Abstract
Heavy metal resistant bacteria are of great interest because of their potential use in bioremediation. Understanding the survival and adaptive strategies of these bacteria under heavy metal stress is important for better utilization of these bacteria in remediation. The objective of this study was to investigate the role of bacterial extracellular polymeric substance (EPS) in detoxifying against different heavy metals in Bacillus sp. S3, a new hyper antimony-oxidizing bacterium previously isolated from contaminated mine soils. The results showed that Bacillus sp. S3 is a multi-metal resistant bacterial strain, especially to Sb(III), Cu(II) and Cr(VI). Toxic Cd(II), Cr(VI) and Cu(II) could stimulate the secretion of EPS in Bacillus sp. S3, significantly enhancing the adsorption and detoxification capacity of heavy metals. Both Fourier transform infrared spectroscopy (FTIR) and three-dimensional excitation–emission matrix (3D-EEM) analysis further confirmed that proteins were the main compounds of EPS for metal binding. In contrast, the EPS production was not induced under Sb(III) stress. Furthermore, the TEM–EDX micrograph showed that Bacillus sp. S3 strain preferentially transported the Sb(III) to the inside of the cell rather than adsorbed it on the extracellular surface, indicating intracellular detoxification rather than extracellular EPS precipitation played an important role in microbial resistance towards Sb(III). Together, our study suggests that the toxicity response of EPS to heavy metals is associated with difference in EPS properties, metal types and corresponding environmental conditions, which is likely to contribute to microbial-mediated remediation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal pollution has become one of the most serious environmental problems around the world [1]. Because of their non-biodegradability, long biological half-lives and bio-accumulation, heavy metals are much more threatening to the natural environment and human health than other organic contaminants [2]. Moreover, with the development of industry and agriculture, the residues generated by mining, industrial processing, and the abuse of chemical fertilizers and pesticides, usually simultaneously, contain a variety of heavy metals, causing serious co-contamination problems [3]. In China, cadmium (Cd), chromium (Cr), copper (Cu) and antimony (Sb) are some of the most common heavy metals in both industrial and agricultural soils, and the concentrations of these heavy metals have significantly exceeded the Grade II environmental quality standard for soils (GB15618-1995) during the past two decades [4,5,6]. How to, efficiently, economically and environmental-friendly, remove these toxic metals from the environments is therefore becoming a significant problem nowadays, receiving notable research attention.
Conventional physico-chemical technologies, including chemical precipitation, filtration, membrane–separation, ion exchange, electrochemical treatment and so on, have been used for removing heavy metals from the polluted environments. Most of them, however, are cost expensive, less efficient, incompletely removable or with greater secondary pollution potential [7]. In recent years, microorganism-mediated bioremediation has been becoming a hotspot due to its great removal efficiency, low operating cost and potential in situ remediation of metal-contaminated environments [8, 9]. In this context, microbial strains capable of surviving at high concentrations of multiple metals make them ideal tools for soil remediation [10]. Meanwhile, a better understanding of their adaptation abilities and resistance mechanisms in multi-metal contaminated environments is a critical prerequisite for the development and practical application of microbe-based remediation strategies.
Several tolerance mechanisms have been proposed for bacteria to cope with the heavy metals. For example, active efflux, intracellular sequestration, enzymatic transformation and oxido-reduction of toxic metal ions are some of the fundamental approaches adopted by bacteria to thrive well under metal-stressed conditions [11]. Apart from these, the extracellular polymeric substance (EPS) production is believed to be another protective strategy for bacteria to survive and grow in the metal-contaminated environment [12, 13]. The EPSs secreted from bacteria mainly consist of polysaccharides, proteins and nucleic acids, and the functional groups in EPS matrix, such as carboxyl and hydroxyl groups, are negatively charged. Thus, the overall negative charge of bacterial EPS plays a significant role in chelating metal cations, and preventing the direct contact between the cells and toxic metals [14, 15]. Although the ability of EPS to remove heavy metals from polluted environment has been widely reported in the literature, focusing mainly on its biotechnological potential [16, 17], information about the effects of heavy metals on EPS production as well as the correlation between EPS production and heavy metal resistance in bacteria is still limited, particularly for exposure to different metals.
Since EPSs serve as the first barrier between the microbial cell and its immediate environment, it has been thought that the metal stressful conditions will induce bacterial EPS production to prevent metals from interacting with vital cellular components [18]. From the physiological point of view, metals can be grouped into two main categories: (i) essential (e.g. Ca, Mg, Fe, Zn and Cu) to many physiological processes and (ii) non-essential (e.g. Cd, Cr, Sb and Pb) to the cell life. Although the binding of metal ions to EPS can prevent, up to a point, the entry of the metals into the cells, it may also affect the metal homeostasis in these organisms [19]. In addition, when in excess, the metal ions enter the cells and their toxicity may be avoided by their reduction to a less toxic oxidation state or intracellular complexing [20]. Thus, it is still not clear how significant is EPS production for bacteria under different types and different concentrations of metals. Moreover, there is still a great controversy regarding the impacts of EPS on metal sorption by microbial. For example, the presence of EPS in Bacillus subtilis and Pseudomonas putida strains can significantly enhance Cu(II) adsorption capacity [21]. However, the extent of Cd adsorption was not significantly affected by EPS in P. putida. [22]. For Pseudomonas aureofaciens, EPS-free cells even showed more remarkable ability to adsorb metals than intact cells [23]. Such a difference in adsorption performance in metal adsorption should be mainly due to different EPS properties, metal species, and environmental conditions [24]. However, it is unclear how exactly these factors are inter-associated and affect the adsorption performances. Therefore, investigating the effects of multiple heavy metal stress on the characteristics and adsorption performance of EPS for the same bacterial species is necessary.
