Abstract
This study evaluated the production of cellulolytic enzymes from different agricultural residues. The crude enzyme extract produced was characterized and applied for saccharification of some agricultural residues. Maximum cellulolytic activities were obtained using soybean hulls. All enzymatic activities were highly stable at 40 °C at a pH range of 4.5–5.5. For stability at low temperatures, the enzyme extract was stored at freezing temperature and cooling for about 290 days without major loss of activity. The Km values found for total cellulase (FPase), endoglucanase (CMCase), and xylanase were 19.73 mg ml−1, 0.65 mg ml−1, and 22.64 mg ml−1, respectively, and Vmax values were 0.82 mol min−1 mg−1, 0.62 mol min−1 mg−1, and 104.17 mol min−1 mg−1 to cellulose, carboxymethyl cellulose, and xylan, respectively. In the saccharification tests, the total amount of total reducing sugars (TRS) released from 1 g of soybean hulls catalyzed by the enzymes present in the crude enzyme extract was 0.16 g g−1 dry substrate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With globalization, the population living in rural areas migrated to urban areas in search of better living conditions. As a result, there is an increase in the rate of industrial waste that is destined for landfills or inappropriate places. Thus, there is a need to reduce these wastes to improve human and environmental health by developing sustainable management based on circular economy [1, 2].
Circular economics emerged as an alternative to the linear economy—based on the use of fossil resources, it becomes ineffective to meet the needs of the population—and has as one of its purposes sustainable consumption and production, its central element being recycling, that is, instead of becoming a waste and not reusing it, are studied ways to reuse it to profit and also not generate as much wastage of matter, thus replacing the resources of linear economy [2, 3].
Lignocellulosic wastes are considered to be promising renewable resources, since they are widely available. The circular economy aims to realize a circuit to potentialize the use of the residues, being able to be used several times [2, 4].
Cellulases, hemicellulases, xylanases, and lignin-modifying enzymes are enzymes that form a cocktail able to act on lignocellulosic materials, promoting its hydrolysis. These enzymes are highly specific and act synergistically to depolymerize plant polysaccharides into glucose and other monosaccharides, arousing interest for industrial-scale production of the second-generation bioethanol [4, 5]. Cellulases also are widely used in the foods, chemicals, detergents, textiles, cosmetics, pulp and paper, and others [6].
The major challenge for bioprocess involving the hydrolysis of biomass is to identify the potential microorganisms, composition of media, and the optimization of various process parameters that influence the microbial growth and production of enzyme in an economically viable way without harming the environment. The majority of microorganisms employed in cellulase production are fungi (as Trichoderma spp.), bacteria, and to a smaller extent actinomycetes, which acts upon under specific (aerobic and anaerobic) conditions [5, 7]. Specifically, Trichoderma reesei, a cellulolytic fungus, is used in industrial scale for cellobiohydrolases’ (CBHI and CBHII) and endo-glucanases’ (GE1 and GE2) production [5,6,7].
Solid-state fermentation (SSF) has been the preferred process for enzyme production, since it presents several advantages, which include less infrastructure, ability to utilize cheaper materials originated from agricultural products, and less skilled labor. In addition, the process requires less energy and less water usage as it occurs in the absence of free-flowing water leading to a more concentrated product [8].
The solid-stated cultivation of such microorganisms in the same substrate to be hydrolyzed is a method to select specific enzymes that are optimal for its hydrolysis. This strategy renders a better cost-efficiency ratio, because the two processes, enzyme selection and substrate hydrolysis, can be co-located and share infrastructure and utilities in the same site [9, 10].
Several industrial and agricultural by-products are abundantly available and rich in polysaccharides, as cellulose and hemicellulose, case of rice husk, soybean hulls, sugarcane bagasse, and powder toothpick yerba mate [9, 10]. The use agricultural waste of low cost as substrates for SSF offers many practical and economic advantages and, therefore, at last decade is an interesting alternative for the production of enzymes for use in several branches of industry, which often uses enzymes costly [11, 12].
