Abstract
Erythromycin A is an important antibiotic. A chemically defined synthetic medium for erythromycin production was systematically optimized in this study. A high-throughput method was employed to reduce the number of components and optimize the concentration of each component. After two round single composition deletion experiment, only 19 components were remained in the medium, and then the concentration of each component was optimized through PB experiment. The optimal medium from the PB experiment was further optimized according to the nitrogen and phosphate metabolic consumption in 5 L bioreactor. It was observed that among the 8 amino acids concluded in the media, 4 amino acids were first consumed, when they are almost depleted, the other 4 amino acids were initiated their consumption afterwards in 5 L bioreactor. The decrease of phosphate concentration would increase qglc and qery. However, when phosphate concentration was too low, the production of erythromycin was hindered. The positive correlation between intracellular metabolite pools and Yery/glc indicated that low phosphate concentration in the medium can promote cell metabolism especially secondary metabolism during the stationary phase; however, if it was too low (5 mmol/L), the cell metabolism and secondary metabolism would both slow down. The erythromycin titer in the optimized medium (medium V) reached 1380 mg/L, which was 17 times higher than the previously used synthetic medium in our lab. The optimized medium can facilitate the metabolomics study or metabolic flux analysis of the erythromycin fermentation process, which laid a solid foundation for further study of erythromycin fermentation process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Erythromycin A, a broad-spectrum antibiotic against pathogenic Gram-positive bacteria is mostly produced by Saccharopolyspora erythraea. The commercial importance of this antibiotic has led to deep research of its biosynthesis, genetic manipulation, and fermentation engineering [1,2,3,4].
Studies of fermentation process of erythromycin A in the past were mostly carried out in complex medium, which would hinder the understanding of the definite relationship between erythromycin A production and environmental parameter. In many other studies, the experiments were carried out in synthetic medium. However, in all these studies the erythromycin titers were too low, so the conclusions drawn from these studies had limit applicability to industrial production of erythromycin [5,6,7,8,9]. Thus the strong need for a synthetic medium with sufficient high erythromycin titer is highly demanding.
The conventional methods of the optimization of the synthetic medium were all conducted with time consuming, low efficiency work. In this study, high-throughput methods for the screening of synthetic medium and determination of erythromycin A titer were employed to reduce both the costs and operation time [10].
Materials and methods
Strains
Two strains were used in this study.
Saccharopolyspora erythraea E3 strain. It was used for the production of erythromycin A and was stored in our laboratory.
Bacillus pumilus CMCC(B)63202. It was used as indicator bacterium for high-throughput determination of erythromycin A and was stored in our laboratory.
Culture medium
Slope solid medium for S. erythraea
Slope solid medium compositions (g/L): corn steep liquor 10, starch 10, NaCl 3, (NH4)2SO4 3, agar 18, CaCO3 6. pH 7.0.
Pre-culture medium for S. erythraea
Pre-culture medium compositions (g/L): starch 40, peptone 20, NaCl 4, dextrin 20, KH2PO4 0.2, CaCO3 3, MgSO4 0.25. pH 7.0.
Synthetic medium for S. erythraea in our previous study
Compositions (g/L): glucose 10, (NH4)2SO4 5, K2HPO4 3, KH2PO4 2, MgSO4 0.4, trace elements (12 ml/L). Trace elements compositions (g/L): MnSO4·H2O 0.2, CoCl2·6H2O 0.25, ZnSO4·7H2O 0.2, FeSO4·7H2O 0.03, Na2MoO4·2H2O 0.02, CaCl2 0.1, CuSO4·5H2O 0.01. pH 7.0.
Solid medium for Bacillus pumilus
Compositions (g/L): peptone 5; beef extract 3; K2HPO4 3; glucose 10; NaCl 5; agar 20. pH 7.0.
Bioactivity assay medium
Compositions (g/L): peptone 5; beef extract 3; K2HPO4 3; glucose 10; NaCl 5. pH 7.0.
Culture conditions for S. erythraea
Slope culture
Inoculate the slope with spore suspension stored in the glycerol stock. The slope was then incubated at 34 °C for 4–6 days.