In the current study, an antimony-oxidizing bacterium Bacillus sp. S3 was applied [25]. The aim of this study was to evaluate the effects of the presence/concentration of several heavy metals commonly found in polluted soils, including Cd(II), Cr(VI), Cu(II) and Sb(III), on the growth/survival, EPS production and composition, and ultrastructure of Bacillus sp. S3 strain. To further explore the binding behaviors and biosorption mechanisms between EPS and the target metals, a combination of Fourier transform infrared spectroscopy (FTIR), three-dimensional excitation–emission matrix (3D-EEM), transmission electron microscopy (TEM) and isothermal adsorption experiments was systematically investigated. The results may contribute to reveal the role of extracellular polymeric substance (EPS) in the toxicity response of microbes to multiple heavy metals, and will help us to implement metal removal systems based on bacterial EPS in the future.
Materials and methods
Bacterial strains and culture conditions
The bacteria Bacillus sp. S3 used in this study was previously isolated from an antimony-mine area, named as Xikuangshan, in Hunan province, China (GPS location: 27°77′32′′N, 111°49′54′′E) [25]. For routine growth and experiments, bacterial cells were aerobically grown in 150 mL Luria broth (LB) media (10.0 g/L tryptone, 5.0 g/L yeast extract, and 5.0 g/L NaCl, pH 7.0–7.2) at 28 °C with a shaking speed of 150 rpm as appropriate.
Metal salts
The metal salts used in this study including CdCl2, K2Cr2O7, Cu(NO3)2·3H2O, and C8H4K2O12Sb2·3H2O were obtained from Shanghai Pharmaceutical Co. Ltd., in China. All chemicals used were of analytical grade. Stock solutions of Cd(II), Cr(VI), Cu(II) and Sb(III) at a concentration of 1000 mg/L were prepared in PBS (pH 6.8). The working solution of metal ion was prepared from serial dilution of the stock solution.
Metal tolerance analysis
To explore the tolerance of Bacillus sp. S3 against heavy metals, we cultured cells in LB alone or supplemented with Cd(II) (0–20 mg/L), Cr(VI) (0–150 mg/L), Cu(II) (0–150 mg/L) and Sb(III) (0–150 mg/L) at 28 °C with shaking at 150 rpm. The optical density of every culture (1 mL) at 600 nm (OD600) was determined at 7 h intervals with ultraviolet (UV) spectrophotometer (Shimadzu UV-2550, Japan). Meanwhile, the minimal inhibitory concentration (MIC) for the isolate Bacillus sp. S3 was also measured as described by Ball et al. to further clarify its capacity of multiple metal tolerance [26]. The MIC tests were carried out in triplicate.
EPS extraction and chemical analysis
Ethylenediaminetetraacetic acid disodium (EDTA) was employed to extract the EPS of Bacillus sp. S3 due to its lower cell lysis and high extraction efficiency [27]. When the bacterial cells (either metal-free or metal-amended) reached the stationary growth phase, they were harvested by centrifuging at 2000g at 4 °C for 15 min. Then, the pellets were washed twice with 0.9% NaCl solution and dissolved in 10 mL sterile distilled water. After addition of 1 mM EDTA, the solution was placed at 4 °C for 3 h. The EPS were harvested by centrifuging at 10,000g at 4 °C for 30 min and then filtrated through 0.22-μm cellulose acetate membrane [28]. To obtain pure EPS, the crude EPS were dialyzed for 24 h at 4 °C using distilled water.
The contents of carbohydrates, proteins and nucleic acids were determined by methods as described in Dubois et al., Frølund et al., and Boonaert et al., respectively [29,30,31]. The three components were added up as the total content of EPS.
Scanning electron microscopy (SEM)
To test whether EDTA treatment was an efficient method for EPS removal in Bacillus sp. S3, the morphological differences between intact cells and EPS-free cells were examined with SEM. The EPS-free cell was prepared via EDTA method as mentioned before. Briefly, bacterial cells were harvested at 4 °C at 2000g for 10 min, which were then fixed at 4 °C in 2.5% glutaraldehyde for about 12 h. After being washed three times with phosphate buffer saline (PBS, 0.1 M and pH 7.4), the cell samples were dehydrated in a series of ethanol (10–100%) and sputter-coated a thin layer of platinum according to standard procedures. JSM 7800 F scanning electron microscopy (JEOL Ltd., Japan) was used to image the morphology of bacterial cells.
Fourier transform infrared (FTIR) spectroscopy analysis
To identify the functional groups related to metal adsorption on the cell surface of Bacillus sp. S3, the FTIR spectra of intact and EPS-free cells before and after reaction with metals were analyzed with a Shimadzu spectrometer (8400, Japan). The wet cell samples were first dried in a vacuum drying oven and then mixed with spectrochemically pure potassium bromide (KBr) at a mass ratio of 1:100. The FTIR spectra were obtained over the range from 500 to 4000 cm−1 at the resolution of 4 cm−1.
