auxiliary muscles are also recruited during CTAR, specifically the sternocleidomastoid muscles. As sternocleidomastoid recruitment did emerge to be a crucial limiting factor for Shaker exercise, it will be clinically valuable to provide hard data verifying that CTAR does not suffer the same constraint. This in turn is in line with the muscle-specificity principle.
Muscle Fatigue and the Two Exercises
Fatigue has been defined as an inability to maintain an expected force or power output under maximal or submaximal sustained muscle contractions [23, p. 7]. This is directly linked to the principle of intensity (sometimes referred to as the principle of overload) because in cases whereby fatigue is exercise-induced, loading on a muscle reduces its ability to produce force or power. Also importantly, from a rehabilitation therapist’s standpoint, fatigue has been conceived as a form of short-term state or state of “acute plasticity,” capable of “modifying [muscular] contractile properties so as to improve the economy or efficiency of contractions [thus leading to muscle strengthening],” as described by Sargeant [2] (p. 116). Evidence demonstrating how fatigue induces changes in the muscle, including muscle growth, was obtained by Burd et al. [24], who found a significant increase in the rate of protein syntheses following fatigue-inducing resistance exercises.
Beyond identifying which muscle region shows maximum activation, rehabilitation through muscle strengthening should be a goal. Since fatigue can precipitate muscle growth and/or strengthening, fatigue analysis of the suprahyoid activity becomes highly relevant for patients with dysphagia secondary to reduced UES opening. Using spectral analyses of sEMG signals, both Ferdjallah et al. [17] and White et al. [19] have provided reliable evidence that the Shaker exercise was effective in fatiguing the suprahyoid muscles. Their data, however, also highlighted that initially greater fatigue was recorded for the sternocleidomastoid muscle, thus reiterating the exercise’s aforementioned limitation on auxiliary muscle recruitment. As for CTAR, there are no published studies appraising its ability to facilitate muscle strengthening using fatigue analysis yet. It is therefore clinically valuable to examine whether CTAR exercise is able to fatigue the suprahyoid muscle group to an equal or greater extent, when compared to the Shaker exercise, without conferring unwanted early fatigue effects on the sternocleidomastoid muscles.
To summarize, the purpose of this study was to extend existing findings by assessing the two therapeutic exercises (CTAR and its traditional counterpart, Shaker exercise), based on their complete 60-s isometric versions. sEMG was simultaneously collected from both suprahyoid muscle group and sternocleidomastoid muscle during the two exercises. To meet the muscle-specificity principle, significantly lower signal amplitude values and less fatigue should be generated by the sternocleidomastoid muscles during CTAR than during the Shaker exercise; but for CTAR to qualify as a suitable therapy alternative, suprahyoid contraction should also generate significantly higher signal amplitude values and greater fatigue, thereby fulfilling the principles of specificity and intensity.
Method
Participants
Prior to the recruitment of participants, this study was approved by the local Institution Review Board. Forty healthy adults volunteered to participate in the experiment. One participant’s data were excluded because a recording for one of the trials could not be read. The eventual sample size was 39 (20 males, 19 females; mean age = 29.82 years, SD = 5.09). A health screening questionnaire was individually administered (Appendix), and none of the participants reported having swallowing problems, muscular disorders, spinal, lungs, thyroid, heart or neurological diseases. Prior to participants performing the two exercises, an oromotor assessment was also individually conducted to ensure that every participant’s oromasculature was structurally and functionally intact. The assessment included asking the participants to (a) smile, (b) raise their eyebrows, (c) puff out their cheeks, (d) open their jaws and move the lower jaw laterally, (d) stick out the tongue and deviate it laterally and vertically, (e) cough, and (f) swallow their saliva. None of the participants had difficulty with the oromotor assessment.
Materials and Apparatus
The MyoTrac Infiniti encoder was used to collect the sEMG signals from the suprahyoid muscle group and sternocleidomastoid muscle. One pre-gelled triode electrode patch (single use) was also attached on each muscle/muscle group for sEMG signal detection, based on recommendations specified by the instrument company to minimize signal pollution [25]. Each electrode patch had three 10-mm silver–silver chloride snap-on style sensor pellets (one reference and two active electrodes fixed in a triangular configuration), embedded within a two-inch diameter circular foam pad with adhesive backing. An EMG cable (Adapter Cable Kit T9801, Thought Technology) connected each electrode patch to the encoder, which was in turn connected to a laptop. For data recording, the encoder’s software Biograph Infiniti [26] was used. The raw signals were then processed using MATLAB [27] for their amplitude, spectral and recurrence quantification characteristics.