Among the so-called alternative energy sources, biofuel has been gaining prominence, because, besides to efficient, it is considered friendly to the environment due to its sustainable characteristics. It is possible to produce biofuel from various residues; however, the use of raw materials such as enzymatic extracts produced by microorganisms from the hydrolysis of agricultural residues performs well, especially as regards their potential to release sugars for production of biofuels [13].
Microorganisms can only metabolize simple sugars, so is necessary that the lignocellulosic biomass passes by pretreatment stages. The cost of hydrolytic enzymes makes the pretreatment unfeasible on a large scale, that way the production of enzymes, cellulose saccharification, and microbial fermentation when performed in a single stage offers a promising solution, since it eliminates steps and presents an economically viable alternative [14].
Based on these aspects, the main objective of this study was the production of cellulolytic enzymes (cellulases and xylanase) from different agricultural residues (rice husk, soybean hulls, sugarcane bagasse, and powder toothpick yerba mate) as substrates by solid-state fermentation and studied the application for enzyme production with substrate of biofuels.
Materials and methods
Microorganism
The microorganism used for the production of cellulase and xylanase was T. reesei NRRL 3652, obtained from ARS Culture Collection (NRRL). The strain of the filamentous fungus was grown on potato dextrose agar (PDA) medium for 7 days at 30 °C. The concentration of the suspension was adjusted to reach 1 × 107 spores/g of dry substrate [15].
Characterization of the substrate
Rice husk (RH), soybean hulls (SH), sugarcane bagasse (SB), and powder toothpick yerba mate (PTY) were obtained in different industries from Rio Grande do Sul (RS), Brazil. The characterization verified the constitution in terms of carbon (C), hydrogen (H), nitrogen (N), and sulfur (S), in duplicate with the equipment CHNS–TRUSPEC Micro (LECO) 4277. The ash content was determined after burning the sample in an oven at 550 °C for 6 h. The oxygen content was calculated according to recommendation of Bech et al. (2009) [16].
Production of cellulase and xylanase
Figure 1 shows a schematic diagram used in our study to evaluate the effect of carbon sources on the growth of the fungus and enzyme production. T. reesei NRRL 3652 was grown by SSF using RH, SH, B, PTY, and the mixture of SH and PTY (1:1) as the main source of substrate (these two residues were mixed, because they were more abundant in local agriculture). The fermentations were performed in polypropylene beakers of 600 ml containing 10 g of dry substrate adjusted to 70% with distilled water [17]. After sterilization (121 °C, 15 min), inoculation proceeded using 2 ml of inoculums and the beakers were incubated in a climate chamber at 30 ± 1 °C with moist air injection [15]. The kinetic behavior of the xylanase and cellulase production process was studied by performing destructive analysis of samples of fermented (0, 3, 6, 9, 12, and 15 days). The secreted enzymes during SSF were extracted with sodium citrate buffer 0.05 M, pH 5.5 in the solid-to-liquid ratio 1:15. These flasks were incubated for 30 min at 50 °C and 100 rpm in an orbital shaker. After incubation, samples were filtered using a nylon cloth and manual pressure to obtain the enzymatic crude extract.
Enzyme assays
Total cellulase (FPase) and endoglucanase (CMCase) activities were determined using Whatman Nº 1 filter paper and carboxymethylcellulose (CMC) as substrates, respectively, according to the described standard by Ghose [18]. The xylanase activity was determined by the amount of reducing sugars released from xylan “birchwood” (Sigma) as described by Bailey et al. [19].
The total reducing sugars (TRS) released during the enzymatic assays were quantified by 3,5-dinitrosalicylic acid (DNS) using glucose (FPase) and xylose (Xylanase) as standard. For all enzymes, one unit of enzyme activity (U) was defined as the amount of enzyme that released 1 µmol of the corresponding product (glucose or xylose) per minute under the assay conditions used. The enzymatic activities were expressed as U g−1.
Partial characterization of cellulase and xylanase
Using a central composite rotational design (CCRD), temperature and pH optimum of enzymes were evaluated. The thermal stability of the enzyme was tested by incubating the crude enzyme extract in sodium citrate buffer 0.05 M pH 4.6 at different temperatures (40, 50, 60, and 70 °C). In addition, samples of the crude enzyme extract were stored at temperatures of − 80, − 10, and 4 °C. In respect to pH, the extract was incubated in sodium citrate buffer 0.05 M at different pH (3.5, 4.5, 5.5, and 6.5) at 55 °C. Samples were withdrawn in certain time intervals by measuring their enzymatic activities.
