Abstract
For an undisturbed operation of two-stage high-pressure fermentation up to 100 bar, a particle-free hydrolysate appears to be necessary. This is even more important if the second stage, i.e., the methane reactor, is designed as fixed bed. Here, we present the potential of microfiltration membranes as separation unit after the first stage, which is the hydrolysis. The study included the selection of membrane material, membrane performance investigations, and long-term-behavior during the filtration period. In a series of experiments, the optimum type of membrane material and the mode of operation [either crossflow (CF) or submerged (S)] were determined. Ceramic membranes proved to be the better option to treat the process stream due to their chemical and temperature resistance. The crossflow filtration achieved a sustainable flux of up to 33 L/(m2 h), while long-term experiments with the submerged membranes confirmed a critical flux of 7 L/(m2 h). Comparative analyses of hydrolysate and permeate showed that the rejected chemical oxygen demand (COD) as well as total organic carbon (TOC) fraction and thereby the loss of organic carbon in the permeate does not reduce the methane yield.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anaerobic two-stage systems with separated hydrolysis and methane reactors have been analyzed intensively in recent years [1, 2]. In addition, the use of two-stage systems with pressurized methane reactors has been described [3,4,5]. However, the application of membrane filtration systems for solid–liquid separation between the two stages has not been described in the literature until now. In fact, all publications concern membrane application for biogas production, generally single-stage anaerobic membrane bioreactors (AnMBRs), utilizing the membrane filtration unit to retain the solid fraction inside the reactor and, therefore, to decouple the solid from the hydraulic retention time. Furthermore, those studies are typically focusing on wastewater treatment at psychro- (< 20 °C) and mesophilic (20–45 °C) conditions, whereas publications on thermophilic (≥ 45 °C) treatment of high particulate feed solutions using micro- or ultrafiltration membrane systems are very limited [6, 7]. Some discuss the benefits of mesophilic temperature conditions, e.g., lower energy demand for heating [8], while others emphasize the potentials of thermophilic treatment with faster reaction rates [9, 10]. Only Lee et al. [11] implemented a membrane unit as an intermediate step between two spatially separated reactors to optimize the hydrolysis rate by retaining the particulate fraction.
Jeison and van Lier [12, 13] discussed the long-term operation of two submerged thermophilic anaerobic membrane bioreactors (AnMBRs) using acidified synthetic wastewater and wastewater, respectively. They concluded that the applicable flux in submerged thermophilic AnMBRs is mainly limited by reversible cake formation on the membrane surface. Further research with thermophilic sidestream AnMBRs operated in crossflow mode exposed a shear rate dilemma [14]. The authors observed that the higher surface shear due to higher superficial velocities resulting in a change of sludge properties and, therefore, not in a sustainable increased back transport of particles. Qiao et al. [15, 16] reported stable operation of a thermophilic AnMBR treating coffee grounds with a total solid (TS) content in the reactor of up to 75 g/L. They identified soluble organic components as the main cause for membrane fouling.
The research conducted on thermophilic as well as anaerobic membrane applications in two-stage systems with separated hydrolysis reactors (HR) and fixed bed methane reactors (MR) is even less pronounced. One of the first manuscripts on coupling membranes and two-stage anaerobic reactors was published by Lee et al. [11]. A pilot scale anaerobic system with a submerged microfiltration (MF) membrane was used for piggery wastewater. The operational flux was stable for 50 days before it declined to 19%. Since inorganic and biological fouling was observed, the flux could be recovered to 89% by cleaning the membrane with sodium hydroxide (NaOH) and hydrochloric acid (HCl) solutions in sequence. Wijekoon et al. [17] operated a thermophilic two-stage system with separated HR and MR. The effluent of the MR was treated with a sidestream microfiltration membrane and the concentrate was pumped back into the methane reactor. However, the authors focused on the reactor and not on the membrane performance. Another research with separated HR and MR was conducted by Mota et al. [18]. The membrane was submerged directly in the MR and the authors defined protein-like substances as most relevant factor for reversible membrane resistance. Chaikasem et al. [19] also treated the effluent of a thermophilic MR with a ceramic microfiltration membrane. Extracellular polymeric substances (EPS) were identified as main cause of membrane fouling, accounting for almost 73% of the reversible fouling.
This study aims to contribute to the development of a membrane separation step, which is able to prevent blocking or clogging of a fixed bed methane reactor. The latter needs a particle-free influent for optimum biogas production. Crossflow and submerged ceramic membranes were operated in a sidestream configuration at thermophilic conditions. Both the unfiltered HR effluent, the so-called hydrolysate, and the permeate from the membrane treatment were analyzed to distinguish the methanogenic potential. Solid–liquid separation with ceramic membranes proved to be a reliable process for removing particulate material from the hydrolysate.