Shake flask culture
Inoculate the shake flask containing 50 mL pre-culture medium with 1 cm2 slope culture. It was cultivated at 34 °C, 220 rpm for 48 h.
48-well plate culture
After 48 h, the pre-culture medium was collected and centrifuged at 4000 rpm for 5 min. Then the cell pellets were washed 3 times with 0.9% NaCl solution. After washing, the cell pellet was resuspended with 50 mL distilled water. 0.1 mL cell pellet suspension was transferred into each well containing 0.9 mL synthetic medium.
5 L-bioreactor culture
The inoculation process of 5 L-bioreactor culture was the same as the 48-well plate culture. The inoculation fraction was 9%. The DO was controlled higher than 40% by adjusting the aeration rate and the stirring speed. The pH was maintained at 7.0 with the addition of NaOH or glucose. The temperature was held at 34 °C.
Determination of erythromycin A
High-throughput method
To facilitate the process of optimization of the synthetic medium, a high-throughput method for determination of erythromycin A was used in this study (Fig. 1). Briefly, the solid culture of Bacillus pumilus was washed with distilled water. Then the cell suspension was diluted until its OD580 reached approximately 1.0. Subsequently, the bioactivity assay medium was inoculated with the cell suspension of Bacillus pumilu (5% (v/v)). After the inoculation of Bacillus pumilu, 225 µL bioactivity assay medium was immediately allocated to each well of a 48-well plate. Then, 25 µL of fermentation broth was transferred into the 48-well plate. The mixture was then incubated at 37 °C, 150 rpm for about 6 h, until the OD580 of the blank well (in which the fermentation broth was replaced with 25 µL distilled water) reached 0.6. At last, 200 µL of the mixture was transferred to a 96-well microtiter plate, and the OD580 of each sample was determined with a thermo scientific microplate reader. Both the single composition deletion experiment and the Plackett–Burman experiment were carried out via this high-throughput method (Fig. 1).
HPLC method
The erythromycin A titer in the 5 L bioreactor was determined with HPLC (Agilent 1100 Series, China). A Hypersil BDS-C18 column (4 mm × 250 mm, 5 mm, Elite, China) was used. Mobile phase: 45% acetonitrile with 0.02 M K2HPO4. Flow rate: 1 mL/min. Oven temperature: 60 °C. Injection volume: 20 µL. Wave length of detector: 215 nm.
Single composition deletion experiment
To reduce the components in the synthetic medium, a single composition deletion method was employed to delete the composition, which have no or have negative effect on erythromycin A production. The original synthetic medium consists of 38 components, which may be beneficial for the growth or the secondary metabolism. In each run of the single composition deletion method, one composition in the original synthetic medium was left out, except that the first run contains all the compositions. The single composition deletion experiments were carried out in a high-throughput cultivation system (Fig. 1). At 120 h, the erythromycin A titer in each run (Ci was the erythromycin A titer of the ith run of the first round of single composition deletion experiment, C1 was the erythromycin titer in the first run) was determined. When Ci/C1 > 1, which means that the addition of the corresponding component was detrimental for the production of erythromycin A, the corresponding component would be removed from the original synthetic medium, and vice versa.
Plackett–Burman method
In this study, the Plackett–Burman method was employed to preliminarily optimize the concentration of each component after the single composition deletion experiment.
Determination of intracellular metabolite pools
The intracellular metabolite pools were measured as the method described in our previous study [11].
Results and discussion
Single composition deletion experiment
As the main purpose of this paper is to get a suitable synthetic medium for the production of erythromycin A, its components should be determined at first. All components of the crude medium were obtained through single composition deletion experiment as described in Sect. 2.5.
The results of the first round of the single composition deletion experiment are listed in Table 1. As can be noticed in Table 1, the Ci/C1 values of 12 components were higher than 1, which indicated that these components were detrimental to the production of erythromycin A. Thus, after the first round of single composition deletion experiment, 12 components were removed from the synthetic medium.