Procedure
Each participant completed two trials of each exercise, Shaker and CTAR. The purpose of having repeated trials was to ensure intra-participant reliability. Upon completion of each exercise trial, there was a mandatory 4-min rest period, before commencing with the following trial. The rest period was included to allow the two muscles to sufficiently recover, before the next trial. During the rest, participants were also asked whether they felt any body discomfort. To minimize transfer effects, the order of the exercises was also counterbalanced using Latin Square and randomly assigned to the participants.
Two demonstration videos with instructions for the two exercises were played to the participants (Video for the Shaker exercise: http://www.mcw.edu/gastrohep/shakerexercise.htm). For the CTAR exercise, the participant performed a 60-s chin tuck to squeeze an inflatable rubber ball (12 cm in diameter) trapped between the base of the chin and the manubrium sterni, tightly. This was carried out in an upright seated position. Participants were reminded that their shoulders should not be slouched forward during the chin tuck. For the Shaker exercise, the participant sustained a 60-s head lift, while lying supine on a 5-mm thick exercise (yoga) foam mat. Participants were also instructed that they should be able to see their toes without having to lift their shoulders during Shaker. For both exercises, participants were reminded to breathe with their mouths closed, while performing the tasks. At the end of the experiment, participants were asked to rate how strenuous each exercise felt on a Likert scale of ‘5’ (‘5’ being ‘very strenuous’, and ‘1’ being ‘not strenuous at all’).
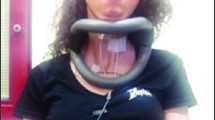
The skin surfaces for the suprahyoid muscle group and sternocleidomastoid muscle were cleaned using alcohol swabs, prior to electrode placement, to improve conduction. One single-use electrode patch was placed on the suprahyoid muscle group and sternocleidomastoid muscleFootnote 2 respectively, following guidelines outlined in Yoon et al. [4], Crary and Groher [28] and Konrad [29] (Fig. 2). For the suprahyoid muscle group, the electrode patch is positioned in between the area of the hyoid bone and the chin. Care was also taken to ensure electrodes on the patch were aligned along the midline of the suprahyoid region (sagittal plane). The electrodes on the suprahyoid muscle group and sternocleidomastoid muscle were not moved between the conditions. During the exercises, muscle activity was recorded at a sampling rate of 2048 Hz. A Notch filter of 50 Hz was applied to the recording. This 50 Hz Notch filter is built into the encoder by default and has no implications on the signal quality.Footnote 3
MATLAB was used to process the sEMG signals offline. Prior to the analyses, sEMG recordings were filtered using low- then high-pass 4th order butterworth filters with cutoff frequencies of 500 and 20 Hz respectively. These filter settings followed De LuCa [30] (who recommended a low-frequency filter of 20 Hz to maintain signal stability and elimination of motion artifacts) and the standard EMG protocol (according to Standards for Reporting EMG Data [31], the EMG power density spectra contains majority of its power within 5–500 Hz). The full duration of the each exercise trial (60-s) was included in the analyses. Power spectral density was computed using the Welch method at a window size of 1024 samples with 50 % overlap [32]. Because each exercise was completed twice per participant, all final values used in the amplitude and fatigue analyses (elaborated below) were averaged across each participant’s two trials.
To study the effects on muscle strength, two amplitude variables were calculated: (a) Maximum sEMG and (b) Root mean square (RMS). Maximum sEMG indicates the level of muscle activation by way of the peak amplitude value obtained for the rectified EMG signal over the 60-s exercise window. RMS, on the other hand, provides insights on amplitude by measuring the mean power of the signal. Including both variables should provide a more accurate examination of the muscle strength, especially if their results converge.
To study muscle fatigue, both spectral and recurrence quantification analyses were carried out. Spectral analyses are the classical means to examine fatigue (see Basmajian & De Luca [33], De Luca [30], or Merletti et al. [34], for a review on the spectral indices). Spectral indices capture the motor unit firing rate during muscle contraction. According to research in muscle physiology, as a muscle fatigues, its motor unit firing rate correspondingly decreases [33]. For the spectral analyses in this paper, we included both traditional spectral indices (overall frequency variables), as well as the regression-based indices (i.e., rate of change in frequency). To compute the regression-based spectral indices, the signal was segmented into 1-s epochs to facilitate the calculation of frequency measures (i.e., mean and median frequencies) at each 1-s interval. Visual inspection shows that the data points generally follow a linear trend. Following Ferdjallah et al. [17] and White et al. [19], these per-second spectral data were then fitted to a linear regression model:
such that t = time and Frequency refers to either mean frequency or median frequency. For regression indices, the variable of interest would be the gradient, i.e., rate(t). Frequency at rest, on the other hand, refers to the frequency calculated at the origin. In essence, four spectral variables were computed to index fatigue. They are: (a) overall mean frequency, (b) overall median frequency, (c) rate of change in mean frequency, and (d) rate of change in median frequency.