Materials and methods
Experimental setup
Membrane tests to evaluate the optimal pore size for the long-term experiments were carried out using polyethersulfone (PES) membranes (pore size = 0.1–0.8 µm). The experimental setup consisted of a Büchner funnel, a suction flask, and a vacuum pump. The different moistened polymeric membranes were placed on the perforated plate of the open filter chamber. The latter was subsequently filled with raw hydrolysate before the permeate was generated by vacuum suction.
Hydrolysis reactor and feed solution
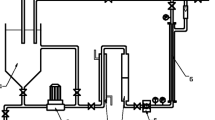
A schematic drawing of the experimental setup designed for this study is shown in Fig. 1. The continuously stirred hydrolysis reactor made of stainless steel had a working volume of 100 L. The hydrolysis reactor (HR) was operated with corn silage and kept at thermophilic conditions of 55 °C in a pH range of 5.6–6.0 as described by Lindner et al. [20]. The effluent was prefiltered with a 100 µm gauze and then fed to the membrane systems. Since the gauze of the HR (cf. Fig. 1) was not able to remove the entire particulate material, the effluent of the hydrolysis reactor contained high quantities of particles and fibrous substances (TS = 19.1 ± 7.5 g/L).
For the initial start-up, the HR was fed with 80 L tap water, 20 L manure, 190 g acetic acid (60%), 140 g lactic acid (90%), 2 kg corn silage as well as 1 kg of fermentation residue from another HR as inoculum. The acidity within the HR was controlled by feeding the reactor with corn silage when the pH exceeded 6.0. The average organic loading rate OLRvs varied between 4 and 6 kg/(m3 day). Total COD concentrations reached levels of 35 g/L directly after start-up, but varied between 17 and 25 g/L during submerged operation and from 5 to 20 g/L during the various crossflow filtration experiments. More detailed information is provided in the third section as well as in Table 1.
Membrane Systems
The crossflow system was operated with tubular ceramic microfiltration membranes (Inopor, Germany) with nominal pore sizes of 0.8 and 0.2 µm. Each membrane had a length of 1000 mm and a diameter of 25 mm. The single membranes were installed in a stainless steel membrane module. The specific membrane area AM is given in Table 2 and varied between 0.13 and 0.31 m2 for the different membrane types. Crossflow shear was induced by a centrifugal pump (Lowara, Germany). Two digital manometers (PKP, Germany) measured the pressure drop between inlet and outlet of the module. The analog pressure gauge (PKP, Germany) in the permeate stream allowed for a manual transmembrane pressure (TMP) calculation. TMP of the crossflow system was regulated with a bypass valve (cf. Fig. 1). With an open bypass, TMP was as low as 200 mbar, while a completely closed bypass would generate TMP values up to 1800 mbar. Permeability measurements were done by gravimetric sampling of permeate for 30–60 s, carried out in triplicates. The crossflow system operation as well as data acquisition was done by the zenon software (COPA-DATA, Germany). Experiments 2 and 3 included backwashing cycles of 15 s every 15 min. Therefore, the permeate stream was reversed with a centrifugal pump (Lowara, Germany). Experiments 1, 4, and 5 were conducted without backwashing (cf. Table 2). The first experiment was done under mesophilic conditions (35 °C); for all the following ones, the membranes were operated under thermophilic conditions (50 °C) with mean crossflow velocities vcf ≥ 1.5 m/s. Since feed temperature is one of the key factors influencing membrane permeability values, results were corrected to a reference temperature (TR = 40 °C) based on the viscosity of water.
For the submerged setup, flat sheet ceramic microfiltration membranes with nominal pore sizes of 0.5 µm and specific membrane areas of 0.11 m2 (Ceraflo, Singapore) were used. The sidestream membrane container had a working volume of 4.5 L. Gas sparging with nitrogen was used to induce shear stress and to minimize particle deposition on the membrane surface. The coarse diffuser was installed directly underneath the membrane to maximize detachment. The hydraulic retention time of the container was set to 12 min to minimize biogas formation in this step, since the solid-free permeate should enhance biogas production in a fixed bed high-pressure MR. Throughout all experiments, the membrane was operated in cycles consisting of three steps: (1) filtration; (2) relaxation; and (3) backwashing. A diaphragm pump (KNF, Germany) was used for filtration and backwashing. The filtration and backwash phases were set to 10 min and 30 s, respectively. A 10 s pause was embedded between the two steps to relax the membrane. Permeate and backwash pressures were measured by pressure transmitters (Huba Control, Switzerland). The respective flows were measured and controlled with mass flow meters (Bronkhorst, The Netherlands). Data acquisition and system operation was done with the LabVIEW software (NI, USA). Critical flux and long-term experiments were done under thermophilic conditions (45 °C). Temperatures of crossflow and submerged system were both controlled with thermostats (Julabo, Germany).