3D-EEM fluorescence spectroscopy analysis
To explore the interaction between metal ions and EPS, a fluorescence spectrophotometer (Cary Eclipse, Varian, USA) was used to measure the 3D-EEM spectra of EPS in the present study. EEM scans were made at the excitation wavelength from 220 to 400 nm and the emission wavelength from 300 to 500 nm. The spectra were recorded at a scan rate of 1200 nm min−1 and the width of the excitation/emission slit was set to 2.5 nm. The software Origin 8.5 (Origin Lab Inc., Hampton, USA) was used to analyze the EEM data.
Metal adsorption studies
To explore the EPS complexing capacity, both intact and EPS-free cells were used for metal adsorption experiments. The bacterial cells were suspended in ultrapure water to form a suspension of 20 g/L (wet mass). The total volume for both adsorption experiments was 20 mL, including 5 mL bacterial suspensions, 0.01 mol/L KNO3 and a series of metal stock. The final metal concentrations in the suspension varied from 10 to 50 mg/L, and the pH of each test tube was adjusted to 5.0. The mixture was shaken at 150 rpm at 28 °C for 3 h, and then filtered through a 0.22 µm cellulose nitrate membrane. The concentrations of heavy metals in the supernatant were analyzed using atomic adsorption spectrometry (AAS Vario 6, Analytikjena Co., Germany). The amount of adsorbed metals by bacterial cells was the difference between the initial metal ion concentration and the remaining metal ion concentration in the supernatant.
Both Langmuir and Freundlich models were used to analyze the adsorption isotherm results in this study. The Langmuir model assumes that adsorption occurs via a monolayer on the surface of adsorbents [32] and the Freundlich model is an empirical equation based on the assumption of multilayer adsorption onto the heterogeneous surface of adsorbents [33]. These models were expressed by Eqs. (1) and (2) as follows:
Linearized Langmuir model:
Linearized Freundlich model:
where qm is the maximum concentration of adsorbed metals (mg/g), qe represents the amount of metal ions adsorbed per unit of dry weight cells at equilibrium (mg/g), Ce is the metal ion concentration at equilibrium (mg/L), b is the Langmuir constant (L/mg), KF (mg/g) and n are the Freundlich constants.
Transmission electron microscope (TEM) and energy-dispersed X-ray (EDX) analysis
The spatial distribution of four different metal ions on the surface and inside the cell of Bacillus sp. S3 was characterized by TEM. After the biosorption experiments, the cell samples were harvested and rinsed twice with phosphate buffer, and then fixed in 1% osmium tetraoxide for 1.5 h. The crude specimens that were dehydrated and embedded in resin were observed using a Joel 2100F transmission electron microscopy [34]. The energy dispersive X-ray spectrometer (EDX) analysis was performed as described by Holmes et al. [35].
Statistical analysis
All the experiments were performed in triplicate and the data were expressed as mean ± standard deviation (SD). Statistical analysis was performed by one-way ANOVA in SPSS version 21.0 (SPSS Inc., Chicago, IL, USA). Any p value less than 0.05 was considered significant.
Results and discussion
Impacts of Cd(II), Cr(VI), Cu(II) and Sb (III) on bacterial growth
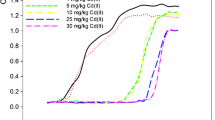
As shown in Fig. 1, the biotoxicity of metals increased with the increase of metal concentrations. Among these tested metals, Cd(II) caused maximum reduction in growth of Bacillus sp. S3, which resulted in a significant lag phase even at low concentration (5 mg/L) (Fig. 1a). In contrast, the other metals such as Cr(VI) and Cu(II) demonstrated a less inhibiting effect on Bacillus sp. S3 especially at low doses (<50 mg/L), while at high doses (>100 mg/L) they exerted a marked discourage impact on the bacterial growths (Fig. 1b, c). However, exogenous addition of 150 mg/L Sb(III) showed slight effect on the bacterial growth, indicating that the bacterial strain Bacillus sp. S3 had a higher degree of resistance to Sb(III) (Fig. 1d). The results of MIC showed that Bacillus sp. S3 was a multi-metal resistant bacterial strain. The maximum tolerance concentration was 320 ± 7.00 mg/L, 140 ± 4.36 mg/L, 80 ± 5.29 mg/L and 20 ± 1.00 mg/L to Sb(III), Cu(II), Cr(VI) and Cd(II), respectively. The whole trend of tolerance capability to heavy metals showed as Sb(III) > Cu(II) > Cr(VI) > Cd(II).
Effects of Cd(II), Cr(VI), Cu(II) and Sb (III) on EPS production and composition
The influence of heavy metals on the EPS production and composition of Bacillus sp. S3 is shown in Fig. 2. Increase of EPS production in the presence of heavy metals was apparent in Bacillus sp. S3 except for exposure to Sb(III) (Fig. 2). For example, the content of EPS produced in the absence of heavy metals was about 36.2 ± 3.33 mg/g dry cell, which comprised of much higher content of protein (80.64 ± 2.06%) than polysaccharides (14.7 ± 1.55%). With the Cd(II) concentration increasing from 0 to 8 mg/L, the EPS content significantly increased from 36.2 ± 3.33 to 60.67 ± 1.73 mg/g dry cell and then, it obviously descended (Fig. 2a). Meanwhile, the content of protein significantly enhanced with increasing Cd(II) from 0 to 8 mg/L, and the ratio of proteins/polysaccharides varied from 5.55 ± 0.76 to 5.77 ± 0.44. Similar patterns were also observed when the bacterial strain was under the Cr(VI) and Cu(II) stress (Fig. 2b, c). The EPS content significantly increased as Cr(VI) and Cu(II) concentrations increase, and were peaked at 30 mg/L (67.17 ± 3.73 mg g −1 dry cell) and 60 mg/L (90.33 ± 5.52 mg g −1 dry cell), respectively. As shown in Fig. 2b, c, the values of proteins/carbohydrates ratio ranged from 5.55 ± 0.76 to 9.27 ± 1.64 for Cr(VI) and from 5.55 ± 0.76 to 8.09 ± 0.91 for Cu(II), respectively. In contrast, the least toxic Sb(III) did not significantly stimulate the production of EPS in Bacillus sp. S3, and the EPS content rapidly decreased to a lower level accompanied by an increase in Sb(III) concentration from 30 to 120 mg/L (Fig. 2d).