The effect of substrate concentration on the rate of enzymatic reactions was performed evaluating the enzymatic activity at different substrate concentrations at a temperature of 50 °C and sodium citrate buffer 0.05 M pH 4.8 to FPase and CMCase, and pH 5.3 for xylanase. The substrates used were Whatman filter paper No 1 (1–200 mg ml−1), carboxymethylcellulose (1–100 mg ml−1), and xylan standard “birchwood” (1–30 mg ml−1) to the activities of FPase, CMCase and xylanase, respectively. Using these dates, the Lineweaver–Burk model was applied for Michaelis–Menten constant determination [20].
Saccharification of agricultural residues biomass
Saccharification process from enzymatic extract (crude enzyme) produced by T. reesei NRRL at SSF condition using soybean hulls was evaluated on lignocellulosic substrate (soybean hulls, sugarcane bagasse, and rice hulls), according to Liu et al. [21].
Assays were performed in Erlenmeyer flasks where 2 g of the respective lignocellulosic residue was added 100 ml of reaction mixture containing: 94 ml of sodium citrate buffer 0.05 M, pH 5.3; 1 ml tetracycline (40 µ ml−1), to inhibit microbial contamination, and 5 ml of the crude enzyme extract. The reactions were performed in an orbital shaker at 150 rpm and 50 °C for a period of 7 days [21] whose samples were removed from the reaction mixture at intervals of 24 h and the TRS and glucose in the hydrolysis were determined using DNS and glucose oxidase/peroxidase (GOD–POD) proposed by Keston [22]. Assays without lignocellulosic substrate and crude enzyme extract were performed as control. The rate of hydrolysis of TRS and glucose were calculated according to Van Dyk and Pletschke [23], as shown in the following equation:
Statistical analysis
A central composite rotational design (CCRD) was used in this study as statistic experimental strategy. Statistical analysis of the different responses obtained during the study was done using the online software Protimiza Experimental Design (http://experimental-design.protimiza.com.br/). It was possible to perform the analysis of variance (ANOVA), of effects, and test of comparison of means (Tukey). The confidence level used in the tests was 95% (p < 0.05).
Results and discussion
Characterization of substrates
For production of cellulolytic enzymes, the lignocellulosic substrate should be inexpensive, available in large quantities throughout the year, and its composition should be adapted to the hydrolysis as well as for the production of these enzymes. Moreover, the choice of the residue is as essential to the success of the fermentation process as the microorganism. In this sense, it is necessary to characterize the substrate used in the process (Table 1).
Overall, among residues evaluated powder toothpick yerba mate and sugarcane bagasse showed the highest carbon content 43.06% and 41.27%, respectively. In the case of sugarcane bagasse, the values obtained were similar to those reported by Varma and Mondal [24] and Balasundram et al. [25]. Already, soybean hulls and powder toothpick mate were presented as the best sources of nitrogen 1.38% and 1.19%, respectively. The levels of oxygen, hydrogen and sulfur showed a little variation between the wastes. Considering the ash content, rice husk had the highest percentage and is in agreement with the results obtained by Cai et al. [26].
The microbial growth in SSF has a strong correlation with the amounts of carbon/nitrogen available [21, 23]; therefore, soybean hulls (C/N = 6.0/1.37), powder toothpick yerba mate (C/N = 43.06/1.19), and the mixture of soybean hulls and powder toothpick yerba mate (C/N = 2.45/0.12) probably offered the best conditions for the growth of T. reesei NRRL 3652 and consequently higher production of enzymes.
Production of cellulase and xylanase
The FPase activity is considered as the total cellulolytic activity. As shown in Fig. 1a, the maximum production FPase occurred after 3 days of fermentation using soybean hulls (6.71 U g−1) maintaining until the ninth day (6.62 U g−1) and after it was observed decline about 30% of production until the fifteenth day. However, for the mixed fermentation of soybean hulls and powder toothpick yerba mate (1:1), was observed maximum yield of 4.06 U g−1 after 6 days, a value 40% lower compared with the use of soybean hulls. When used only powder toothpick yerba mate as substrate, practically no production of FPase. For bagasse and rice husk, the activities of 1.02 U g−1 and 0.75 U g−1 were obtained at 3 and 6 days, respectively; after these times, the yields of the enzymes decreased gradually.
