Abstract
Objectives
To investigate the contribution of direct electron transfer mechanisms to electricity production in microbial fuel cells by physically retaining Shewanella oneidensis cells close to or away from the anode electrode.
Results
A maximum power output of 114 ± 6 mWm−2 was obtained when cells were retained close to the anode using a dialysis membrane. This was 3.5 times more than when the cells were separated away from the anode. Without the membrane the maximum power output was 129 ± 6 mWm−2. The direct mechanisms of electron transfer contributed significantly to overall electron transfer from S. oneidensis to electrodes, a result that was corroborated by another experiment where S. oneidensis cells were entrapped in alginate gels.
Conclusion
S. oneidensis transfers electrons primarily by direct electron transfer as opposed to mediated electron transfer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Significant amounts of money are spent treating large volumes of wastewater every year because of the high energy (and hence cost) involved. The wastewater could, however, be used as a resource saving energy and money as most wastewaters contain organic matter that can be oxidised to generate electricity while at the same time cleaning up the wastewater. It is estimated that wastewater contains 10 times more energy than the energy required for its treatment. While not all the energy can be recovered, there is a potential to make the treatment process at least self-sufficient from an energy perspective.
Anaerobic digestion has been used to treat and recover energy (as biogas) from industrial wastewater but the technology is unsuitable for dilute streams (chemical oxygen demand <1 kgm−3) or those with high ammonium nitrogen. Microbial fuel cells (MFCs) are a possible viable way of treating wastewater sustainably while at the same time produce electricity (Lee et al. 2010; Harnisch et al. 2011; Fernando et al. 2012).
MFCs utilise microorganisms (exoelectrogens) e.g. Shewanella, Geobacter, Rhodoferax, yeasts etc. to catalyse an oxidation and reduction reaction at an anode and cathode electrode respectively and can producing electricity when connected to a load/resistor via an external circuit.
A number of studies have been done on treatment of various wastewater types but the electrical energy recovery from these systems was very poor, generally less than 150 Wm−3 of the anode volume (Fernando et al. 2012; Logan 2008; Oliveira et al. 2013). For cost-effectiveness the energy recovery needs to reach 1000 W/m3, an energy output that would be competitive with anaerobic digesters.
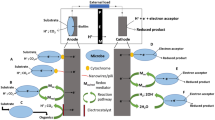
Three possible mechanisms of electron transfer from microorganisms to anodes have been suggested (Fig. 1): directly using a cascade of membrane proteins and/or conduction by pilus-like appendages (bacterial nanowires, Wrighton et al. 2011), and mediated electron transfer.
Mediated electron transfer involves the use of soluble redox-active molecules such as flavin mononucleotide or phenazines to shuttle electrons from the electron transport chain to solid electrodes (Okamoto et al. 2012).
Low extracellular electron transfer efficiency between exoelectrogens and anodes remains one of the major bottlenecks in the practical application of MFC. Assuming more than one electron transfer mechanism is operating in a given microorganism, it would be useful if the relative contribution of these mechanisms to electron transfer could be quantified. Efforts could then be geared towards improving the efficiency of that mechanism if its contribution is found to be relatively large by comparison to other mechanisms. This study therefore investigated the contribution of direct electron transfer mechanisms to electricity production by physically retaining S. oneidensis cells close to or away from the anode electrode using a dialysis membrane as well as immobilising the cells in alginate.
Materials and methods
Chemicals
All chemicals were of analytical grade. Ficodox Plus mixed chemical oxygen demand (COD) reagent was purchased from Fisher Scientific (UK).
Bacteria strains, maintenance and culture
Shewanella oneidensis strain 700,550 was from ATCC. The strain was first grown in LB medium containing (per litre) 10 g tryptone, 5 g yeast extract and 5 g NaCl at 30 °C for 48 h; followed by sub-culturing in minimal salt medium (MSM, see below) supplemented with 500 mg glucose l−1. Before inoculation into the microbial fuel cell (MFC), the strain was grown on LB medium supplemented with 15 g agar l−1 and plated for enumeration.