EPS act as the first barrier to protect cell from direct contact with heavy metals, and therefore reducing the toxic effect of heavy metals on microorganisms [36]. Moreover, heavy metal ions can be precipitated onto the cell surfaces by functional groups of EPS [22], bacteria, therefore, have been thought to produce more EPS under metal stress [37]. Similar to previous studies [36,37,38], our data also showed that Bacillus sp. S3 produced more EPS under relatively low concentration of Cd(II), Cr(VI), and Cu(II) (Fig. 2). In addition, the larger proportion of protein in EPS and the marked changes of protein content before and after heavy metal stimulation suggested that extracellular proteins played a major role in metal resistance for Bacillus sp. S3 [27]. At high concentrations, however, these toxic heavy metals might decrease the metabolism activity of Bacillus sp. S3 and, accordingly, reduce the production of EPS (Fig. 2). This agreed with results reported by Sheng et al. [27] and Ding et al. [38], who found that the exceeded concentration of heavy metal would cause toxicity to cell at physiological and molecular levels, resulting in enzyme inactivation and impeding EPS synthesis.
In contrast, the Sb(III) stress did not significantly induce enhanced production of EPS in Bacillus sp. S3 as expected, even at low concentrations of Sb(III) (Fig. 2d). In our previous study, Bacillus sp. S3 has been proved to be a novel antimony-oxidizing bacteria with extremely high Sb(III) oxidation efficiency [25], which can mitigate the toxicity of Sb(III) by converting it to a less toxic form (Sb(V)) through oxidation reaction [39, 40]. Based on the results of MIC data mentioned above, the bacterial strain Bacillus sp. S3 showed the highest degree of resistance to Sb(III) compared to other metals, indicating that intracellular detoxification rather than extracellular EPS precipitation might play an important role in boosting microbial resistance towards Sb(III) [41, 42].
The surface properties of EPS
As shown in Fig. 3a, the intact cells of Bacillus sp. S3 were covered with a flocculated layer of EPS, which made the cell surfaces fold. Meanwhile, some slimy substances were observed on the cell surfaces, forming a polymeric network and connecting the rod-shaped cells (Fig. 3a). However, the outer layer and fibrous materials on the cell surfaces disappeared after the EDTA treatment, indicating that the EDTA treatment method was suitable for EPS removal in this study (Fig. 3b).
Effects of Cd(II), Cr(VI), Cu(II) and Sb (III) on FTIR spectra of EPS
The spectra of FTIR for intact and EPS-free Bacillus sp. S3 before and after metal exposure were recorded in Fig. 4. The information about FTIR bands are summarized in Table 1 [43,44,45]. As shown in Fig. 4a, Bacillus sp. S3 possessed many functional groups including carboxyl and amino as well as phosphate on the bacterial surface (Fig. 4a). Moreover, the peak positions of FTIR bands between EPS-free cells and intact bacteria were similar (Fig. 4b), indicating that the EPS of Bacillus sp. S3 shares similar components with the cell wall and removal of EPS from cell surfaces did not affect the cell wall-functional group chemistry [22, 46].
For both intact and EPS-free cells, exposure to metals resulted in slight changes in the position of the band in the FTIR spectra but without band missing or new band appearing (Fig. 4), indicating that metal stress did not affect the types of functional groups of EPS in Bacillus sp. S3. Amine groups played an important role in metal ions binding in both intact and EPS-free cells because of the shifting of the peak at ~ 3447 cm−1, ~ 1643 cm−1 and ~ 1548 cm−1 after Cd(II), Cr(VI), Cu(II) and Sb(III) loaded. Moreover, broadening and shifting in the position of the band at ~ 1400 cm−1 were observed for Bacillus sp. S3 after exposure to Sb(III), indicating that Sb(III) was complexed directly with carboxyl groups. As demonstrated in Fig. 4, other changes of peak positions, such as slight variations at ~ 1081 cm−1 and ~ 1235 cm−1 suggested that phosphate groups were also responsible for the binding of metal ions. In general, our data suggested that many functional groups, such as C=O, N–H, P=O and O–H on cell surface might be responsible for the metal ion binding, and proteins were the predominant contributors in metal ion uptake [47].