Finally, the recurrence variable percentage of determinism (%DET) was calculated to enable recurrence quantification analyses [35]. Recurrence quantification analyses are included because of the growing body of evidence that this alternative approach provides an even more sensitive assessment of fatigue, compared to the traditional spectral variables [see 36, 37]. The recurrence variable, %DET, captures the synchrony of neuronal firing rates. It is observed that the firing rates become progressively more regular as a muscle fatigues [38]. In this study, a %DET value was computed for every 1-s chunk. The lag parameter was set as the time of the first zero-crossing of the auto-correlation of each data chunk [39], and the embedding dimension D was set as the standard value of 15 [40]. The threshold for recurrence detection was set such that it yielded a recurrence rate of 1 %. The last variable rate of change in %DET was then calculated using the procedure described earlier for regression-based spectral indices. However, instead of the frequency measures, %DET was obtained for each 1-s epoch and inserted as appropriate in each step.
SPSS software was used to compute the repeated-measures ANOVA and post hoc analyses for the amplitude and fatigue measures. Bonferroni’s correction was also applied for the post hoc comparisons.
Results
Perceptual Ratings
Descriptive statistics for the perceptual ratings are as follow: For CTAR Exercise, M = 3.23, SD = 1.08; for Shaker exercise, M = 3.79, SD = 0.98. Paired samples t test revealed a significance difference between the two ratings [t(38) = 2.72, p < .01, d = 0.44]. To rule out the possibility that the order of the exercises was a confound, a 2 × 2 (order × exercise) analysis was also conducted. Result for this two-way interaction was not significant (p = .15).
Amplitude Analyses
Table 1 presents the descriptive statistics for the maximum sEMG activation levels and RMS for each muscle type during the two exercises.
For each variable, a 2 × 2 (exercise × muscle type) repeated-measures ANOVA was performed. Same pattern of interaction effects were observed in both variables [For maximum sEMG: F(1, 38) = 53.20, p < .001, MSe = 54772.00, \( \eta_{\text{partial}}^{2} \) = .58; for RMS: F(1, 38) = 89.61, p < .001, MSe = 1503.34, \( \eta_{\text{partial}}^{2} \) = .70]. Follow-up analyses found that significantly higher amplitude values were registered for the suprahyoid muscles during CTAR compared to Shaker exercise; but for the sternocleidomastoid, the reverse was found, such that significantly higher amplitude values were registered during the Shaker compared to the CTAR exercise (see Table 1).
Fatigue Analyses
Table 2 presents the descriptive statistics for the spectral and recurrence variables (and their respective regression-based indices) obtained for each muscle during the two exercises.
Traditional Frequency Indices (Mean and Median Frequencies)
2 × 2 (exercise × muscle type) repeated-measures ANOVA was conducted on all six variables (traditional frequency indices, regression-based indices and the recurrence variables). The same interaction pattern was replicated across both traditional frequency indices [For mean frequency: F(1, 38) = 18.13, p < .001, MSe = 241.70, \( \eta_{\text{partial}}^{2} \) = .32; for median frequency: F(1, 38) = 8.54, p = .006, MSe = 283.87, \( \eta_{\text{partial}}^{2} \) = .18]. Follow-up analyses showed that significantly lower frequency values were obtained for the suprahyoid muscle group during CTAR compared to Shaker exercise; but for the sternocleidomastoid muscle, the opposite trend was found, such that significantly lower frequency values were obtained for the Shaker compared to the CTAR exercise (see Table 2).
Recurrence Quantification Analysis (Mean %DET)
For mean %DET, the exercise × muscle-type interaction was observed [F(1, 38) = 34.28, p < .001, MSe = 0.002, \( \eta_{\text{partial}}^{2} \) = .47]. Follow-up analyses (see Table 2) revealed that a significantly higher recurrence value was obtained for the suprahyoid muscle group during CTAR compared to Shaker exercise, but the opposite pattern occurred for the sternocleidomastoid muscle, whereby a significantly higher recurrence value was obtained during Shaker compared to the CTAR exercise.