Critical flux determination
The critical flux concept is based on the balance between convection towards and back transport away from the membrane surface [21]. According to Field et al. [22], the critical flux can be defined in its strong or weak form as a flux below which a decline with time does not occur. In this study, the critical flux was determined in the weak form using the flux-step method as described by Le-Clech et al. [23]. The strong form is the flux at which the TMP deviates from the linear pure water line (PWL). The weak form assumes very rapid fouling at start-up; therefore, the flux–TMP relationship is below the PWL and the critical flux is then the point, where the flux–TMP line becomes non-linear. Duration of the flux steps was set to 10 min, followed by a 30 s backwashing phase. The different flow rates and flux-step heights for the critical flux experiments are shown in Table 3. Experimental data were recorded every 5 s during critical flux experiments and every 20 s during long-term operation with the submerged system. Two approaches to determine the mean critical flux value can be used, (1) calculating the fouling rate (dTMP/dt) as well as (2) the average TMP for a certain flux can be used. Both values are used in this study.
Membrane cleaning procedure
Membrane cleaning procedures were performed when the flux of the crossflow membranes declined below 7 L/(m2 h) or TMP values exceeded 900 mbar and the TMP of the submerged system reached 500 mbar, respectively. In both cases, membrane fouling was found to be severe. One physical and two chemical membrane cleaning procedures were used to recover the permeability. Physical cleaning was done by backwashing the membrane for 15 min with pure water. Chemical cleaning with the crossflow membranes was done by submerging them externally into a NaOH solution (pH 13) for 12 h. The submerged membranes were backwashed with a NaOH solution (pH 12) for 12 min, adding 18 min of soaking. Subsequently, the procedure was repeated with an HCl solution (pH 2).
Membrane-resistance determination
The fouling effects on filtration performance can be expressed by determining the hydrodynamic resistance. The total hydraulic filtration resistance RT (m−1) was calculated by Darcy’s law:
where TMP is the measured transmembrane pressure (Pa), η is the dynamic viscosity (Pa s) and J is the permeate flux (m s−1). The total filtration resistance RT can be divided in partial resistances, resulting in the simplified resistance-in-series model:
where Rm represents the intrinsic membrane resistance, Rrev is the reversible, and Rirr is the irreversible fouling [11, 24,25,26,27]. Total membrane resistance RT was defined after stopping the respective experiment as the one occurring under the most severe conditions at the end of each long-term operation. Rm, Rrev, and Rirr were determined by recording different stable flux rate steps for 10 min with pure water at 40 °C. Intrinsic membrane resistance Rm was measured for each unused membrane before starting a new experiment. Rrev and Rirr were measured in two steps after an experiment was terminated. During the first step, the membrane was backwashed for 15 min with pure water and the reversible membrane resistance Rrev (+ Rm + Rirr) was obtained subsequently by recording the pure water line. Afterwards, the membrane was chemically cleaned as described in the previous section. Following this procedure, the pure water line was recorded again to obtain the irreversible fouling resistance Rirr (+ Rm) of the specific membrane.
Analytical methods
The chemical oxygen demand (COD) and the soluble COD (sCOD) were measured using test kits (Hach Lange, Germany). Total organic carbon (TOC) and dissolved organic carbon (DOC) concentrations were measured with a TOC Analyzer (Shimadzu TOC-V CPN, Japan). For the sCOD and DOC measurements, the samples were prefiltered with a 0.45 µm membrane filter. An ion chromatography (IC) system (Metrohm, Switzerland) was used to determine organic acid concentrations. Electric conductivity and pH were measured with a portable multimeter (WTW Multi 350i, Xylem, USA). The concentration of total solids (TS) and volatile solids was measured by drying (105 °C, 24 h) and volatilization (550 °C, 8 h) [28].
Methane potential tests
Methane potential of hydrolysate and permeate was analyzed with the automatic methane potential test system (AMPTS, Bioprocess Control, Sweden). The system automatically analyzes the accumulated methane volume over time for small continuously stirred batch reactors (37 °C) with a total volume of 500 mL, consisting of 400 mL liquid and 100 mL head space. Digested sludge from the wastewater treatment plant Heidelsheim was used as inoculum. All measurements were carried out as triplicates. The baseline measurement was conducted by adding 400 mL of digested sludge into the batch reactors. For the measurements of hydrolysate and permeate, 300 mL of inoculum were mixed with 100 mL of hydrolysate and permeate, respectively.