Effects of Cd(II), Cr(VI), Cu(II) and Sb(III) on 3D-EEM fluorescence spectra of EPS
Fluorescence spectrum provided special information about the chemical composition of EPS samples. As shown in Fig. 5a and Supplementary Table S1, two typical peaks of EEM fluorescence spectra were observed in control metal-free cells, which are located at Ex/Em (excitation/emission) wavelengths of 225/350 nm and 315/405 nm, respectively. These peaks indicated that the EPSs of Bacillus sp. S3 were mainly composed of aromatic protein-like substances (peak I) and fulvic-like substances (peak II) [15, 48, 49]. After addition of toxic Cd(II), Cu(II), Cr(VI) or Sb(III), the fluorescence intensity at the two peaks significantly decreased (Fig. 5, and Supplementary Table S1), indicating that chem-physical reaction occurred between those heavy metal ions and EPSs [50]. Moreover, the peak I disappeared under Cu(II) and Cr(VI) stress, indicating that the structure of aromatic proteins in EPSs was destroyed by an exposure to the high concentrations of Cu(II) and Cr(VI) [51]. It has been demonstrated that complexation with copper and chromium ions quenched protein-like fluorophores of EPS [52, 53]. However, the potential fluorescence quenching mechanisms (static or dynamic quenching) relating to these processes need to be further explored.
Furthermore, fluorescence peaks shifted towards shorter (blue shift) or longer wavelengths (red shift) along the Ex axis and Em axis after exposure to metals (Fig. 5 and Table S1). Usually, the red shift is associated with the carbonyl, hydroxyl, alkoxyl, amino groups, and carboxyl-containing substances [54], whereas the blue shift results from decomposition of condensed aromatic moieties and break up of large molecules into smaller fragments [55]. Under Cd(II) stress, peaks I and II were blue-shifted by 10 nm and 5 nm along Em axis, respectively. However, peak I under Sb(III) stress was red shifted by 35 nm along the Em axis and blue shifted 45 nm along the Em axis. Meanwhile, peak II exhibited a red shift of 60 nm and 70 nm along the Ex and Em axes, respectively. This difference suggests that exposure to different metal ions made the components in the EPS chemically different.
Adsorption isotherms of Cd(II), Cr(VI), Cu(II) and Sb (III) on EPSs
A comparison between the Langmuir and Freundlich models is shown in Table 2. Based on the correlation coefficient (R2), the Langmuir isotherm fitted better with the experimental data for intact and EPS-free cells than Freundlich isotherm, indicating monolayer biosorption and a homogeneous distribution of active surface sites [56]. The capacities of metal adsorption for both intact and EPS-free Bacillus sp. S3 cells are shown in Figs. 6 and 7. The adsorption capacity of intact cells increased with the increase of initial metal concentrations but it would not increase when the metal concentration exceeded a certain value. In general, the biosorption process is defined by two Langmuir constants qm and b together [57]. The predicted maximum biosorption capacity (qm) was 11.76, 12.28, 11.56 and 3.49 mg/g for Cd(II), Cr(VI), Cu(II) and Sb (III), respectively (Table 1). Meanwhile, the values of constant b for Cd(II), Cr(VI) and Cu(II) in Bacillus sp. S3 cells were also higher than that for Sb(III) (Table 2), indicating that Bacillus sp. S3 was more efficient in adsorbing Cd(II), Cr(VI) and Cu(II) than Sb(III). However, the maximum amounts of metal adsorbed in Bacillus sp. S3 did not significantly decrease after the EPS was fully removed. Instead, it was found that the adsorption capacity to Sb(III) of intact cells was relatively lower than that of EPS-free cells (Table 2).
EPS could show different levels of metal affinity due to differences in composition of functional groups [24]. Increasing of metal adsorption in the presence of EPS has been reported in a number of studies. For example, the amounts of Cu(II) adsorbed by B. subtilis and P. putida were decreased by 37.8% and 25.4%, respectively when the bacterial EPS was removed [21]. The corresponding EPS-free cells of Shewanella oneidensis showed a significantly lower Zn and Pb adsorption relative to the intact cells [58]. Additionally, the phosphate groups of EPS in Acidithiobacillus ferrooxidans have been proved to play an important role in uranium absorption, although they accounted for only a small part of the EPS components [59]. However, if the external EPSs are mainly composed of hydroxyl, ester, and aliphatic components, it may become a physical (diffusion) barrier for metal ions to approach the cell wall. González et al. (2010) found that the content of carboxylate groups in the EPS of P. aureofaciens was much less than that in the cell wall, therefore significantly reducing its metal adsorption ability [23]. Ueshima et al. (2008) also showed that the EPS layer of P. putida did not enhance the Cd binding. EPS may result in the cell aggregation, thereby decreasing the available surface area on the bacterial cells and lowering the adsorption sites for metal ions [22]. Meanwhile, dissolved organic carbon (DOC) that is released from EPS layer may compete with heavy metal ions for the binding sites [24].
Metal distribution in bacterial cells
To intuitively show the locations of accumulated metals within the cells, as well as the possible ultrastructural changes of the cells, TEM and EDX analyses were performed. As shown in Fig. 8, the metal-free cells revealed smooth and intact cell wall and relatively homogenous cytoplasm, while the metal-treated cells showed different degrees of cytoplasmic disintegration and shrinkage, which was a typical response to metal toxicity [60]. Large amounts of metal ions were adsorbed on the extracellular surface of the bacterial cells (Fig. 8, arrowheads). Meanwhile, the intracellular bioaccumulation can be perceived as electron-dense deposits in the cell cytoplasm (Fig. 8, arrows).