The evaluation of the production of CMCase can be seen in Fig. 1b, where the maximum activity was found in 6 days (20.77 ± 0.02 U g−1) for fermentation with mixed substrate. For powder toothpick yerba mate, the activity remained stable from the third day until the twelfth day of fermentation (7.58 ± 0.01–6.61 ± 0.03 U g−1, respectively). The maximum activity for soybean hulls (5.45 ± 0.02 U g−1) and rice husk (3.91 U g−1) was found at 15 days of fermentation. The production using soybean hulls was about 70% less compared with the mixed substrate fermentation. The bagasse maximum activity (0.77 U g−1) was observed in 9 days of fermentation.
Figure 1c shows high xylanase production for soybean hulls on the third day; however, the maximum value of 1,130.70 U g−1 was obtained in 6 days of fermentation. After that, the activity decreases by about 40% (648.38 U g−1) on the fifteenth day. For the fermentation with the mixed substrate observed maximum production of 581.72 U g−1 at 3 days and after that, the yields of the enzyme gradually decreased. When used alone, powder toothpick yerba mate as the substrate was observed the same behavior that to FPase, the low yield of this enzyme. The tests showed that, in the mixed substrate fermentation, the soybean hulls were the substrate used by the microorganism for production of xylanase, since, in general, the activity values were almost half. Bagasse with maximum activity of 77.61 U g−1 and rice husk with 32.08 U g−1 was not shown to be good substrates for the production of xylanase.
According to Yadav [27] and Xu et al. [28], agricultural wastes have cellulosic components that can induce cellulase production when used as carbon sources for the growth of fungi. Cellulolytic enzymes of fungal source catalyze reactions aim at the total hydrolysis of cellulose, being very useful in the conversion of cellulose and hemicelluloses in reducing sugars, which are of industrial interest, such as glucose and xylose. Furthermore, according to the authors, the production of cellulase is dependent on the other essential nutrients used in the culture media. Such nutrients may be required for growth of the microorganism and thus to produce cellulase enzyme.
Xu et al. [28] studied the bioconversion of lignocellulosic residues by Inonotus obliquus for the production of cellulolytic enzymes in SSF, using birch branch, beech branch, rice straw, wheat straw, wheat bran, sugarcane bagasse, cassava peel, and peanut shell as substrates. Of all the residues evaluated and sampled on day 7, using wheat bran as a carbon source, which reported maximum activity of CMCase (17.66 ± 0.36 U g−1), and for sugarcane bagasse, which reported the maximum the FPase (5.55 ± 0.01 U g−1). These results are lower for FPase (6.71 U g−1) and higher for CMCase (4.30 U g−1), compared with this study.
Delabona et al. [29] reported a maximum production of CMCase 160.1 U g−1 in 3 days using soybean meal and low production of 16.71 U g−1 in 4 days when used bagasse as substrate for the growth of A. fumigatus.
About xylanase, similar results to these were obtained by Ncube et al. [30], which studied the kinetics for the production of xylanase in Jatropha curcas seed cake as substrate in SSF by Aspergillus niger. In the kinetic study, the highest production was achieved on the second day of fermentation, and Bajaj et al. [31] obtained maximum production of xylanase when Bacillus pumilus SS1 was grown in wheat bran as the only carbon source. However, rice bran, sawdust, and sugarcane bagasse did not induce the production of xylanase; according to the authors, this is probably due to the complex nature of these substrates.
Song et al. [32] studied the combined use the cellulases and xylanases to produce total reducing sugars, using agricultural residues, the mixture of enzymes resulted in the increase of sugar yield by 133% corn cob, 164% corn stover, and 545% rice straw.