Results and discussion
The main hydrolysate characteristics are given in Table 1. Average COD and TOC concentrations of 21 and 6 g/L were found, respectively. The dissolved fraction, measured as sCOD, accounted with approximately 10 g/L for about 50% of the total COD. The DOC concentration was measured with concentrations as high as 4 g/L. When analyzed with the IC, five organic acids contributed mainly to the DOC. Those were, namely, acetic, propionic, isobyturic, butyric, and valeric acids. Acetic and butyric acid concentrations of 2.5 and 1.5 g/L were regularly found, respectively. Propionic acid concentrations averaged 0.8 g/L, valeric as well as isobutyric acid were always below 0.4 g/L. The latter values are not shown in Table 1. COD could be measured without any time delay and pretreatment (no prefiltration and no dilution) of the hydrolysate and was, therefore, chosen as sum parameter for the evaluation of hydrolysate and permeate in this study.
Screening test of membranes
The membrane tests were carried out with raw hydrolysate from the hydrolysis reactor to pre-assess the impact of the filtration step, especially to determine the optimal pore size. The main idea of the membrane filtration was to eliminate the solid fraction and collect the dissolved organic matter, primarily organic acids as permeate. The results based on different polymeric MF membranes are shown in Fig. 2. While Fig. 2a depicts the sum parameters COD, total carbon (TC), TOC, and DOC, Fig. 2b displays the main organic acids of the raw hydrolysate and the different permeates. TOC could only be measured for the raw hydrolysate and the 0.8 µm permeate; when filtrated with 0.45 µm, it is defined as DOC. The 0.8 µm membrane eliminates approximately 40% of the COD, the membranes with smaller pore sizes (0.1–0.45 µm) detain about 10% more. TC and TOC, respectively, and DOC concentrations for permeate ≤ 0.45 µm are rejected by approximately 50%. Acetic, propionic, butyric, isobutyric, and valeric acids are all reduced by approximately 15%, without any remarkable margin between the different permeates. Since the analyzed acid molecules are too small to be eliminated by the used pore sizes, it is assumed that the removed percentage of organic acids is adsorbed on the surface of the particulate matter of the hydrolysate and thereby partly removed with the membrane. The filtration with pore sizes ≤ 0.1 µm resulted in an acid elimination of more than 30%. Therefore, in this case, the minimum reasonable membrane cutoff for long-term experiments should not be lower than 0.2 µm.
Crossflow filtration experiments
As the experiments with the different MF membranes do not show significant differences, the crossflow experiments were carried out with ceramic membranes in the range of 0.2–0.8 µm. The first two crossflow long-term experiments were carried out with 0.8 µm ceramic membranes (Inopor, Germany). A comparison of the long-term experiments is given in Table 2. Since the crossflow step is planned to be located between the thermophilic hydrolysis reactor (55–60 °C) and the mesophilic fixed bed methane reactor (37 °C) the influence of the temperature on the filtration performance was tested. Therefore, the first experiment was done under mesophilic conditions at 35 °C, all the following crossflow experiments under thermophilic ones at 50 °C. Figure 3a shows experiment 1, which was carried out under mesophilic conditions with a 0.8 µm membrane in crossflow mode. Experiments 1, 4, and 5 were carried out without a backwash procedure. Directly after start-up, the permeability declined steadily in the first 5 days until it stabilized at a mean value of approximately 45 L/(m2 h bar) after 15 days of operation. The COD concentration of the hydrolysate (feed) alternated during the whole experiment between 8 and 12 g/L. The COD fraction in the permeate varied together with the feed, resulting in an overall COD rejection of approximately 46%. The experiment was stopped after 40 days.
The second and third experiment were conducted under thermophilic conditions with a physical backwash procedure for 15 s every 15 min of filtration. During experiment 2, carried out with a 0.8 µm membrane under thermophilic conditions, a heavy decline of permeability was observed after 5 days of filtration. Backwashing was done with permeate, which was stored in a separate tank. Due to the substrate conditions, a biomass accumulation in the permeate tank was detected 3 days after start-up. The microorganisms faced optimum growth conditions and formed a dense fouling layer on the permeate side of the membrane. The formation led to a constant decline of the permeability throughout the experiment. The average flux was found to be 7.2 L/(m2 h) compared to 23.4 L/(m2 h) in experiment 1 without backwashing (cf. Table 2).
To avoid the accumulation of suspended biomass in the permeate tank, experiment 3 was carried out with a 0.2 µm membrane to minimize the contamination of the permeate with microorganisms. However, the biomass accumulation in experiment 3 was nearly comparable to experiment 2. The average flux in 15 days was again as low as 8.1 L/(m2 h). The fouled membrane from experiment 3 was chemically cleaned and used in experiment 4. Chemical cleaning was done by submerging the membrane externally into a NaOH solution (pH 13) for 12 h. Due to the severe fouling, the permeability could only be recovered by 75%.