Cd(II) is a non-essential toxic heavy metal for microorganisms and the Cd accumulation caused thickening and unevenness of the surface of cell walls (Fig. 8c, d). Meanwhile, the micrograph showed that electron-dense deposits were mainly present on the cell surface rather than the cell interior, indicating that the accumulated Cd(II) was mainly located within the EPSs and on the cell wall in Bacillus sp. S3. The present observation was in line with an earlier report, which confirmed extracellular adsorption on the cell surface of Bacillus cereus RC-1 was the predominant mechanism for limit Cd(II) toxicity, followed by a relatively slow bioaccumulation process inside the cell [61]. The presence of Cr(VI) and Cu(II) resulted in extensive morphological abnormalities, with the appearance of irregular cell walls and the separation of cell membranes from the cell walls (Fig. 8e–h). The electron dense granules of Cr(VI) and Cu(II) mainly deposited on the EPS cell wall–periplasmic cell membrane components, indicating that Bacillus sp. S3 cells could enhance the resistance of Cr(VI) and Cu(II) tolerance by sequestrating these metals in and around the cell periphery and preventing, at least partially, the entry of metal into the cytoplasm [62]. The Sb(III) accumulation did not change the shape of cells (Fig. 8f). Compared to Cd(II)-, Cr(VI)- and Cu(II)-treated cells, most of the electron-dense deposits were in the cytoplasm of the Sb(III)-treated cells (Fig. 8i, j). According to our previous research, Bacillus sp. S3 was a novel antimony-oxidizing bacteria isolated from Sb mine soils, which occupied extremely high Sb(III) oxidation efficiency [25]. The preferential intracellular deposition rather than extracellular adsorption indicated that the oxidation of Sb(III) inside the cells was the predominant detoxification mechanism of Bacillus sp. S3 for Sb(III) [39, 40].
Conclusion
In this study, we demonstrated that the isolated bacterial strain Bacillus sp. S3 was highly resistant to multiple heavy metals, especially to Sb(III), Cu(II) and Cr(VI). The EPS production of Bacillus sp. S3 was significantly induced under Cd(II), Cr(VI) and Cu(II) stress, which could indirectly reduce the toxicity of these heavy metals by complexation with surface functional groups. FTIR and 3D-EEM analysis further confirmed that proteins were the main compounds of EPS for metal binding under metal stress. Meanwhile, the results of adsorption experiments showed that EPS of Bacillus sp. S3 had a higher combined ability with Cd(II), Cr(VI) and Cu(II) than Sb(III). In contrast, the production of EPS was not stimulated by the toxic Sb(III) stress, and Bacillus sp. S3 strain preferentially transported the Sb(III) to the inside of cell rather than adsorbed it on the bacterial cell surface, indicating intracellular detoxification rather than extracellular EPS precipitation played an important role in microbial resistance towards Sb(III). Hence, our research indicated that the role of EPS in metal detoxification was quite different, depending on the EPS properties, metal types and corresponding environmental conditions. Further research is needed to promote microbes to produce specific EPS types and therefore improve their biosorption capability.
References
Fu FL, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manag 92:407–418
Pourret O, Bollinger JC (2017) "Heavy metal"—what to do now: to use or not to use? Sci Total Environ 610–611:419–420
Giovanella P, Cabral L, Costa AP, Fa DOC, Gianello C, Bento FM (2017) Metal resistance mechanisms in Gram-negative bacteria and their potential to remove Hg in the presence of other metals. Ecotoxicol Environ Saf 140:162–169
Wei BG, Yang LS (2010) A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchem J 94:99–107
Li ZY, Ma ZW, Kuijp TJVD, Yuan ZW, Huang L (2014) A review of soil heavy metal pollution from mines in China: pollution and health risk assessment. Sci Total Environ 468–469:843–853
Yang QJ, Li ZY, Lu XN, Duan QN, Huang L, Bi J (2018) A review of soil heavy metal pollution from industrial and agricultural regions in China: pollution and risk assessment. Sci Total Environ 642:690–700
Naja G, Volesky B (2011) The mechanism of metal cation and anion biosorption. In: Kotrba P, Mackova M, Macek T (eds) Microbial biosorption of metals. Springer, Dordrecht, pp 19–58
Mishra A, Malik A (2013) Recent advances in microbial metal bioaccumulation. Crit Rev Environ Sci Technol 43:1162–1222
More TT, Yadav JS, Yan S, Tyagi RD, Surampalli RY (2014) Extracellular polymeric substances of bacteria and their potential environmental applications. J Environ Manag 144:1–25
Mohite BV, Koli SH, Patil SV (2018) Heavy metal stress and its consequences on exopolysaccharide (EPS)-producing pantoea agglomerans. Appl Biochem Biotechnol 186:199–216
Bruins MR, Kapil S, Oehme FW (2000) Microbial resistance to metals in the environment. Ecotoxicol Environ Saf 45:198–207
Wei X, Fang L, Cai P, Huang Q, Chen H, Liang W, Rong X (2011) Influence of extracellular polymeric substances (EPS) on Cd adsorption by bacteria. Environ Pollut 159:1369–1374