After the first round of single composition deletion experiment, there were still 26 components left. To further decrease the components, a second round of single composition deletion experiment was carried out. In the second round of single composition deletion experiment, the initial medium contains 26 components as the results of the first round of single composition deletion experiment. The results of the second round of single composition deletion experiment are listed in Table 2, in which C′i was the erythromycin A titer of the ith run of the second round of single composition deletion experiment. In the second round, the C′i/C′1 of 7 components were higher than 1. And the corresponding components were deleted.
After two rounds of single composition deletion experiment, 19 components in the initial synthetic medium were removed.
Plackett–Burman experiment
Once the components of the crude medium was obtained, the concentration of each component still needs to be optimized. The 19 components left in the single composition deletion experiment were then subject to the Plackett–Burman experiment (PB) to optimize their concentrations. There were 20 runs in the PB experiment and it was designed as in Table 3.
The effect and optimal concentration of each factor in the PB experiment are shown in Table 4. The results showed that K2HPO4 have the highest negative effect on erythromycin production, and aspartate have the highest positive effect on erythromycin production.
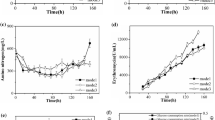
The optimized medium was then used in a 5 L bioreactor to produce erythromycin (Fig. 2). The erythromycin titer at 120 h was 1110 mg/L.
Optimization of the synthetic medium in 5 L bioreactor by the nitrogen and phosphate regulation
After the crude synthetic medium was obtained via the high-throughput method, the metabolism behavior of S. erythraea in the synthetic medium was carefully observed. To eliminate the second growth phenomenon, 4 amino acids, which was used by cell only after the other 4 amino acids in the medium were depleted, were left out of the crude medium. Then 5 experiments were carried out to observe the nitrogen and phosphate effect on erythromycin production (Table 5).
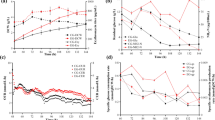
The cell concentration has a positive effect on the erythromycin production, and nitrogen source is very essential for the cell growth, thus the consumption behavior of the major nitrogen sources, e.g., amino acids during the erythromycin production process was carefully studied. The amino acid consumption behavior in the optimal synthetic medium of the PB experiment is illustrated in Fig. 3. It was observed that the 8 amino acid were not consumed at the same time. Before 12 h only 4 kinds of amino acids (this group of amino acids were then called the group A, which contains alanine, serine, cysteine and arginine) were absorbed and after 12 h the other 4 kinds of amino acids were then absorbed (this group of amino acids was then called the group B, which contains aspartate, threonine, leucine and isoleucine). This suggested that the cell were inclined to consume the amino acids in group A first. The amino acids were all used up before the stationary phase, during which most of the erythromycin were produced, thus the amino acids were just used for cell growth and had very little effect on erythromycin production. Three of the amino acids in group A were transformed into pyruvate, the other one was transformed into glutarate. These two metabolites are useful for cell growth. The amino acids in group B would transform into other metabolites which are less useful for cell growth. These results may suggest that cell are inclined to consume amino acids that can be transformed into more useful metabolites for cell growth. The above results suggested that amino acids in group B were not necessary in the medium. To decrease the components in the medium and eliminate the second growth (Fig. 2, 48 h), the amino acids in group B were left out in the following medium (medium II in Table 5).
Beside nitrogen source, phosphate source is also very important for cell growth and erythromycin production. As to further optimize the synthetic medium for erythromycin production, the optimal medium in the PB experiment was further optimized (Table 5). The fermentation results of medium I to medium V are illustrated in Fig. 4, the optimal medium for erythromycin production was medium V. As high phosphate concentration would inhibit the secondary metabolism [12], thus the phosphate in medium II were lowered, which produced medium III. The erythromycin production in medium III was higher than in medium II (Fig. 4a). The phosphate concentration was further decreased in medium IV. However, the erythromycin production in medium IV was lower than medium III (Fig. 4a). The low cell concentration in medium IV indicated that low phosphate concentration had inhibited cell growth thus decreased erythromycin production. The phosphate concentration in medium III was suitable for cell growth and erythromycin production.