Regression-Based Indices (Rate of Change in Mean and Median Frequencies, Rate of Change in %DET)
Identical patterns of findings were generally observed for all three regression-based indices. Crucially, an interaction was found for all three regression-based indices [For the rate of change in mean frequency: F(1, 38) = 14.02, p = .001, MSe = 0.072, \( \eta_{\text{partial}}^{2} \) = .27; for the rate of change in median frequency: F(1, 38) = 9.50, p = .004, MSe = 0.087, \( \eta_{\text{partial}}^{2} \) = .20; for the rate of change in %DET: F(1, 38) = 9.77, p = .003, MSe = 1.38 × 10−6, \( \eta_{\text{partial}}^{2} \) = .20]. In both regression-based spectral indices, the negative decline in the frequency gradients for the sternocleidomastoid muscle was significantly steeper during Shaker compared to those of the CTAR exercise, but there was no significant difference between the frequency gradient indices for the suprahyoid muscle group during the two exercises (see Table 2 for the respective follow-up analyses). As for the rate of change in %DET, follow-up analyses showed that the increment in %DET for the sternocleidomastoid muscle during Shaker was significantly sharper compared to the CTAR exercise (see Table 2). However, no significant difference was observed in the rate of change in %DET between the two exercises for the suprahyoid muscle group (p = ns).
Lastly, to make sure the order at which the exercises were taken had no impact on the results, an additional set of 2 × 2 × 2 (order × exercise × muscle type) analyses was run on each of the nine variables (three amplitude variables, two traditional frequency indices, mean %DET, and all three regression-based indices). None of the three-way interactions was significant (ps > .10).
General Discussion
The purpose of this study was to assess the effectiveness of two therapeutic interventions developed to restore functional swallowing, through the exercise of suprahyoid muscles. sEMG data were recorded while the participants performed the two exercises, Shaker and CTAR, in counterbalanced order. Participants also rated how strenuous each exercise was. Based on the principles of specificity and intensity, a successful therapeutic intervention treating reduced UES opening would be one that is (1) more specific in targeting and (2) sufficiently intensive to fatigue the suprahyoid muscle group. Following this thread of reasoning, in an ideal therapy exercise, the suprahyoid muscle group should be highly activated (thus, register high amplitude readings) and show substantial fatigue. The reverse should apply to a non-targeted muscle, such as the sternocleidomastoids, if the exercise was effective. In the following, we explain in depth how our data support this dissociative pattern between the suprahyoid muscle group and sternocleidomastoid muscle, when CTAR was contrasted against the Shaker exercise.
On average, participants perceived CTAR to be significantly less strenuous compared to the Shaker exercise, based on the ratings collected. This is consistent with the subjective feedback derived for the 10-s CTAR trials reported in Yoon et al. [4]. Thus, similar perceptions on the ease of performing CTAR prevail, even after the exercise duration was increased to the full 60-s.
This perceived ease for CTAR is likely to be important for treatment compliance, reportedly limited for the elderly participating in the Shaker exercise regimen [20]. However, the focus of the study was on objective measures of signal properties collected from the two muscles. These were examined using amplitude and fatigue analyses. sEMG amplitude indices specify the level of muscle activation (e.g., maximum sEMG activation) and the amount of force (e.g., RMS) generated by the muscle [30, p. 149]. Across the two amplitude measures, significantly greater maximum activation and force were generated by the suprahyoid muscle group during CTAR than the Shaker exercise. These findings suggest that flexing down the head with a ball as resistance is capable of delivering a significantly greater impact on the suprahyoid muscles, compared to raising the head against gravity from a supine position. Equally important, is the finding that during CTAR, the sternocleidomastoid muscle appeared to generate significantly less activity (maximum sEMG activation) and amplitude (RMS), when compared against the Shaker exercise. This dissociation in results between the muscles suggests that compared to Shaker exercise, CTAR demonstrates higher precision in targeting the suprahyoid muscles, without recruiting the sternocleidomastoid muscles to as large an extent. The muscle-specificity principle thus appeared to be upheld by CTAR, based on the amplitude analysis.
Amplitude analyses inform us which muscle sites were electrically active, but it was also important to examine whether those activated muscles were also physiologically affected by the exercises, and might be strengthened as a corollary. Evidence of fatigue is therefore necessary to demonstrate whether the exercises in question have a training effect on the suprahyoid muscle group. Two broad approaches were utilized to analyze muscle fatigue: (1) classical spectral analyses, represented by (a) traditional frequency indices and the (b) regression-based indices; and (2) recurrence quantification analysis (and its regression-based index).