Li WW, Yu HQ (2014) Insight into the roles of microbial extracellular polymer substances in metal biosorption. Bioresour Technol 160:15–23
Sheng GP, Yu HQ, Li XY (2010) Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: a review. Biotechnol Adv 28:882–894
Wu XL, Wu XY, Shen L, Li JK, Yu RL, Liu YD, Qiu GZ, Zeng WM (2019) Whole genome sequencing and comparative genomics analyses of Pandoraea sp.XY-2, an ewspecies capable of biodegrade tetracycline. Front Microbiol https://doi.org/10.3389/fmicb.2019.00033
Guibaud G, Comte S, Bordas F, Dupuy S, Baudu M (2005) Comparison of the complexation potential of extracellular polymeric substances (EPS), extracted from activated sludges and produced by pure bacteria strains, for cadmium, lead and nickel. Chemosphere 59:629–638
Pagnanelli F, Mainelli S, Bornoroni L, Dionisi D, Toro L (2009) Mechanisms of heavy-metal removal by activated sludge. Chemosphere 75:1028–1034
Sheng GP, Yu HQ, Yue ZB (2005) Production of extracellular polymeric substances from Rhodopseudomonas acidophila in the presence of toxic substances. Appl Microbiol Biotechnol 69:216–222
Baptista MS, Vasconcelos MT (2006) Cyanobacteria mtal interactions: requirements, toxicity, and ecological implications. Crit Rev Microbiol 32:127–137
Nies DH (1999) Microbial heavy-metal resistance. Appl Microbiol Biotechnol 51:730–750
Fang LC, Wei X, Cai P, Huang QY, Chen H, Liang W, Rong XM (2011) Role of extracellular polymeric substances in Cu(II) adsorption on Bacillus subtilis and Pseudomonas putida. Bioresour Technol 102:1137–1141
Ueshima M, Ginn BR, Haack EA, Szymanowski JES, Fein JB (2008) Cd adsorption onto Pseudomonas putida in the presence and absence of extracellular polymeric substances. Geochim Cosmochim Acta 72:5885–5895
González AG, Shirokova LS, Pokrovsky OS, Emnova EE, Martínez RE, Santanacasiano JM, Gonzálezdávila M, Pokrovski GS (2010) Adsorption of copper on Pseudomonas aureofaciens: protective role of surface exopolysaccharides. J Colloid Interface Sci 350:305–314
Tourney J, Ngwenya BT, Mosselmans JWF, Magennis M (2009) Physical and chemical effects of extracellular polymers (EPS) on Zn adsorption to Bacillus licheniformis S-86. J Colloid Interface Sci 337:381–389
Li JK, Yu H, Wu XL, Shen L, Liu YD, Qiu GZ, Zeng WM, Yu RL (2018) Novel hyper antimony-oxidizing bacteria isolated from contaminated mine soils in China. Geomicrobiology 35:713–720
Ball MM, Carrero P, Castro D, Yarzábal LA (2007) Mercury resistance in bacterial strains isolated from tailing ponds in a gold mining area near El Callao (Bolívar State, Venezuela). Curr Microbiol 54:149–154
Sheng GP, Yu HQ, Yu Z (2005) Extraction of extracellular polymeric substances from the photosynthetic bacterium Rhodopseudomonas acidophila. Appl Microbiol Biotechnol 67:125–130
Joshi PM, Juwarkar AA (2009) In vivo studies to elucidate the role of extracellular polymeric substances from Azotobacter in immobilization of heavy metals. Environ Sci Technol 43:5884–5889
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Frølund B, Griebe T, Nielsen PH (1995) Enzymatic activity in the activated-sludge floc matrix. Appl Microbiol Biotechnol 43:755–761
Boonaert CJP, Dufrêne YF, Derclaye SR, Rouxhet PG (2001) Adhesion of Lactococcus lactis to model substrata: direct study of the interface. Colloids Surf B 22:171–182
Langmuir I (2015) The adsorption of gases on plane surfaces of glass, mica and platinum. J Chem Phys 40:1361–1403
Freundlich H (1906) Über die adsorption in lösungen. Zeitschrift Fã¼r Physikalische Chemie 57U: 385–470
Tyagi AK, Malik A (2010) In situ SEM, TEM and AFM studies of the antimicrobial activity of lemon grass oil in liquid and vapour phase against Candida albicans. Micron 41:797–805
Holmes JD, Smith PR, Evans-Gowing R, Richardson DJ, Russell DA, Sodeau JR (1995) Energy-dispersive X-ray analysis of the extracellular cadmium sulfide crystallites of Klebsiella aerogenes. Arch Microbiol 163:143–147
Aquino SF, Stuckey DC (2004) Soluble microbial products formation in anaerobic chemostats in the presence of toxic compounds. Water Res 38:255–266
Mikes J, Siglova M, Cejkova A, Masak J, Jirku V (2005) The influence of heavy metals on the production of extracellular polymer substances in the processes of heavy metal ions elimination. Water Sci Technol 52:151–156
Ding PF, Song WF, Yang ZH, Jian JY (2018) Influence of Zn(II) stress-induction on component variation and sorption performance of extracellular polymeric substances (EPS) from Bacillus vallismortis. Bioprocess Biosyst Eng 41:781–791
Hamamura N, Fukushima K, Itai T (2013) Identification of antimony- and arsenic-oxidizing bacteria associated with antimony mine tailing. Microbes Environ 28:257–263
Nguyen TA, Ngo HH, Guo WS, Zhang J, Liang S, Yue QY, Li Q, Nguyen TV (2013) Applicability of agricultural waste and by-products for adsorptive removal of heavy metals from wastewater. Bioresour Technol 148:574–585
Li J, Wang Q, Zhang SZ, Qin D, Wang GJ (2013) Phylogenetic and genome analyses of antimony-oxidizing bacteria isolated from antimony mined soil. Int Biodeterior Biodegrad 76:76–80