In an overview of the results obtained, the production of lignocellulolytic enzymes investigated here showed that the soybean hull was the most suitable substrate for the production of FPase (6.71 U g−1) and xylanase (1130.70 U g−1). The highest CMCase production (20.77 U g−1) was observed using the mixed substrate of soybean and powder toothpick yerba mate (1:1). In this sense, it is difficult to compare the production of enzymes with different growing conditions and microorganisms used in each study; the values presented here using different agricultural wastes were able to demonstrate that soybean hulls can be used to produce enzymes such as FPase, CMCase, and xylanase from T. reesei NRRL 3652 in SSF.
Partial characterization of cellulase and xylanase
Effect of temperature and pH on enzyme activity
The matrix of the experimental design, as well as the actual values of the independent variables and coded the responses to activities of the respective enzymes of the crude extract produced in soybean hulls are shown in Table 2. The values for FPase activity obtained experimentally ranged from 0.02 to 0.18 FPU ml− 1.
As can be seen in assays 1, 2, 5, and 7, the maximum activity FPase was obtained in the temperature range of 40–55 °C and a broad pH range (3.0–6.0). The CMCase activities ranged from 0.09 to 0.31 U ml−1, and the maximum activity was obtained at the center point, pH and temperature of 4.6 and 55 °C, respectively. The activities of xylanase ranged from 18.56 to 33.34 U ml−1 and the maximum activity was also obtained at the center point.
Xu et al. [28] evaluated the influence of pH (3.0–8.0) on enzymatic activity; for CMCase at pH 3.0–3.5, the maximum activity of the enzyme was obtained; for FPase, the optimum pH remained in the range of 3.5–4.5, with maximum activity pH 4.0.
Effects of pH and temperature optimum of activity are fundamental to understanding the enzymatic behavior and possibly determine their optimal working conditions. In general, the cellulases produced by filamentous fungi are excellent in the acid pH range (3.6–5.0), while bacterial cellulases produce highly active in alkaline pH. Enzymes that catalyze reactions in extreme environments are important in industrial processes, since obtaining cellulases besides expensive presents low stability [27, 33]. To this end, optimum temperatures for your performance are mostly above 40 °C.
Effect of thermal and pH stability of the enzymes
In this study, the thermal stability consisted of incubation of the crude enzyme extract for 96 h at temperatures ranging from 40 to 70 °C. The results showed that FPase, CMCase, and xylanase showed increased stability at 40 °C. Reduction of about 25% in the activity of the respective enzymes after 60 h of reaction was verified. At 96 h of incubation, FPase, CMCase, and xylanase presented the reductions of 44%, 66%, and 65%, respectively. In addition, at 40 °C, there was no significant loss in the activity of xylanase for a period of 8 h. For the temperature of 50 °C, all the enzymes studied showed a reduction of 50% after 8 h of reaction and drastic loss were observed for all enzymes at temperatures of 60 and 70 °C. These results indicate that the temperature range of 40–50 °C is most suitable for the industrial application of the enzyme obtained in the study of soybean hulls using T. reesei NRRL 3652. These results were similar to the studies done by Xu et al. [28]; the ideal temperature for CMCase and FPase activity was 55 and 40 °C, respectively. The authors observed that the activity of the enzymes decreased gradually in relation to the temperature increase; at 70 °C, the CMCase activity decreased by approximately 47%, demonstrating that the enzymes produced in this study offer good conditions of use in industrial processes that do not occur at high temperatures.
Regarding the effect of enzyme stability at low temperatures, it was observed that there was not a distinct behavior among different temperatures. The three forms of storage (− 80, − 10, and 4 °C), showed that after 290 days about 60% of FPase activity at 4 °C and about 20% of the xylanase and CMCase activity was lost compared with its initial activity.
FPase and xylanase showed higher stability at pH 4.5, a reduction of approximately 50% activity after 20 and 16 h, respectively. CMCase with 45% reduction after 16 h of reaction was more stable at pH 5.5. To pH 3.5 was observed complete loss of activity for all the enzymes in a few hours of reaction, probably because this pH is too close to the isoelectric point of the protein, which can lead to denaturation of the enzyme.