As a result of these findings, experiments 4 and 5 were carried out without backwashing. Compared to experiment 1, the pore size was reduced to 0.2 µm and the temperature during filtration was set to thermophilic conditions.
In Fig. 3b, the results of experiment 5 are shown. The feed COD started with a mean value of 14.5 g/L. About 3 weeks after filtration start-up, the stirrer of the hydrolysis reactor had to be exchanged and the reactor operation had to be stopped. During this period, a part of the hydrolysate was stored in external tanks and the whole permeate was cycled back to the feed tank for 10 days. This led to a significant decline in the COD feed concentration, ending up at 8 g/L. After the reactor was restarted with the stored hydrolysate, the initial COD concentrations could not be recovered quickly. The rest of experiment 5 was carried out with feed concentrations between 7 and 9 g/L. During the first 10 days, the permeability dropped more than 50% from over 300 L/(m2 h bar) to less than 150 L/(m2 h bar). The next 10 days, the trend was stable and the permeability decreased 35% more until it reached a solid level of approximately 100 L/(m2 h bar), before dropping to less than 75 L/(m2 h bar). To recover the permeability without a backwash cycle, the bypass valve was closed for 1 min (see bypass in Fig. 1). By doing so, the blocked membrane channels were flushed and the membrane regained lost filtration area. The effect of this flushing can be seen after 60 days of filtration (Fig. 3b). The permeability could be restored to a stable value of 100 L/(m2 h bar). During all crossflow experiments, the COD elimination was quite steady and averaged 45%. The use of the chemically cleaned membrane in experiment 4 resulted in a little bit lower COD removal of approximately 40%.
Critical flux and long-term filtration experiments with submerged membranes
For the long-term filtration experiments with the submerged system (exp. 6–10), ceramic flat sheet membranes (Ceraflo, Singapore) with nominal pore sizes of 0.5 µm were used. In advance, critical flux experiments were carried out using the flux-step method as described by Le-Clech et al. [23] to determine the optimal filtration parameters. Critical flux experiments were all done with superficial gas sparging velocities of us = 65 m/h (≈ 2.3 Nm3/(m2 h)) at temperatures of 45 °C. The permeate flow rate and the flux-step size of each run are given in Table 3. At each flux step, filtration was performed for 10 min ahead of a 30 s backwash cycle, subsequently moving to the next higher flux. During the individual steps, the TMP development over time was recorded and the (1) fouling rate dTMP/dt as well as the (2) average TMP for each flux step were calculated to determine the mean critical flux. A comparison of both approaches is given in Fig. 4a, b. In Fig. 4a, the step size was set to 0.2 L/h resulting in a mean critical flux of 8.61 L/(m2 h). By decreasing the step size to 0.1 L/h, the calculated mean critical flux diminished to 7.1 L/(m2 h), as can be seen in Fig. 4b. This result shows the importance of choosing the right filtration mode with optimal step sizes, respectively, and heights to ensure no under- or overestimation of the actual critical flux value [24].
Experiment 6, the first long-term experiment with the submerged system (cf. Table 2), was carried out under sub-critical flux conditions of 4.7 L/(m2 h). As in the critical flux measurements, filtration was carried out at 45 °C with a superficial gas sparging velocity of 65 m/h. Due to the experiences with permeate fouling while operating the crossflow system, the retention time of the permeate generated by the submerged setup was minimized to 16 min. To achieve that, the permeate for the backwash was stored directly in the permeate tubes. The excess permeate was directly circulated back into the hydrolysis reactor. Despite this precautions, a severe accumulation of biomass in the system periphery, especially in the permeate tubes, was observed from day 6 onward. Due to the submerged system cycle of 10 min filtration, 30 s backwash, and 10 s relaxation, the biomass was continuously transported back to the permeate side of the membrane. After 11 days, the experiment had to be stopped because of intense biomass accumulation on the permeate side of the system and consequently the blocking of the permeate suction pump.
To decrease the retention time of the backwash permeate stored in the tubes even further and to test the critical flux performance of the membranes, the flux was accordingly increased to 7 L/(m2 h) in experiment 7. Figure 5 shows the results.
As mentioned above, filtration was carried out at 45 °C with a superficial gas sparging velocity of 65 m/h. TMP increased slightly in the first 3 weeks from 10 to 25 mbar. After the third week, a more intensified biomass accumulation on the permeate side was observed, resulting in a further TMP increase of up to 50 mbar. To flush the biomass out of the permeate tubes, the flux was increased to 9.34 L/(m2 h) for approximately 70 h. After 35 days, TMP values reached levels of 100 mbar and the flux was reduced again to relieve the system. Since the fouling on the permeate side was already severe, a further reduction of the flux to the sub-critical value of experiment 6 did not help. The experiment was stopped on day 44.