Experimental design
The experiment on the contribution of direct electron transfer mechanisms on electricity production was studied for 11 days under strictly anaerobic-anodic conditions in two-chamber MFCs. The inoculum (3.4 × 109 CFU) made up 10 % (v/v) of the anode working volume. S. oneidensis cells were physically retained close to or away from the anode electrode using a dialysis membrane (MWCO 12,000 Da) (Fig. 2). The pore size of the membrane is small enough to prevent cells from going through but large enough to allow movement of proteins, redox shuttles and metabolites. The dialysis tubing used (Sigma) was made from cellulose and is not electrically conducting. The tubing was also electrically isolated from the anode. The experiment was conducted in triplicate.
Schematic of the MFC set up (anode chambers only). S. oneidensis cells were restrained using a dialysis membrane close to the anode (A Direct) or away from the anode (B mediated) or freely suspended in the anode (C combined). C–B and A–B reflect the actual contribution of direct electron transfer mechanisms
S. oneidensis cells were also immobilised in alginate beads and added to the anode chamber to keep them separate from the anode itself. Beads were prepared by mixing equal volumes (20 ml) of S. oneidensis cells (3.4 × 109 CFU) with sodium alginate (4 % w/v) and releasing drops into 20 g CaCl2 l−1 to entrap the cells. Controls involved cells inoculated into the anolyte without restriction (meaning electrons could be transferred by direct and mediated electron transfer mechanisms) as well as anodes without microorganisms. The total volume in the anode chamber with beads was displaced by about 50 ml compared to the control. The beads settled to the bottom of the anode chamber and were not in direct contact with the anode. The experiment was conducted in triplicate.
The performance of the MFCs was investigated with respect to degradation performance (COD removal efficiency) and electrochemical performance (i.e. voltage outputs, maximum power generation and coulombic efficiency (CE).
MFC setup and operation
H-type MFCs were constructed with two identical Duran bottles and were held together with an external metal clip. The anode and cathode compartments were separated with a cation-exchange membrane (CMI-7000, Membranes International USA). Two rubber gaskets were used to ensure a seal. The electrodes were constructed from carbon cloth. The cathodes contained no Pt catalyst layer and the electrodes had a projected surface area of 25 cm2. An external load of 1000 Ω was utilised for all experiments and the potential across the resistors was recorded using a Picolog ADC-24 (Pico Technology, UK) online data logging system.
The minimal salts medium (MSM) used was adapted from Fernando et al. (2012) and consisted of 0.46 g NH4Cl l−1, 0.225 g KCl l−1, 0.117 g MgSO4·7H2O l−1, 2.5 g NaH2PO4 l−1, 4.11 g Na2HPO4 l−1, 0.225 g (NH4)2SO4 l−1 with addition of vitamins and trace mineral solutions each at 1 % (v/v) (see Fernando et al. 2012). The anolyte used was MSM supplemented with 500 mg casein hydrolysate l−1 and 2.2 g sodium pyruvate l−1 as the primary carbon source and the catholyte used was 50 mM (pH 7) phosphate buffer containing 0.1 M potassium ferricyanide, without aeration. During start-up operation, actively growing S. oneidensis (10 % v/v of the total anolyte volume) were inoculated according to the scheme in Fig. 2.
The MFC components and all media solutions were sterilised by autoclaving. The experiments were conducted in batch mode with a working volume of 200 ml in each MFC compartment. The anolyte was purged with nitrogen gas for 10 min through a 0.22 µm pore size diameter filter prior to inoculation.
All experiments were studied at 30 °C using a Stuart 160 incubator (Fisher Scientific, U.K.).
Analytical procedures
COD removal
COD removal was determined using the closed reflux titrimetric method as described in the Environment Agency (UK) standard method 5220D (Westwood 2007). Briefly, appropriately diluted 1 ml sample (so resulting COD < 500 mg l−1) were used for each determination. The COD removal was calculated by the following expression:
where COD represents the amount of oxygen consumed per litre of sample, Vb and Vs are ferrous ammonium sulphate (FAS) titrant volumes for the blank and the sample respectively, DF is the sample dilution factor and M is the molarity of FAS titrant.
where CODi and CODt are initial and final COD values of samples at the beginning and end of the investigation respectively.