One of the critical steps in the manufacturing process of ethanol production is the pretreatment which can be carried out using different methods at different pHs, and this is followed by saccharification of biomass using enzymes or chemical catalysts. In the case of using enzymatic hydrolysis after pretreatment, adjustment of pH to the range of action of the biological catalyst is required. Thus, when enzymes are stable in a pH range from 4.0 to 7.0, it has a positive feature for use in the production of bioethanol.
Determination of the Michaelis–Menten constant
The effect of the substrate concentration on the velocity of the enzymatic activity, FPase, CMCase, and xylanase was evaluated at 50 °C and of 0.05 M blood citrate in pH 4.8 for FPase and CMCase, and in pH 5.3 for xylanase. The substrates used were Whatman No. 1 (1–200 mg ml−1) filter paper, carboxymethylcellulose (1–100 mg ml−1), and standard bilellum xylan (1–30 mg ml−1) for FPase activity, CMCase, and xylanase, respectively. The determination of the kinetic parameters was described by Lineweaver–Burk model (1934). The determination of the kinetic parameters Km and Vmax have obtained by the analysis of the initial reaction rates at different substrate concentrations established for this study. In all cases which were considered, r2 > 0.95 value.
The Km values found for FPase, CMCase, and xylanase were 19.73 mg ml−1, 0.65 mg ml−1, and 22.64 mg ml−1, respectively, and Vmax values were 0.82 mol min−1 mg−1, 0.65 mol min−1 mg−1, and 104.17 mol min−1 mg−1 to cellulose (Whatman No.1 paper), carboxymethylcellulose, and xylan, respectively.
Saccharification of biomass
Xu et al. [28] have observed in their studies that saccharification is not only related to the properties of the substrates; the amount of each enzymatic component also affects the saccharification process.
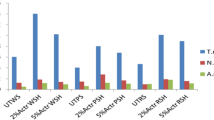
In the present study, the saccharification from enzymatic extract produced by T. reesei on soybean hulls, sugarcane bagasse, and rice hulls was evaluated. Figure 2 shows the yields of TRS and the released glucose experimentally. The release TRS increased rapidly in the initial phase (0 and 24 h), especially for rice hulls and soybean hull, and the highest percentage (16.8%) was obtained after 120 h of reaction using soybean hulls. Rice husk showed the best performance in glucose primarily in the first 72 h (4%); thereafter, the increase is very small (about 1%) (Fig. 3).
Furthermore, no yield of glucose was obtained for soybean hulls and sugarcane bagasse. Thus, the total amount of TRS released from 1 g of soybean hulls catalyzed by the enzymes present in the crude enzyme extract obtained from the fermentation of soybean hulls was 0.16 g g−1 dry substrate.
Jain and Agrawal [34] obtained the interesting results using sugarcane bagasse pretreated with acid; however, the maximum values of sugar release were obtained with the crude enzymatic extracts, and according to the authors, this fact is related to the pretreatment which crystallizes the lignocellulosic layer of the bagasse. Analyzing the reducing sugars released during the reaction, the microorganisms with higher performance, Talaromyces australis and Penicillum verruculosum, the two demonstrated to produce highly specific cellulases, since, after 72 h, the first microorganism released 5.99 ± 0.80 g of glucose/mg, while the second released 6.32 ± 0.95 g of glucose/mg of protein, indicating the enzymes produced exhibit tolerance and stability to the products generated during the saccharification reaction.
For obtaining high yields, saccharification of lignocellulosic biomass is extremely important to study different processes of pretreatment and this is because the lignin present in the plant cell wall hemicellulose forms a barrier to enzymatic action [35].
Conclusions
This study investigated the use of T. reesei NRRL 3652 on the feasibility of producing enzymes on agricultural waste by solid-state fermentation. Among lignocellulolytic substrates, soybean hulls were the more effective for the production of xylanase and FPase. The highest production of CMCase was observed using a mixed culture of soybean hulls and powder toothpick yerba mate.
The application of enzymes on an industrial scale still presents a challenge, since studies on the production of highly efficient and stable enzymes to these processes are still considered recent. Cellulolytic enzymes are used for lignocellulosic biomass to be converted into products with highly added value. For this purpose, microorganisms with the capacity to produce enzymes or to cause the hydrolysis of this type of material, such as agricultural residues from the production of sugarcane (bagasse), can convert hundreds of kilograms of bagasse into bio-available cellulosic material for the generation of biofuels and electricity, on circular economy approach.