Due to the strong accumulation of biomass on the permeate side, the whole membrane system periphery (pump, tubes etc.) was chemically cleaned for 3 consecutive days. In addition, a new membrane was used for the rest of the experiment. As a result of the unsteady feed conditions in the first half of the experiment (cf. next paragraph), this was necessary to evaluate the overall membrane performance. Besides the higher COD feed concentration at start-up, the second half of the experiment, especially the TMP development, was almost a replica of the first one. The TMP increase of the first 30 days was as smooth as before resulting in mean values of less than 50 mbar. As previously, the biomass accumulation on the permeate side became more severe after 3 weeks of operation. Despite keeping the flux constant this time, the TMP increased to more than 400 mbar after 6 weeks. The experiment was stopped when TMP values of 500 mbar were recorded.
In the first part of experiment 7, heavy foaming in hydrolysis reactor and membrane tank occurred, resulting in a loss of biomass in the feed from day 5 to day 20. To increase the puffer capacity of the hydrolysate, 20 L liquid manure was added to the hydrolysis reactor. This led to the sudden increase in feed COD from 13 to 20 g/L and permeate from 5 to 8 g/L on day 21. For the first 5 days, COD elimination was at 40%, increasing steadily to more than 60%. For the second membrane, the feed concentrations were more stable at average values of 20 g/L leading to a mean elimination rate of 57%. These findings were confirmed by experiments 8 and 9. The main characteristics of feed and permeate are compared in Table 4 as mean values during all long-term experiments with the submerged membrane system.
Membrane resistance
The results of the filtration resistance analysis are outlined in Table 5. Total membrane resistance RT was as high as RT,s = 39.31 × 1012 (m−1) for the submerged and as high as RT,cf = 16.45 × 1012 (m−1) for the crossflow system, respectively. The reversible fouling resistance Rrev accounted for almost Rrev,s = 96% and Rrev,cf = 87% of the total fouling resistance. Irreversible fouling Rirr was as low as Rirr,s = 1.3% and Rirr,cf = 8.3% when chemical cleaning procedures were applied as described in the material and methods section. These results indicate that the filtration resistance is mainly attributed to reversible fouling formation due to sludge, colloids, particle deposition, and biofilm formation on the membrane surface. Consequently, it was found that organic and inorganic fouling was essentially reversible. EPS has been reported as a potential organic fouling agent in thermophilic AnMBR systems by Lin et al. [29], Visvanathan et al. [30] and Chaikasem et al. [19]. The former analyzed the thermophilic sludge cake layer in detail and detected a higher content of extracellular polymeric substances (EPS) as well as a more compact sludge cake layer when compared to a mesophilic operated membrane setup. Based on the findings presented in this section, reversible fouling is the main component of total filtration resistance.
Methane potential analysis
Two results of methane potential measurements carried out with hydrolysate and permeate during experiment 4 and experiment 7 can be seen in Fig. 6a, b, respectively. The depicted curves are qualitatively representative for all conducted AMPTS experiments with hydrolysate and permeate. Most of the methane is produced in the first 24 h. Despite the elimination of up to 50% of COD and approximately 25% of organic acids by membrane filtration, the permeate produced always as much or even more CH4 than the hydrolysate (see Table 6). Calculated on basis of the input COD during the batch reactor experiments, the permeate/inoculum solution produced 0.31 L CH4/CODin compared to 0.24 L CH4/CODin of the hydrolysate/inoculum broth after t = 24 h experiment time. The implemented MF membranes with pore sizes in the vicinity of 0.45 µm led mainly to the fractioning of dissolved constituents from fibrous substances and particles. MF membranes are not able to eliminate organic acids due to their molecular size. The organic acid elimination rate (approx. 15%) indicates that this part might be adsorbed to particulate matter and, therefore, retained by the microfiltration unit together with the solid fraction. Those adhered acids seem to be hardly accessible for the microorganisms. Since dissolved fatty acids are easily available for methanotrophic bacteria, the microfiltration separation results in a faster and more efficient conversion rate of the organic carbon sources in the permeate. That balances the fact that the carbon sources in the permeate are present in lower concentrations than in the hydrolysate. First results of the coupled crossflow system with a high-pressure methane reactor showed methane contents of more than 94%, which opens up new possibilities for the utilization of biogas [31].
Conclusions
Both crossflow and submerged ceramic membranes have been used to treat hydrolysate from an anaerobic hydrolysis reactor. The crossflow system could be operated with fluxes of higher than 30 L/(m2 h) under stable conditions when no backwash cycles were applied. The backwash cycles were found to limit the operation time to under a week due to fouling on the permeate side. A more efficient way to recover lost filtration area is to flush the membrane channels by increasing the crossflow velocity for less than a minute.