Detection of degradation products using gas chromatography
Degradation products of glucose were identified by GC with flame ionisation detection. Samples (1.5 ml) for analysis were centrifuged at 15,000×g for 30 min. The supernatant was transferred into a 2 ml vial tube and run on a GC system. The mobile phase was He at 2 ml min−1; the injector was at 260 °C. The oven was initially set at 35 °C for 5 min and then ramped to 170 °C over 10 min. The detector was at 250 °C. The presence of degradation metabolites ethanol, acetic acid and butyric acid was confirmed using the retention time of the respective standard compounds.
Electrochemical monitoring
Polarisation curves for measuring power density vs current density plots were constructed using a range of external resistances ranging from 10 Ω to 1 MΩ. The closed external circuit of the MFC system for each test was opened to connect various external resistances when the system exhibited a stable voltage across the initial 1000 Ω external resistor. The current flowing through each external load was calculated using Ohm’s law: \( I = \frac{E}{R} \), where E is the potential across the resistor (mV), I is the current flowing through the load (mA) and R is the external resistance (Ω). The power generated was calculated with the expression: \( P = E*I \) where P is the power produced (µW), E is the potential difference between anode and cathode (mV) and I is the current generated (mA). The power density and current density values were calculated by normalising power and current values to the projected surface area of the anodic electrode (25 cm2).
Coulombic efficiency was calculated by integrating the measured current over time based on the observed COD removal by using the criteria outlined in Zhao and Slade (2009). CE is a measure of the amount of electrons generated via substrate oxidation that are reflected as current.
Statistical analysis
Statistical analyses were performed using Prism Graph Pad 5.0 with α = 0.05. Each experiment was performed in triplicate. All data is presented as means of triplicate experiments and the error bars represent the standard deviation of the mean.
Results and discussion
Experiment involving use of a dialysis membrane to retain cells
Voltage–time profiles and polarisation curves
The voltage–time profile, polarisation and power density curves for the contribution of direct electron transfer mechanism to electricity production are shown in Figs. 3 and 4. MFCs utilising the direct mechanisms of electron transfer (DET) generated the highest voltage throughout the study. The maximum voltage generated across a 1000 Ω resistor by MFCs utilising DET, mediated electron transfer mechanism (MET) and those utilising both mechanisms (combined, CM) were 586 ± 5, 400 ± 6 and 470 ± 6 mV respectively. Similarly, DET produced the highest average voltage of 485 ± 7 mV, followed by CM of 323 ± 5 mV and MET of 317 ± 6 mV. At day 8 there was an exponential increase in voltage production by CM after a gradual reduction of voltage to 157 mV ± 3.
Power density tests were conducted on the second day when all the MFCs were in their pseudo-steady-state conditions. As shown in Fig. 4, the CM system generated the highest maximum power density of 129 ± 6 mWm−2; DET study generated 114 ± 6 mWm−2 while MET produced the least maximum power density of 32 ± 8 mWm−2.
COD degradation and coulombic efficiency
Table 1 shows a comparison of substrate degradation as COD and amount of electron recovery on day 11 of the investigation. The CM system gave the highest substrate utilization efficiency of 57 ± 3 % which was more than 2 fold higher than DET which gave 21 ± 2 %. However, with regard to CE, DET gave 36 ± 1 % and was 4 fold higher than CM of 9 ± 1 %. MET gave 46 ± 3 % COD reduction with a CE of 11 ± 2 %.
Metabolites of substrate degradation
Acetic acid and butyric acid were the main degradation products with acetic acid produced in larger amounts than butyric acid (Table 2).