References
Allesch A, Brunner PH (2014) Assessment methods for solid waste management: a literature review. Waste Manag Res 36:461–473
Liguori R, Faraco V (2016) Bioresource technology biological processes for advancing lignocellulosic waste biorefinery by advocating circular economy. Bioresour Technol 215:13–20
Velis CA (2015) Circular economy and global secondary material supply chains. Waste Manag Res 33:389–391
Yoo J, Alavi S, Vadlani P, Amanor-Boad V (2011) Thermo-mechanical extrusion pretreatment for conversion of soybean hulls to fermentable sugars. Bioresour Technol 102:7583–7590
Pirota RDPB, Tonelotto M, Delabona PS, Fonseca RF, Paixão DAA, Baleeiro FCF, Bertucci Neto V, Farinas CS (2016) Bioprocess developments for cellulase production by Aspergillus oryzae cultivated under solid-state fermentation. Braz J Chem Eng 33:21–31
Sajith S, Priji P, Sreedevi S, Benjamin S (2016) An overview of fungal cellulases with an industrial perspective. J Nutr Food Sci 6:461
Behera SS, Rayb RC (2016) Solid state fermentation for production of microbial cellulases: Recent advances and improvement strategies. Int J Biol Macromol 86:656–669
Thota SP, Badiya PK, Yerram S, Vadlani PV, Pandey M, Golakoti NR, Belliraj SK, Dandamudi RB, Ramamurthy SS (2017) Macro-micro fungal cultures synergy for innovative cellulase enzymes production and biomass structural analyses. Renew Energy 103:766–773
Gasparotto JM, Werle LB, Mazutti MA (2015) Production of cellulolytic enzymes and application of crude enzymatic extract for saccharification of lignocellulosic biomass. Appl Biochem Biotechnol 175:560–572
Orencio-Trejo M, Torres-Granados J, Rangel-Lara A, Beltrán-Guerrero E, García-Aguilar S, Moss-Acosta C, Valenzuela-Soto H, De la Torre-Zavala S, Gastelum-Arellanez A, Martinez A, Tiessen A, Diaz-Mireles E, Lozoya-Gloria E (2016) Cellulase and xylanase production by the Mexican strain Talaromyces stollii LV186 and its application in the saccharification of pretreated corn and sorghum stover. Bioenergy Res 9:1034–1045
Falkoski DL, Guimarães VM, de Almeida MN, Alfenas AC, Colodette JL, de Rezende ST (2013) Chrysoporthe cubensis: a new source of cellulases and hemicellulases to application in biomass saccharification processes. Bioresour Technol 130:296–305
Waghmare PR, Kadam AA, Saratale GD, Govindwar SP (2014) Enzymatic hydrolysis and characterization of waste lignocellulosic biomass produced after dye bioremediation under solid state fermentation. Bioresour Technol 168:136–141
Joshi G, Pandey JK, Rana S, Rawat DS (2017) Challenges and opportunities for the application of biofuel. Renew Sustain Energy Rev 79:850–866
Jiang Y, Xin F, Lu J, Dong W, Zhang W, Zhang M, Wu H, Ma J, Jiang M (2017) State of the art review of biofuels production from lignocellulose by thermophilic bacteria. Bioresour Technol 245:1498–1506
Dhillon GS, Oberoi HS, Kaur S, Bansal S, Brar SK (2011) Value-addition of agricultural wastes for augmented cellulase and xylanase production through solid-state tray fermentation employing mixed-culture of fungi. Ind Crops Prod 34:1160–1167
Bech N, Jensen PA, Dam-Johansen K (2009) Determining the elemental composition of fuels by bomb calorimetry and the inverse correlation of HHV with elemental composition. Biomass Bioenergy 33:534–537
Deswal D, Khasa YP, Kuhad RC (2011) Optimization of cellulase production by a brown rot fungus Fomitopsis sp. RCK2010 under solid state fermentation. Bioresour Technol 102:6065–6072
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59:257–268
Bailey MJ, Biely P, Poutanen K (1992) Interlaboratory testing of methods for assay of xylanase activity. J Biotechnol 23:257–270