The critical flux measurements with the submerged membrane system predicted a sustainable flux of approximately 7 L/(m2 h). Long-term operations confirmed these findings. Similar to the crossflow system, the filtration performance was mainly restricted by the high fouling potential of the permeate leading to biomass accumulation on the permeate side of the membrane. The lower sustainable flux in the submerged system can be compensated when gas sparging with hydrolysis gas is applied, since this gas cannot be utilized in any step of a two-stage biogas process.
COD removal was as high as 46% and as high as 60% when operated with the crossflow or submerged system, respectively. Despite the high elimination rate of COD, methane potential tests showed that the loss of organic carbon in the permeate does not reduce the methane yield.
Solid–liquid separation of first-stage hydrolysate with ceramic microfiltration membranes can be a reliable tool to avoid clogging and/blocking in a fixed bed methane reactor.
References
Lindner J, Zielonka S, Oechsner H, Lemmer A (2016) Is the continuous two-stage anaerobic digestion process well suited for all substrates? Biores Technol 200:470–476. https://doi.org/10.1016/j.biortech.2015.10.052
Zielonka S, Lemmer A, Oechsner H, Jungbluth T (2010) Energy balance of a two-phase anaerobic digestion process for energy crops. Eng Life Sci 10(6):515–519. https://doi.org/10.1002/elsc.201000071
Lemmer A, Chen Y, Lindner J, Wonneberger AM, Zielonka S, Oechsner H, Jungbluth T (2015) Influence of different substrates on the performance of a two-stage high pressure anaerobic digestion system. Biores Technol 178:313–318. https://doi.org/10.1016/j.biortech.2014.09.118
Merkle W, Baer K, Haag NL, Zielonka S, Ortloff F, Graf F, Lemmer A (2017) High-pressure anaerobic digestion up to 100 bar: influence of initial pressure on production kinetics and specific methane yields. Environ Technol 38(3):337–344. https://doi.org/10.1080/09593330.2016.1192691
Merkle W, Baer K, Lindner J, Zielonka S, Ortloff F, Graf F, Kolb T, Jungbluth T, Lemmer A (2017) Influence of pressures up to 50 bar on two-stage anaerobic digestion. Biores Technol 232:72–78. https://doi.org/10.1016/j.biortech.2017.02.013
Abeynayaka A, Visvanathan C (2011) Performance comparison of mesophilic and thermophilic aerobic sidestream membrane bioreactors treating high strength wastewater. Biores Technol 102(9):5345–5352. https://doi.org/10.1016/j.biortech.2010.11.079
Duncan J, Bokhary A, Fatehi P, Kong F, Lin H, Liao B (2017) Thermophilic membrane bioreactors: A review. Biores Technol 243:1180–1193. https://doi.org/10.1016/j.biortech.2017.07.059
Martinez-Sosa D, Helmreich B, Netter T, Paris S, Bischof F, Horn H (2011) Anaerobic submerged membrane bioreactor (AnSMBR) for municipal wastewater treatment under mesophilic and psychrophilic temperature conditions. Biores Technol 102(22):10377–10385. https://doi.org/10.1016/j.biortech.2011.09.012
van Lier JB, Hulsbeek J, Stams AJM, Lettinga G (1993) Temperature susceptibility of thermophilic methanogenic sludge: Implications for reactor start-up and operation. Biores Technol 43(3):227–235. https://doi.org/10.1016/0960-8524(93)90035-A
Van Lier JB, Martin JLS, Lettinga G (1996) Effect of temperature on the anaerobic thermophilic conversion of volatile fatty acids by dispersed and granular sludge. Water Res 30(1):199–207. https://doi.org/10.1016/0043-1354(95)00107-V
Lee S-m, Jung J-y, Chung Y-c (2001) Novel method for enhancing permeate flux of submerged membrane system in two-phase anaerobic reactor. Water Res 35(2):471–477. https://doi.org/10.1016/S0043-1354(00)00255-4
Jeison D, van Lier JB (2007) Thermophilic treatment of acidified and partially acidified wastewater using an anaerobic submerged MBR: Factors affecting long-term operational flux. Water Res 41(17):3868–3879. https://doi.org/10.1016/j.watres.2007.06.013
Jeison D, van Lier JB (2008) Feasibility of thermophilic anaerobic submerged membrane bioreactors (AnSMBR) for wastewater treatment. Desalination 231(1–3):227–235. https://doi.org/10.1016/j.desal.2007.11.048
Jeison D, Telkamp P, van Lier JB (2009) Thermophilic sidestream anaerobic membrane bioreactors: the shear rate dilemma. Water Environ Res 81(11):2372–2380. https://doi.org/10.2175/106143009X426040
