Abstract
In this study, the microalgae Scenedesmus rubescens were cultivated under the following nitrogen sources, nitrogen concentrations, and nitrogen feeding times (NFTs). This was to help assess biomass and lipid productivity. Scenedesmus rubescens can grow well by adhering to the cellulose acetate membrane in five kinds of nitrogen medium: KNO3, urea, NaNO3, (NH4)2CO3, and NH4NO3. Under the criteria of bio-productivity and lipid productivity, urea was the optimal nitrogen source. Among different urea concentrates, biomass productivity and lipid content of S. rubescens cultivated in 0.27 g/L urea medium were optimized at 8.8 g/(m2 day) and 31.1%, respectively. With attached cultivation, the highest biomass of 9.4 g/m2 was obtained at NFTs of 4 days. These results showed that culturing S. rubescens using urea as sole nitrogen source by improving nitrogen uptake with attached cultivation is more efficient.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microalgae can accumulate considerable amounts of biomass and lipids under different nutrient conditions, making them one of the most promising sustainable sources for biofuel production [1, 2]. Under natural growth conditions, phototrophic algae absorb sunlight and assimilate carbon dioxide and nutrients from air and aquatic habitats, respectively. Inorganic nutrients required for algae production include nitrogen, phosphorus, and other nutrients. Nitrogen was the most abundant nutrient under photosynthetic autotrophic conditions. While some algae species can fix nitrogen from air in the form of NO x , most microalgae required nitrogen in soluble form with urea as the best source [3, 4].
Although algal biofuels possess many potential advantages, such as the ability to produce petroleum fuel substitutes, and can undergo oil extraction to produce valuable co-products such as proteins and residual biomass, which may serve as feed or fertilizer [5], profitable production remains unrealized [6, 7]. Generation of 1 kg biodiesel required 3726 kg water, 0.33 kg nitrogen, and 0.71 kg phosphate. Fertilizer demands features clear effects on both energy use and greenhouse gases (GHG) emissions, and 50% of energy use and GHG emissions were associated with fertilizer production, which failed to receive much attention from researchers. For this reason, nutrient delivery represented a significant opportunity for improving the overall sustainability of large-scale algae cultivation [8, 9].
Many cultivation modes, such as open pond, raceway, and inclined surface systems, have been established for the mass production of microalgae. However, microalgal biomass recovery, which generally required one or more solid–liquid separation steps, was a challenging phase in algal biomass production and accounts for 20–30% of total production cost [10]. The processes involved include flocculation, filtration, flotation, and centrifugal sedimentation. Some of these methods were highly energy-intensive. Low cell densities and small size of some algal cells, which typically measure within 0.3–5 g/L (when light penetration is limited) and 2–40 mm, respectively, posed difficulties in biomass recovery [11, 12].
Previous studies have characterized constraints on algal biofuel production technologies [13]. In this study, ‘attached cultivation’ was applied to improve microalgal cultivation modes with higher biomass productivity. In contrast to conventional suspended cultivation, microalgal cells separate from most of an aqueous medium in attached cultivation systems (Fig. 1). Dewatering of biomass harvested from attached culture requires 0.075 MJ of additional energy, which was 0.3% of the energy required in raceway pond harvests [14, 15]. Many studies have assessed effects of nitrogen sources and nitrogen concentration on microalgae in different cultivation systems. However, most of these studies used conventional aqueous-suspended cultivation systems, and derived optimal values may not necessarily be true for attached cultivation systems. In this research, to maximize nitrogen source and concentration and to reduce costs of nutrient medium, effects of nitrogen source, nitrogen concentration and nitrogen feeding times (NFTs) on biomass and oil production from Scenedesmus rubescens were studied with an inexpensive and efficient attached cultivation method.
The schematic diagrams of attached cultivation devices. a Attached cultivation module of the photobioreactor, the residual medium was recycling. The medium was propelled to the system by a peristaltic pump when it flowed through the chamber during the cultivation. b The detailed structure of the cultivation surface of the attached photobioreactor. c The actual photograph for the photobioreactor of attached cultivation used in the research
Materials and methods
Algal strain and broth seed culture for inoculum preparation
Scenedesmus rubescens was purchased from the Institute of Hydrobiology, Chinese Academy of Sciences (Wuhan, China) and purified aseptically for further studies. The strain was maintained in a BG-11 medium. The alga was firstly cultivated in glass columns (0.5 m long, 0.05 m inner diameter, with 0.7 L working volume) under continuous illumination of 100 ± 10 µmol photons/(m2 s) for 7 days to prepare the inocula for attached bioreactors. A freshwater S. rubescens could be well grown as one of the best oil producer for large-scale cultivations. Algal cells also experienced a brief lag phase in broth mediums. Alga in the broth was harvested at late exponential phase (approximately 7 days after inoculation) by 12 h gravity sedimentation (data was not shown) to prepare the inocula for attached bioreactors. Algal culture was aerated continuously with CO2-enriched air flow and an inoculating temperature 25 ± 1 °C. Air bubble that contained 1% CO2 (v/v) was continuously injected into the bottom of the columns with a speed of 1 vvm to agitate the algal broth as well as supply carbon resource.
Attached cultivation system and culture conditions
The attached cultivation system used in this research was similar to that described by Cheng et al. [16]. Single-layer vertical plates were attached to photobioreactors. In brief, a glass chamber containing a glass plate and an algal disk attachment was placed on an iron rack and tilted at a certain angle against the horizontal plane. The medium was propelled (~ 10 mL/min) with a peristaltic pump (TP12DC 12V, Guangzhou JU PlasFitting Technology Co., LTD., China) to circulate the medium inside the system. Light intensity inside the chamber at the position of attached algal cells totaled 100 ± 10 µmol/(m2 s). Continuous airflow containing 1% CO2 (v/v) was injected into the glass chamber at a speed of 0.1 v/(v min) to supply carbon, and the temperature inside the glass chamber measured 25 ± 1 °C during the experimental periods. For the sake of accurate measurement, each culture period was maintained for 8 days for all attached cultivations (data was not shown).