For the traditional frequency indices, fatigue is represented by lower values. This is because during isometric contractions, the sEMG power spectrum shifts predictably left towards the lower values when the muscle fatigues. This shift in the signal energy towards lower frequencies is likely driven by the decrease in motor unit firing rates (which are quantified by the mean and median power frequencies; see [33], pp. 213–219, or [34], for a review of the evidence). Separate analyses based on mean and median frequencies indicated that the suprahyoid muscle group was significantly more fatigued during CTAR, when compared to the Shaker exercise, but the sternocleidomastoid muscle showed significantly less fatigue during CTAR than the Shaker exercise.
Traditional frequency indices are stationary quantifications of the overall muscle activity. Fatigue is however a dynamic system. More recently, researchers have advocated using recurrence quantification analysis to detect the changes in muscular state. The recurrence variable, percentage of determinism (%DET), characterizes the regularity in muscle contractile patterns (or in terms of neuronal activity, the synchrony of firing rates). The rationale for quantifying recurring patterns was based on research observation that during fatigue, the rhythm of muscle contraction tends to get repeated, i.e., more regular (or when translated to neuronal activity, the firing rates become more synchronized [39]). Thus, higher %DET values detected represent higher levels of fatigue [38]. Results based on %DET suggested that the suprahyoid muscle group was significantly more exhausted during CTAR compared to the Shaker exercise, and the opposite was found for the sternocleidomastoids, with significantly less fatigue located during the CTAR exercise.
To capture the changes in fatigue across time, regression-based indices of mean and median frequencies, as well as %DET, were also computed. Across these three variables, the rate of fatigue was found to be significantly higher for the sternocleidomastoid muscle when participants performed the Shaker exercise than doing the chin tuck. This means that CTAR was more effective in avoiding the recruitment and training of non-targeted muscle, the sternocleidomastoid, and thus did not convey as sharp an increase in fatigue as the Shaker exercise. No difference in the fatigue rates was detected on the suprahyoid muscle group between the two exercises, across all three regression indices, suggesting that both exercises were equally effective in evoking a similar amount of change in fatigue across time.
Overall, based on all six fatigue variables, it is clear that CTAR achieved similar or more intensive physiological impact on the suprahyoid muscle group, compared to the Shaker exercise. Notably, CTAR did not appear to fatigue the sternocleidomastoid muscle, the muscle auxiliary to the exercise goal, to the same extent that Shaker exercise did. These findings converge to provide a strong indication that CTAR resulted in more specificity, i.e., was able to activate the suprahyoid muscle group, and more effective in avoiding the recruitment and training of a non-targeted muscle, the sternocleidomastoid muscle. Additionally, CTAR was as equally effective as Shaker in fatiguing (thus strengthening) targeted muscle group, i.e., the suprahyoid muscle group. This supports the view that in terms of training the target muscle group, CTAR is also a sufficiently demanding exercise, which is in keeping with the principle of intensity.
Interestingly, it appears that the rates of change in fatigue across time induced on the suprahyoid muscle group are similar across the two exercises. This is in spite of the significant difference noted on the suprahyoid muscles, using overall mean figures (i.e., mean/median frequency and mean %DET). There are no clear reasons for this null finding, but we can offer two possibilities. First, this could be a consequence of suprahyoid muscles’ intrinsic muscular properties such that this muscle group shows inconsistent degrees of fatigue during contraction across time. To the best of our knowledge, there is scant literature explicitly studying the structural physiology of suprahyoid muscle group; thus, it remains to be seen whether this proposition stands up to scrutiny (Cobos et al. [41] did publish a paper on the muscle fiber types in the suprahyoid muscles, but their research was based on the rat). A second possibility pertains to the individual differences in coping with the resistance load (be it against gravity or compressing against the ball) across time. This is attested by the considerable variance obtained for the three regression indices specified for the suprahyoid muscle group during the two exercises (Table 2). Large variance sizes were similarly reported by Ferdjallah et al. [17], suggesting that our findings are not atypical. In fact, this variance is likely to reflect the huge variation in initial coping when participants were first tasked to contract the suprahyoid muscles. Aside from daily swallowing and the tangential neck movements, the suprahyoid muscles are not a typical muscle group that is purposefully trained in the gymnasium. For this reason, the varied capacity of the participants’ suprahyoid muscles to cope with a given load should not come as a surprise, and would be expected for individuals suddenly tasked to engage in an exercise that deliberately recruits a muscle group otherwise ignored. It might then be worthwhile to examine whether this considerable variance would truncate after a few weeks of CTAR exercise training. The reduction in variance would be further evidence that muscle strengthening occurred, because a smaller variance obtained following a muscle-specific intervention indicates that the suprahyoid muscles have developed sufficient power to cope against resistance, and this coping is consistent across the exercise time.