Qiao W, Takayanagi K, Niu Q, Shofie M, Li YY (2013) Long-term stability of thermophilic co-digestion submerged anaerobic membrane reactor encountering high organic loading rate, persistent propionate and detectable hydrogen in biogas. Biores Technol 149:92–102. https://doi.org/10.1016/j.biortech.2013.09.023
Qiao W, Takayanagi K, Shofie M, Niu Q, Yu HQ, Li Y-Y (2013) Thermophilic anaerobic digestion of coffee grounds with and without waste activated sludge as co-substrate using a submerged AnMBR: System amendments and membrane performance. Biores Technol 150:249–258. https://doi.org/10.1016/j.biortech.2013.10.002
Wijekoon KC, Visvanathan C, Abeynayaka A (2011) Effect of organic loading rate on VFA production, organic matter removal and microbial activity of a two-stage thermophilic anaerobic membrane bioreactor. Biores Technol 102(9):5353–5360. https://doi.org/10.1016/j.biortech.2010.12.081
Mota VT, Santos FS, Amaral MCS (2013) Two-stage anaerobic membrane bioreactor for the treatment of sugarcane vinasse: Assessment on biological activity and filtration performance. Biores Technol 146:494–503. https://doi.org/10.1016/j.biortech.2013.07.110
Chaikasem S, Jacob P, Visvanathan C (2015) Performance improvement in a two-stage thermophilic anaerobic membrane bioreactor using PVA-gel as biocarrier. Desalin Water Treatm 53(10):2839–2849. https://doi.org/10.1080/19443994.2014.931531
Lindner J, Zielonka S, Oechsner H, Lemmer A (2015) Effect of different pH-values on process parameters in two-phase anaerobic digestion of high-solid substrates. Environ Technol 36(2):198–207. https://doi.org/10.1080/09593330.2014.941944
Wu D, Howell JA, Field RW (1999) Critical flux measurement for model colloids. J Membr Sci 152(1):89–98. https://doi.org/10.1016/S0376-7388(98)00200-2
Field RW, Wu D, Howell JA, Gupta BB (1995) Critical flux concept for microfiltration fouling. J Membr Sci 100(3):259–272. https://doi.org/10.1016/0376-7388(94)00265-Z
Le-Clech P, Jefferson B, Chang IS, Judd SJ (2003) Critical flux determination by the flux-step method in a submerged membrane bioreactor. J Membr Sci 227(1–2):81–93. https://doi.org/10.1016/j.memsci.2003.07.021
Bacchin P, Aimar P, Field RW (2006) Critical and sustainable fluxes: theory, experiments and applications. J Membr Sci 281(1):42–69. https://doi.org/10.1016/j.memsci.2006.04.014
Wu J, Le-Clech P, Stuetz RM, Fane AG, Chen V (2008) Novel filtration mode for fouling limitation in membrane bioreactors. Water Res 42(14):3677–3684. https://doi.org/10.1016/j.watres.2008.06.004
Meng F, Zhang H, Yang F, Liu L (2007) Characterization of Cake Layer in Submerged Membrane Bioreactor. Environ Sci Technol 41(11):4065–4070. https://doi.org/10.1021/es062208b
Meng F, Shi B, Yang F, Zhang H (2007) New insights into membrane fouling in submerged membrane bioreactor based on rheology and hydrodynamics concepts. J Membr Sci 302(1–2):87–94. https://doi.org/10.1016/j.memsci.2007.06.030
VDI Society Energy and Environment (2016) VDI 4630:2016-11: Fermentation of organic materials - Characterization of the substrate, sampling, collection of material data, fermentation tests
Lin HJ, Xie K, Mahendran B, Bagley DM, Leung KT, Liss SN, Liao BQ (2009) Sludge properties and their effects on membrane fouling in submerged anaerobic membrane bioreactors (SAnMBRs). Water Res 43(15):3827–3837. https://doi.org/10.1016/j.watres.2009.05.025
Visvanathan C, Choudhary MK, Montalbo MT, Jegatheesan V (2007) Landfill leachate treatment using thermophilic membrane bioreactor. Desalination 204(1):8–16. https://doi.org/10.1016/j.desal.2006.02.028
Bär K, Merkle W, Tuczinski M, Saravia F, Horn H, Ortloff F, Graf F, Lemmer A, Kolb T (2018) Development of an innovative two-stage fermentation process for high-calorific biogas at elevated pressure. Biomass Bioenerg 115:186–194. https://doi.org/10.1016/j.biombioe.2018.04.009
Acknowledgements
This research was supported by the German Ministry for Education and Research (BMBF), funding code 03EK3526B.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tuczinski, M., Saravia, F. & Horn, H. Treatment of thermophilic hydrolysis reactor effluent with ceramic microfiltration membranes. Bioprocess Biosyst Eng 41, 1561–1571 (2018). https://doi.org/10.1007/s00449-018-1983-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-018-1983-3