Relationship between intracellular metabolite pools and phosphate concentration in the medium
To get a more detailed understanding of relationship between the phosphate concentration and erythromycin production, the intracellular metabolite pools were determined.
It could be noticed that high phosphate concentration was good for cell growth (Fig. 4c), but low phosphate concentration was good for erythromycin production (Fig. 4a, b). In the batch fermentation process, the added phosphate source in the medium cannot be largely consumed, thus initial phosphate concentration is very vital for the whole fermentation process. The only difference among medium II, medium III and medium IV was the phosphate concentration. It could be observed that with the decrease of phosphate concentration the specific glucose consumption rate and specific erythromycin production rate both increased (Table 6), which indicated that the decrease of phosphate concentration facilitate the consumption of glucose and the production of erythromycin. However, the yield of erythromycin to glucose (Yery/glc) increased at first, and then decreased as the phosphate concentration further decreased (Table 6). The increase of Yery/glc from medium II (0.0015 mol/mol) to medium III (0.0062 mol/mol) indicated that low phosphate concentration can increase the proportion of glucose used for erythromycin. However, further decrease of phosphate from medium III to medium IV led to the decrease of Yery/glc (from 0.0062 to 0.0046 mol/mol), which meant that more glucose was used for maintenance. It could be observed that from medium II to medium IV, pool sizes of most intracellular metabolites were positively correlated with Yery/glc at 72 h (Fig. 5). These result indicated that low phosphate concentration in the medium can promote cell metabolism especially secondary metabolism during the stationary phase. However, if the phosphate concentration in the medium was too low (5 mmol/L), the primary metabolism and secondary metabolism would both slow down. These results suggested that the phosphate concentration is not the lower the better, and there is an optimal level.
The comparison of the optimized medium (medium V) and previously used medium
The erythromycin titer in the optimized medium (medium V) reached 1380 mg/L, which is 17 times higher than the previously used synthetic medium in our lab (Fig. 6) [13]. The optimized medium can facilitate the metabolomic study or metabolic flux analysis of the erythromycin fermentation process, which laid a solid foundation for further study of fermentation process or cell metabolism.
Conclusion
A synthetic medium for erythromycin production was successfully developed and optimized in this study. At first, a synthetic medium consists of 38 components, which may be beneficial for the growth or the secondary metabolism, was designed. Then a high-throughput method was employed to reduce the number of components and optimize the concentration of each component. After two round single composition deletion experiment, only 19 components were left in the medium, and then the concentration of each component was optimized through PB experiment. The optimal medium from the PB experiment was further optimized through the nitrogen and phosphate regulation in 5 L bioreactor. It was observed that the 8 amino acid were consumed at different stages in 5 L bioreactor, so the four amino acids in group B were eliminated in the recipe. The decrease of phosphate concentration would increase qglc and qery. However, when phosphate concentration was too low (5 mmol/L), the production of erythromycin was hindered. The best phosphate concentration in this study was determined, which was employed in the medium III. The positive correlation between intracellular metabolite pools and Yery/glc indicated that low phosphate concentration in the medium can promote cell metabolism especially secondary metabolism during the stationary phase, but if the phosphate concentration in the medium was too low, the cell metabolism and secondary metabolism would both slow down. It could also be noticed that the phosphate concentration in the medium could impact the intracellular metabolite concentration. When phosphate concentration was too high (28 mmol/L), the most of the intracellular metabolite pool were low; and when phosphate concentration decreased to 6.6 mmol/L, the intracellular metabolites were dramatically increased. However, when phosphate concentration further decreased to 5 mmol/L, the intracellular metabolite pools decreased again. These results indicated that the phosphate concentration has major impact on cell metabolism and a proper phosphate can benefit cell metabolism. The erythromycin titer in the optimized medium (medium V) reached 1380 mg/L, which is 17 times higher than the previously used synthetic medium in our lab (Fig. 6). The optimized medium can facilitate the metabolomics study or metabolic flux analysis of the erythromycin fermentation process, which laid a solid foundation for further study of erythromycin fermentation process or cell metabolism.