This study represents a nascent attempt to further validate a relatively new pharyngeal dysphagia rehabilitation exercise using healthy adults. Converging data from different spectral and fatigue analyses corroborate that CTAR is more specific in activating the suprahyoid muscles, and is equally effective in fatiguing this target muscle group, when compared against the more traditional Shaker exercise. Moreover, the perceived level of muscle strain was significantly lower for CTAR. Two directions are identified for future research. First, clinical trials with elderly patients diagnosed with dysphagia secondary to diminished UES opening are critical. Despite the promising findings yielded for CTAR, the research data thus far are still based on healthy adults. CTAR’s efficacy for patients with dysphagia, the elderly population’s compliance, and their perception of CTAR’s ease of execution remain to be empirically tested. Second, there are crucial details in CTAR that require fine-tuning. These include whether different individuals are suited for balls of different diameters or hardness, and how the principle of progressive intensity can be incorporated into the exercise (e.g., by adjusting the air pressure within the rubber ball). Neck anthropometry (specifically, the broadness of chin and length of neck), systematic manipulations of the rubber ball’s properties (size, pressure), and exercise timings will be important considerations as follow-up.
Notes
The term ‘suprahyoid muscles’ used in this paper refers to suprahyoid muscle group. For textual fluidity, ‘suprahyoid muscles’ is also used.
The electrode position for the sternocleidomastoid muscle is also generally consistent with Falla et al.’s (2002) recommendations [42]. The placement is perpendicular to the one-third distance from the sternal notch.
The use of a 50 Hz notch filter may shift the median frequency measures to slightly higher frequencies. However the filter was applied to all conditions and participants, making a potential frequency shift identical across conditions, and therefore would not affect differences between the conditions [43]. Previously published studies on muscle fatigue have also employed the 50 Hz notch filter [44, 45].
References
Burkhead LM. Applications of exercise science in dysphagia rehabilitation. In: SIG 13 Perspectives on Swallowing and Swallowing Disorders (Dysphagia), vol.18, 2009; pp. 43–8. doi:10.1044/sasd18.2.43.
Sargeant AJ. Human power output and muscle fatigue. Int J Sports Med. 1994;15(3):116–21. doi:10.1055/s-2007-1021031.
Shaker R, Kern M, Bardan E, Taylor A, Stewart ET, Hoffmann RG, Arndorfer RC, Hoffmann C, Bonnevier J. Augmentation of deglutitive upper esophageal sphincter opening in the elderly by exercise. Am J Physiol. 1997;272(6 Pt 1):G1518–22.
Yoon WL, Khoo JKP, Rickard Liow SJ. Chin Tuck against Resistance (CTAR): new method for enhancing suprahyoid muscle activity using a Shaker-type exercise. Dysphagia. 2014;29(2):243–8. doi:10.1007/s00455-013-9502-9.
Kern M, Hofmann C, Bardan E, Ren J, Arndorfer R, Shaker R. Comparison of upper esophageal sphincter opening in healthy asymptomatic young and elderly volunteers. Ann Otol Rhinol Laryngol. 1999;108(10):982–9. doi:10.1177/000348949910801010.
Cook IJ, Dodds WJ, Dantas RO, Massey B, Kern MK, Lang IM, Brasseur JG, Hogan WJ. Opening mechanisms of the human upper esophageal sphincter. Am J Physiol. 1989;257(5 Pt 1):G748–59.
Pearson WG Jr, Langmore SE, Zumwalt AC. Evaluating the structural properties of suprahyoid muscles and their potential for moving the hyoid. Dysphagia. 2011;26(4):345–51. doi:10.1007/s00455-010-9315-z.
Crary MA, Carnaby GD, Groher ME. Biomechanical correlates of surface electromyography signals obtained during swallowing by healthy adults. J Speech Lang Hear Res. 2006;49(1):186–93. doi:10.1044/1092-4388(2006/015).
Perlman AL, Palmer PM, McCulloch TM, VanDaele DJ. Electromyographic activity from human laryngeal, pharyngeal, and submental muscles during swallowing. J Appl Physiol. 1999;86(5):1663–9.
Goyal RK, Martin SB, Shapiro J, Spechler SJ. The role of cricopharyngeus muscle in pharyngoesophageal disorders. Dysphagia. 1993;8(3):252–8. doi:10.1007/BF01354547.