Lineweaver H, Burk D (1934) The determination of enzyme dissociation constants. J Am Chem Soc 56(3):658–666
Liu D, Zhang R, Yang X, Wu H, Xu D, Tang Z, Shen Q (2011) Thermostable cellulase production of Aspergillus fumigatus Z5 under solid-state fermentation and its application in degradation of agricultural wastes. Int Biodeterior Biodegrad 65:717–725
Keston A (1956) Paper 31C, 129th meeting of the American Chemical Society
Van Dyk JS, Pletschke BI (2012) A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes-Factors affecting enzymes, conversion and synergy. Biotechnol Adv 30:1458–1480
Varma AK, Mondal P (2017) Pyrolysis of sugarcane bagasse in semi batch reactor: Effects of process parameters on product yields and characterization of products. Ind Crops Prod 95:704–717
Balasundram V, Ibrahim N, Kasmani RM, Isha R, Hamid MKA, Hasbullah H, Ali RR (2018) Catalytic upgrading of sugarcane bagasse pyrolysis vapours over rare earth metal (Ce) loaded HZSM-5: Effect of catalyst to biomass ratio on the organic compounds in pyrolysis oil. Appl Energy 220:787–799
Cai W, Liu R, He Y, Chai M, Cai J (2018) Bio-oil production from fast pyrolysis of rice husk in a commercial-scale plant with a downdraft circulating fluidized bed reactor. Fuel Process Technol 171:308–317
Yadav SK (2017) Technological advances and applications of hydrolytic enzymes for valorization of lignocellulosic biomass. Bioresour Technol 245:1727–1739
Xu X, Lin M, Zang Q, Shi S (2018) Solid state bioconversion of lignocellulosic residues by Inonotus obliquus for production of cellulolytic enzymes and saccharification. Bioresour Technol 247:88–95
Delabona PS, Farinas CS, da Silva MR, Azzoni SF, Pradella JGC (2012) Use of a new Trichoderma harzianum strain isolated from the Amazon rainforest with pretreated sugarcane bagasse for on-site cellulase production. Bioresour Technol 107:517–521
Ncube T, Howard RL, Abotsi EK, van Rensburg ELJ, Ncube I (2012) Jatropha curcas seed cake as substrate for production of xylanase and cellulase by Aspergillus niger FGSCA733 in solid-state fermentation. Ind Crops Prod 37:118–123
Bajaj BK, Khajuria YP, Singh VP (2012) Agricultural residues as potential substrates for production of xylanase from alkali-thermotolerant bacterial isolate. Biocatal Agric Biotechnol 1:314–320
Song HT, Gao Y, Yang YM, Xiao WJ, Liu SH, Xia WC, Liu ZL, Yi L, Jiang ZB (2016) Synergistic effect of cellulase and xylanase during hydrolysis of natural lignocellulosic substrates. Bioresour Technol 219:710–715
Brijwani K, Oberoi HS, Vadlani PV (2010) Production of a cellulolytic enzyme system in mixed-culture solid-state fermentation of soybean hulls supplemented with wheat bran. Process Biochem 45:120–128
Jain L, Agrawal D (2018) Performance evaluation of fungal cellulases with dilute acid pretreated sugarcane bagasse: a robust bioprospecting strategy for biofuel enzymes. Renew Energy 115:978–988
Mood SH, Golfeshan AH, Tabatabaei M, Jouzani GS, Najafi GH, Gholami M, Ardjmand M (2013) Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Renew Sustain Energy Rev 27:77–93
Acknowledgements
This work was supported by CAPES, CAPES-PNPD, CNPq, and FAPERGS for the financial support. This work was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Super Grant number PROAP and PNPD and Conselho Nacional de Desenvolvimento Científico e Tecnológico Grant number 306558/2014-9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Astolfi, V., Astolfi, A.L., Mazutti, M.A. et al. Cellulolytic enzyme production from agricultural residues for biofuel purpose on circular economy approach. Bioprocess Biosyst Eng 42, 677–685 (2019). https://doi.org/10.1007/s00449-019-02072-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-019-02072-2