References
Oliynyk M, Samborskyy M, Lester JB, Mironenko T, Scott N, Dickens S, Haydock SF, Leadlay PF (2007) Complete genome sequence of the erythromycin-producing bacterium Saccharopolyspora erythraea NRRL23338. Nat Biotechnol 25:447–453
Mironov VA, Sergienko OV, Nastasyak IN, Danilenko VN (2004) Biogenesis and regulation of biosynthesis of erythromycins in Saccharopolyspora erythraea. Appl Biochem Micro 40:531–541
Li YY, Chang X, Yu WB, Li H, Ye ZQ, Yu H, Liu BH, Zhang Y, Zhang SL, Ye BC, Li YX (2013) Systems perspectives on erythromycin biosynthesis by comparative genomic and transcriptomic analyses of S. erythraea E3 and NRRL23338 strains. Bmc Genomics 14:523
Wu J, Zhang Q, Deng W, Qian J, Zhang S, Liu W (2011) Toward improvement of erythromycin A production in an industrial Saccharopolyspora erythraea strain via facilitation of genetic manipulation with an artificial attB site for specific recombination. Appl Environ Microbiol 77:7508–7516
Flores ME, Sánchez S (1985) Nitrogen regulation of erythromycin formation in Streptomyces erythreus. Fems Microbiol Lett 26:191–194
Caffrey P, Bevitt DJ, Staunton J, Leadlay PF (1992) Identification of DEBS 1, DEBS 2 and DEBS 3, the multienzyme polypeptides of the erythromycin-producing polyketide synthase from Saccharopolyspora erythraea. Febs Lett 304:225–228
Hsieh YJ, Kolattukudy PE (1994) Inhibition of erythromycin synthesis by disruption of malonyl-coenzyme A decarboxylase gene eryM in Saccharopolyspora erythraea. J Bacteriol 176:714–724
Bermúdez O, Padilla P, Huitrón C, Elenaflores M (1998) Influence of carbon and nitrogen source on synthesis of NADP+-isocitrate dehydrogenase, methylmalonyl-coenzyme A mutase, and methylmalonyl-coenzyme A decarboxylase in Saccharopolyspora erythraea CA340. Fems Microbiol Lett 164:77–82
Zhang Q, Chen Y, Hong M, Gao Y, Chu J, Zhuang Y-P, Zhang S-l (2014) The dynamic regulation of nitrogen and phosphorus in the early phase of fermentation improves the erythromycin production by recombinant Saccharopolyspora erythraea strain. Biores Bioprocess 1:15
Tan J, Chu J, Hao YY, Wang YH, Yao SC, Zhuang YP, Zhang SL (2013) A high-throughput screening strategy for accurate quantification of erythromycin. J Taiwan Inst Chem E 44:538–544
Hong M, Mou H, Liu X, Huang M, Chu J (2017) (13)C-assisted metabolomics analysis reveals the positive correlation between specific erythromycin production rate and intracellular propionyl-CoA pool size in Saccharopolyspora erythraea. Bioproc Biosyst Eng 40:1–12
Bibb MJ (2005) Regulation of secondary metabolism in streptomycetes. Curr Opin Microbiol 8:208–215
Hong M, Huang M, Chu J, Zhuang Y, Zhang S (2016) Impacts of proline on the central metabolism of an industrial erythromycin-producing strain Saccharopolyspora erythraea via C labeling experiments. J Biotechnol 231:1–8
Acknowledgements
This work was financially supported by a grant from the Major State Basic Research Development Program of China (973 Program, no. 2012CB721006), National Natural Science Foundation of China (no. 21276081), partially supported by NWO-MoST Joint Program (2013DFG32630).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Rights and permissions
About this article
Cite this article
Hong, M., Liao, J. & Chu, J. High-throughput optimization of the chemically defined synthetic medium for the production of erythromycin A. Bioprocess Biosyst Eng 41, 1529–1538 (2018). https://doi.org/10.1007/s00449-018-1980-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-018-1980-6