McConnel FMS, Cerenko D, Jackson RT, Hersh T. Clinical application of the manofluorogram. Larnygoscope. 1988;98(7):705–11. doi:10.1288/00005537-198807000-00003.
Easterling C, Shaker R. UES opening muscle dysfunction. In: Shaker R, Belafsky PC, Postma GN, Easterling C, editors. Principles of deglutition: a multidisciplinary text for swallowing and its disorders. New York: Springer; 2013. p. 529–35.
Autunes EB, Lunet N. Effects of the head lift exercise on the swallow function a systematic review. Gerodontology. 2012;29(4):247–57. doi:10.1111/j.1741-2358.2012.00638.x.
Easterling C. Shaker exercise. In: Shaker R, Easterling C, Belafsky PC, Postma GN, editors. Manual of diagnostic and therapeutic techniques for disorders of deglutition. New York: Springer; 2013. p. 257–68.
Shaker R, Easterling C, Kern M, Nitschke T, Massey B, Daniels S, Grande B, Kazandjian M, Dikeman K. Rehabilitation of swallowing by exercise in tube-fed patients with pharyngeal dysphagia secondary to abnormal UES opening. Gastroenterology. 2002;122(5):1314–21. doi:10.1053/gast.2002.32999.
Logemann JA, Rademaker A, Pauloski BR, Kelly A, Stangl-McBreen C, Antinoja J, Grande B, Farquharson J, Kern M, Easterling C, Shaker R. A randomized study comparing the Shaker exercise with traditional therapy: a preliminary study. Dysphagia. 2009;24(4):403–11. doi:10.1007/s00455-009-9217-0.
Ferdjallah M, Wertsch JJ, Shaker R. Spectral analysis of surface electromyography (EMG) of upper esophageal sphincter-opening muscles during head lift exercise. J Rehabil Res Dev. 2000;37(3):335–40.
Kendall FP, McCreary EK, Provance PG, Rodgers MM, Romani WA. Muscles: testing and function with posture and pain. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2005.
White KT, Easterling C, Roberts N, Wertsch J, Shaker R. Fatigue analysis before and after Shaker exercise: physiologic tool for exercise design. Dysphagia. 2008;23(4):385–91. doi:10.1007/s00455-008-9155-2.
Easterling C, Grande B, Kern M, Sears K, Shaker R. Attaining and maintaining isometric and isokinetic goals of the Shaker exercise. Dysphagia. 2005;20(2):133–8. doi:10.1007/s00455-005-0004-2.
Forsberg C-M, Hellsing E, Linder-Aronson S, Sheikholeslam A. EMG activity in neck and masticatory muscles in relation to extension and flexion of the head. Eur J Orthod. 1985;7(3):177–84. doi:10.1093/ejo/7.3.177.
Adler SS, Beckers D, Buck M. PNF in practice: an illustrated guide. 4th ed. Berlin: Springer Medizin; 2014.
Williams CA, Ratel S. Definitions of muscle fatigue. In: Williams CA, Ratel S, editors. Human muscle fatigue. Oxon: Routledge; 2009. p. 3–16.
Burd NA, West DWD, Staples AW, Atherton PJ, Baker JM, Moore DR, Holwerda AM, Parise G, Rennie MJ, Baker SK, Phillips SM. Low-load high volume resistance exercise stimulates muscle protein synthesis more than high-load low volume resistance exercise in young men. PLoS One. 2010;5(8):e12033. doi:10.1371/journal.pone.0012033.
Thought Technology. Basics of surface electromyography applied to physical rehabilitation and biomechanics. Montreal: Thought Technology Ltd; 2010.
Thought Technology. BioGraph infiniti [computer software]. Montreal: Thought Technology Ltd; 2009.
MathWorks. MATLAB [computer software]. Natick: MathWorks; 2014.
Crary MA, Groher ME. Basic concepts of surface electromyographic biofeedback in the treatment of dysphagia: a tutorial. Am J Speech-Lang Pathol. 2000;9(2):116–25. doi:10.1044/1058-0360.0902.116.
Konrad P. The ABC of EMG: a practical introduction to kinesiological electromyography. Arizona: Noraxon; 2005.
De Luca CJ. The use of surface electromyography in biomechanics. J Appl Biomech. 1997;13(2):135–63.
Standards for Reporting EMG Data. J Electromyogr Kinesiol 2014; 24(3): I–II. doi:10.1016/S1050-6411(14)00082-0.