Experiment involving immobilised Shewanella oneidensis cells
Voltage–time profiles and polarisation curves
The voltage–time profile, polarisation and power density curves for the contribution of mediated electron transfer mechanism to electricity production are shown in Figs. 5 and 6. MFCs utilising MET generated the highest voltage throughout the study (average voltage of 347 ± 4 mV for MET and 267 ± 6 mV for CM).
The maximum voltage generated under 1000 Ω by MET and CM were 445 ± 6 and 395 ± 5 mV respectively. At day 10.4 there was an exponential increase in voltage production by CM after a gradual reduction of voltage to 239 mV ± 4.
Power density tests were conducted on the second day when all the MFCs were in their pseudo-steady-state conditions. As shown in Fig. 6, the CM system generated maximum power density of 105 ± 4 mWm−2 while MET produced a maximum power density of 36 ± 6 mWm−2.
COD degradation and coulombic efficiency
Table 3 shows a comparison of substrate degradation as COD and amount of electron recovery on day 11 of the investigation. The CM system gave the highest substrate utilization of 43 ± 2 % with a CE of 13 ± 2 % whereas MET gave 36 ± 3 % COD reduction with a CE of 20 ± 4 %.
There are a number of mechanisms through which extracellular electron transfer in Shewanella might occur, including direct electron transfer and transfer using redox shuttles (Gralnick and Newman 2007). The experiment aimed to investigate the contribution of direct electron transfer mechanism to electricity production in microbial fuel cells utilising Shewanella as biocatalyst. This mechanism is thought to involve four key proteins—CymA, MtrA, MtrB and MtrC (Fig. 7)—which form a conduit for electron transfer from the quinone pool to the outside of the cell. Other direct electron transfer pathways e.g. the MtrFDE pathway have also been suggested (Kracke et al. 2015). Conductive appendages (pili) might also be involved.
The results indicated that in the case where a dialysis membrane was used DET makes a significant contribution, \( \frac{{\left[ {\frac{(114\, + \,129)}{2}} \right]\, - \,32}}{{\left[ {\frac{(114\, + \,129)}{2}} \right]}}\, = \,74\,\% \), to overall electricity production. When cells were retained close to the anode, direct electron transfer was assumed to be the main mechanism although we do recognise that this does not stop mediated electron transfer from operating hence the averaging of the two maximum power density values 114 and 129 mWm−2. By subtracting the maximum power density value associated with mediated electron transfer from the one when both direct and mediated electron transfer are operational, we get the contribution of DET mechanisms to overall electron transfer. When cells were immobilised in alginate the corresponding contribution was \( \frac{(105 - 36)}{105} = 66\,\% \). The results are, however, in direct contrast to the work of Kotloski and Gralnick (2013) who showed that DET accounted for ca. 25 % of the ability of Shewanella to reduce insoluble substrates. They showed this by generating mutants of Shewanella that could no longer secrete redox shuttles (flavins) and characterising the mutants for reduction of Fe3+ in comparison with wild type strains. The reduced electron transfer in the riboflavin-deficient mutant in this case could also have been due to reduced cell growth rate reducing substrate turnover rate as riboflavin is necessary for growth. In our study, cell viability is not expected to be different in the systems tested; one of the advantages of immobilised cells is that they can be reused over and over in batch and in continuous systems. For all the systems tested, substrate was continuously utilised although to different extents because of diffusion limitations in entrapped immobilisation systems (Bickerstaff 2009).
Table 1 indicates that DET gives more CE than other mechanisms possibly because the retaining of the cells close to the anode helps to overcome resistances to electron transport from bacteria to the anode by the formation of biofilms on the anode and/or by direct contact of cells to the anode via pili (Mohan et al. 2008). In the case of CM and MET, the observed diminished CE can possibly be due to diversion of electrons for biomass growth (Zhuang et al. 2012).
Table 2 indicates a low concentration of butyric acid produced by CM which could be explained by the voltage increasing after a gradual decline (see Fig. 3, day 8), suggesting a metabolic shift. Butyrate could have been reused as substrate as was also observed by Finch et al. (2011) in the case of Clostridium acetobutylicum.