Yamamura S, Amachi S (2014) Microbiology of inorganic arsenic: from metabolism to bioremediation. J Biosci Bioeng 118:1–9
Drake LR, Lin S, Rayson GD, Jackson PJ (2009) Chemical modification and metal binding studies of Datura innoxia. Environ Sci Technol 30:110–114
Loukidou MX, Zouboulis AI, Karapantsios TD, Matis KA (2004) Equilibrium and kinetic modeling of chromium(VI) biosorption by Aeromonas caviae. Colloids Surf A 242:93–104
Eboigbodin KE, Biggs CA (2008) Characterization of the extracellular polymeric substances produced by Escherichia coli using infrared spectroscopic, proteomic, and aggregation studies. Biomacromol 9:686–695
Zhao WQ, Walker SL, Huang QY, Cai P (2015) Contrasting effects of extracellular polymeric substances on the surface characteristics of bacterial pathogens and cell attachment to soil particles. Chem Geol 410:79–88
Guibaud G, Tixier N, Bouju A, Baudu M (2003) Relation between extracellular polymers’ composition and its ability to complex Cd, Cu and Pb. Chemosphere 52:1701–1710
Chen W, Westerhoff P, Leenheer JA, Booksh K (2003) Fluorescence excitation-emission matrix regional integration to quantify spectra for dissolved organic matter. Environ Sci Technol 37:5701–5710
Qu F, Liang H, He J, Ma J, Wang ZZ, Yu HR, Li GB (2012) Characterization of dissolved extracellular organic matter (dEOM) and bound extracellular organic matter (bEOM) of Microcystis aeruginosa and their impacts on UF membrane fouling. Water Res 46:2881–2890
Pan XL, Jing L, Zhang DY (2010) Binding of phenanthrene to extracellular polymeric substances (EPS) from aerobic activated sludge: a fluorescence study. Colloids Surf B 80:103–106
Miao L, Wang C, Hou J, Wang P, Ao Y, Li Y, Yao Y, Lv B, Yang Y, You G (2017) Response of wastewater biofilm to CuO nanoparticle exposure in terms of extracellular polymeric substances and microbial community structure. Sci Total Environ 579:588–597
Wang Z, Gao M, Wang S, Xin Y, Dong M, She Z, Zhe W, Chang Q, Yun R (2014) Effect of hexavalent chromium on extracellular polymeric substances of granular sludge from an aerobic granular sequencing batch reactor. Chem Eng J 251:165–174
Sheng GP, Xu J, Li WH, Yu HQ (2013) Quantification of the interactions between Ca2+, Hg2+ and extracellular polymeric substances (EPS) of sludge. Chemosphere 93:1436–1441
Senesi N (1990) Molecular and quantitative aspects of the chemistry of fulvic acid and its interactions with metal ions and organic chemicals: Part I. The electron spin resonance approach. Anal Chim Acta 232:51–75
Chen J, Gu B, Leboeuf EJ, Pan H, Dai S (2002) Spectroscopic characterization of the structural and functional properties of natural organic matter fractions. Chemosphere 48:59–68
Paul ML, Samuel J, Das SB, Swaroop S, Chandrasekaran N, Mukherjee A (2012) Studies on Cr(VI) removal from aqueous solutions by nano-alumina. Ind Eng Chem Res 51:15242–15250
Davis TA, Volesky B, Mucci A (2003) A review of the biochemistry of heavy metal biosorption by brown algae. Water Res 37:4311–4330
Ha JY, Gélabert A, Spormann AM, Brown GE Jr (2010) Role of extracellular polymeric substances in metal ion complexation on Shewanella oneidensis: batch uptake, thermodynamic modeling, ATR-FTIR, and EXAFS study. Geochim Cosmochim Acta 74:1–15
Merroun ML, Ben CK, Arias JM, Gonzálezmuñoz MT (2003) Lanthanum fixation by Myxococcus xanthus: cellular location and extracellular polysaccharide observation. Chemosphere 52:113–120
Nithya C, Gnanalakshmi B, Pandian SK (2011) Assessment and characterization of heavy metal resistance in Palk Bay sediment bacteria. Mar Environ Res 71:283–294
Huang F, Dang Z, Guo CL, Lu GN, Gu RR, Liu HJ, Zhang H (2013) Biosorption of Cd(II) by live and dead cells of Bacillus cereus RC-1 isolated from cadmium-contaminated soil. Colloids Surf B 107:11–18
Choudhary S, Sar P (2009) Characterization of a metal resistant Pseudomonas sp. isolated from uranium mine for its potential in heavy metal (Ni2+, Co2+, Cu2+, and Cd2+) sequestration. Bioresour Technol 100:2482–2492
Acknowledgements
J.K.L., W.M.Z. G.Z.Q and F.L. conceived and designed the experiments. F.L. performed the experiments. J.K.L., W.M.Z., F.L., C.C.W., R.L.Y., X.L.W., L.S., Y.D.L. analyzed the data. J.K.L. and F.L. wrote and revised the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China [Grant Numbers: 31470230, 51320105006, 51604308]; The Youth Talent Foundation of Hunan Province of China [Grant Number: 2017RS3003]; Natural Science Foundation of Hunan Province of China [Grant Number: 2019JJ40361]; Key Research and Development Projects in Hunan Province [Grant Number: 2018WK2012]; Fundamental Research Funds for the Central Universities of Central South University [Grant Number: 2018zzts763].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zeng, W., Li, F., Wu, C. et al. Role of extracellular polymeric substance (EPS) in toxicity response of soil bacteria Bacillus sp. S3 to multiple heavy metals. Bioprocess Biosyst Eng 43, 153–167 (2020). https://doi.org/10.1007/s00449-019-02213-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-019-02213-7