Welch PD. The use of fast Fourier transform for the estimation of power spectra: a method based on time averaging over short, modified periodograms. IEEE Trans Audio Electroacoust. 1967;15(2):70–3. doi:10.1109/TAU.1967.1161901.
Basmajian JV, De Luca CJ. Muscles alive: their functions revealed by electromyography. 5th ed. Baltimore: Williams & Wilkins; 1985.
Merletti R, Lo Conte LR, Orizio C. Indices of muscle fatigue. J Electromyogr Kinesiol. 1991;1(1):20–33. doi:10.1016/1050-6411(91)90023-X.
Marwan N, Romano MC, Thiel M, Kurths J. Recurrence plots for the analysis of complex systems. Phys Rep. 2007;438(5–6):237–329. doi:10.1016/j.physrep.2006.11.001.
Farina D, Fattorini L, Felici F, Filligoi G. Nonlinear surface EMG analysis to detect changes of motor unit conduction velocity and synchronization. J Appl Physiol. 2002;93(5):1753–63. doi:10.1152/japplphysiol.00314.2002.
Yassierli, Nussbaum MA. Utility of traditional and alternative EMG-based measures of fatigue during low-moderate level isometric efforts. J Electromyogr Kinesiol. 2008;18(1):44–53. doi:10.1016/j.jelekin.2006.08.003.
Ito K, Hotta Y, Abe T. Detection of muscle fatigue during low-level isometric contraction using recurrence quantification analysis. In: Long M, editor. World congress on medical physics and biomedical engineering, IFMBE Proceedings, vol. 39. Berlin: Springer; 2013. p. 509–12.
Filligoi G, Felici F. Detection of hidden rhythms in surface EMG signals with a nonlinear time-series tool. Med Eng Phys. 1999;21(6):439–48.
Morana C, Ramdani S, Perrey S, Varray A. Recurrence quantification analysis of surface electromyographic signal: sensitivity to potentiation and neuromuscular fatigue. J Neurosci Methods. 2009;177(1):73–9. doi:10.1016/j.jneumeth.2008.09.023.
Cobos AR, Segade LAG, Feutes I. Muscle fibre types in the suprahyoid muscles of the rat. J Anat. 2001;198(3):283–94. doi:10.1046/j.1469-7580.2001.19830283.x.
Falla D, Alba PD, Rainoldi A, Merletti R, Jull G. Location of innervation zones of sternocleidomastoid and scalene muscles—a basis for clinical and research electromyography applications. Clin Neurophysiol. 2002;113(1):57–63. doi:10.1016/S1388-2457(01)00708-8.
Szeto GPY, Straker LM, O’Sullivan PB. EMG median frequency changes in the neck–shoulder stabilizers of symptomatic office workers when challenged by different physical stressors. J Electromyogr Kinesiol. 2005;15(6):544–55. doi:10.1016/j.jelekin.2005.06.004.
Abdul-Latif AA, Cosic I, Kumar DK, Polus B and Da Costa C. Power changes of EEG signals associated with muscle fatigue: the root mean square analysis of EEG bands. In: Proceedings of the 2004 intelligent sensors, sensor networks and information processing conference 2004; pp. 531–4. doi:10.1109/ISSNIP.2004.1417517.
Koumantakis GA, Arnall F, Cooper RG, Oldham JA. Paraspinal muscle EMG fatigue testing with two methods in healthy volunteers. Reliability in the context of clinical applications. Clin Biomech. 2001;16(3):263–6. doi:10.1016/S0268-0033(00)00113-3.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Appendix: Health Screening Questionnaire
Appendix: Health Screening Questionnaire
Respondent ID: __________________
Please circle Yes or No:
1. Have you ever had any swallowing problem(s)? | Yes/No |
2. Have you ever had a major surgery on or above the neck or on the spine? | Yes/No |
3. Have you ever had any muscle disease(s)? | Yes/No |
4. Have you ever had any heart or lung disease(s)? | Yes/No |
5. Have you ever had a thyroid disease? | Yes/No |
6. Have you ever had a neurological disease? | Yes/No |
7. Have you ever had head injury with loss of consciousness? | Yes/No |
8. Are you on any medication now? If yes, please state: | Yes/No |
Rights and permissions
About this article
Cite this article
Sze, W.P., Yoon, W.L., Escoffier, N. et al. Evaluating the Training Effects of Two Swallowing Rehabilitation Therapies Using Surface Electromyography—Chin Tuck Against Resistance (CTAR) Exercise and the Shaker Exercise. Dysphagia 31, 195–205 (2016). https://doi.org/10.1007/s00455-015-9678-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-015-9678-2