Less COD reduction observed by DET, as shown in Table 1, could be due to the diffusion limitation of substrate across the dialysis membrane limiting the availability of substrate that can be readily consumed.
Conclusion
The contribution of direct electron transfer mechanism to electricity production in microbial fuel cells is shown by physically retaining Shewanella oneidensis close to or away from the anode electrode using a dialysis membrane and by immobilising the cells in alginate. 66–74 % of the electrons transferred could be attributed to direct electron transfer. Studies that would enhance direct electron transfer in Shewanella spp are suggested as future work.
References
Bickerstaff GF (2009) Immobilisation of enzymes and cells. In: Walker JM, Rapley R (eds) Molecular biology and biotechnology. RSC Publishing, Cambridge
Fernando E, Keshavarz T, Kyazze G (2012) Enhanced bio-decolourisation of acid orange 7 by Shewanella oneidensis through cometabolism in a microbial fuel cell. Int Biodeter Biodegrad 72:1–9
Finch AS, Mackie TD, Sund CJ, Summer JJ (2011) Metabolite analysis of Clostridium acetobutylicum fermentation in a microbial fuel cell. Bioresour Technol 102:312–315
Gralnick JA, Newman DK (2007) Extracellular respiration. Mol Microbiol 65:1–11
Harnisch F, Aulenta F, Schroder U (2011) Microbial fuel cells and bioelectrochemical systems: industrial and environmental biotechnologies based on extracellular electron transfer. In: Moo-Young M (ed) Comprehensive biotechnology, 2nd edn. Elsevier, Amsterdam, pp 643–659
Kotloski NJ, Gralnick JA (2013) Flavin electron shuttles dominate extracellular electron transfer by Shewanella oneidensis. MBio 4:e00553
Kracke F, Vassilev I, Kromer JO (2015) Microbial electron transport and energy conservation—the foundation for optimising bioelectrochemical systems. Front Microbiol. doi:10.3389/fmicb.2015.00575 (Article 575)
Lee SW, Jeon BW, Park DH (2010) Effect of bacterial cell size on electricity generation in a single-compartimented microbial fuel cell. Biotechnol Lett 32:483–487
Logan BE (2008) Microbial fuel cells. Wiley-Interscience, Hoboken
Mohan SV, Rhagavulu SV, Sarma P (2008) Influence of anodic biofilm growth on bioelectricity production in single chambered mediatorless microbial fuel cell using mixed anaerobic consortia. Biosens Bioelectr 24:41–47
Okamoto A, Hashimoto K, Nakamura R (2012) Long-range electron conduction of Shewanella biofilms mediated by outer membrane c-type cytochromes. Bioelectrochemistry 85:61–65
Oliveira VB, Simoes M, Melo LF, Pinto AMFR (2013) Overview on the developments of microbial fuel cells. Biochem Eng J 73:53–64
Westwood D (2007) The determination of chemical oxygen demand in waters and effluents. Environment Agency, National Laboratory Service
Wrighton K et al (2011) Evidence of direct electron transfer by a gram positive bacterium isolated from a microbial fuel cell. Appl Environ Microbiol 77:7633–7639
Zhao F, Slade RC (2009) Techniques for the study and development of microbial fuel cells: an electrochemical perspective. Chem Soc Rev 38:1926–1939
Zhuang L, Chen Q, Zhou S, Yuan Y, Yuan H (2012) Methanogenesis control using 2-bromoethanesulfonate for enhanced power recovery from sewage sludge in air-cathode microbial fuel cells. Int J Electrochem Sci 7:6512–6523
Acknowledgments
Segun Fapetu wishes to thank his family for sponsoring his PhD studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fapetu, S., Keshavarz, T., Clements, M. et al. Contribution of direct electron transfer mechanisms to overall electron transfer in microbial fuel cells utilising Shewanella oneidensis as biocatalyst. Biotechnol Lett 38, 1465–1473 (2016). https://doi.org/10.1007/s10529-016-2128-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-016-2128-x