Experimental design
Attached cultivation of Scenedesmus rubescens with different kinds of nitrogen source
Considering NaNO3 concentration at 1.5 g/L in fresh water BG11 medium, that is, N concentration of 17.6 mM, as standard concentration, five kinds of nitrogen source were selected as medium based on equimolar N concentrations. Concentrations of NaNO3, KNO3, urea, (NH4)2CO3, and NH4NO3 measuring 1.50, 1.78, 0.53, 0.85, and 0.71 g/L, respectively, were selected. Culture media constituted for different nitrogen sources with the same volume were placed in the attached device. Scenedesmus rubescens seed broth in logarithmic phase was centrifuged, and algal solution with the same concentration was filtered to the cellulose acetate membrane and attached to the plate of attached device. Initial inoculum sizes of S. rubescens solution were the same in the five nitrogen sources. Inocula were cultivated for 8 days and sampled once a day to measure cell biomass and lipid accumulation in algal cells. Thus, optimal nitrogen source types can be selected based on bioproductivity and lipid productivity during algal growth together with market price of the five nitrogen sources.
Attached cultivation of Scenedesmus rubescens with different concentrations of nitrogen source medium
According to test data in above research and market prices of nitrogen sources, optimal nitrogen source was selected for configuration into six concentrations, namely, 1 (standard concentration), 3/4, 1/2, 1/5, 1/10, and 1/20. Scenedesmus rubescens seed broth in logarithmic phase was centrifuged, and algal solution with the same concentration was filtered with an air pump to the cellulose acetate membrane attached to the plate of attached device. Initial inoculum sizes of S. rubescens solution were the same in six treatment groups. Inocula were cultivated for 8 days and sampled once a day to measure cell biomass and lipid accumulation of algal cells.
Attached cultivation of Scenedesmus rubescens with different nitrogen feeding times (NFTs)
Given the specificity of the device, algal cells were separated from liquid culture medium during attached cultivation. BG11 culture medium can be directly removed to replace the nitrogen-deficient medium without centrifugal separation of algal cells after a certain cultivation time (concentrations of other nutritive salts were constant). To further optimize the amount of nitrogen usage and improve efficiency, based on tests from above sections, optimal nitrogen source and its concentration were selected with 8 days as cultivation period. The following NFTs were set under the same conditions: (1) NFT2, 2 days of nitrogen feeding before cultivation and 6 days of nitrogen deficiency after cultivation; (2) NFT4, 4 days of nitrogen feeding before cultivation and 4 days of nitrogen deficiency after cultivation; (3) NFT6, 6 days of nitrogen feeding before cultivation and 2 days of nitrogen deficiency after cultivation. Scenedesmus rubescens seed broth in logarithmic phase was centrifuged, and algal solution with the same concentration was filtered with an air pump to the cellulose acetate membrane attached to the plate of the attached device. Similar initial inoculum sizes of S. rubescens in three groups of different NFTs were used to measure growth conditions of algal cells.
Analytical procedures
Biomass estimation
The biomass was determined by the gravimetric method [14]. During the experiments, two algae disks were collected every day. Algal cells from the filter membrane were flushed with distilled water and then filtered to a pre-weighed GF/C filter membrane (Whatman, England; DW0). The area size of the cellulose acetate membrane was 10 cm2, i.e., 0.001 m2, and the pore size was 0.45 μm. The membrane was oven dried at 80 °C for about 24 h and then cooled down to room temperature to measure dry weight (DW1). Finally, their average was used. The DW was calculated as follows:
where 0.001 represented the footprint area of the ‘algal disk’ (m2).
Lipid extraction
The attached algal cells were harvested by washing down with de-ionized water and centrifugation at 3800g for 10 min (Allegra X-22R, Beckman coulter, USA). The algal pellets were washed three times with de-ionized water to remove any attached salt. Then the total lipid was measured according to Bligh and Dyer’s method [17] and Cheng et al. [16]. Approximately 50 mg of dried algae powder was mixed with 5 mL chloroform/methanol (1:2, v/v) at 65 °C for 1 h. The mixture was then centrifuged at 948 g for 5 min. The supernatant was collected and residual biomass was extracted twice more. The supernatants were combined, and chloroform and 1% sodium chloride solution were added to a final volume ratio of 1:1:0.9 (chloroform/methanol/water). The solution was allowed to settle, and carefully transferred to a vial and dried to constant weight at 60 °C under nitrogen flow. The total lipid content was calculated as a percentage of the dry weight of the algae.
Statistical analysis
All experiments were performed in triplicates, and data were presented by mean of three independent replicates. One-way ANOVA was performed using Microsoft Office Excel 2010 (Microsoft, USA) to determine variations in the means.
Results and discussion
Influence of different nitrogen sources on attached growth and lipid accumulation of Scenedesmus rubescens
Influence of different nitrogen sources on attached growth of Scenedesmus rubescens
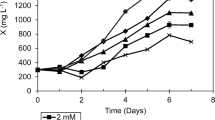
Figure 2 shows the growth curve of S. rubescens under different nitrogen source conditions. Initial inoculum biomass of S. rubescens measured was 7.4 g/m2 in each treatment group. Under each cultivation condition, biomass of S. rubescens significantly increased, and algal cells growth in each treatment group showed a rapid increase in the initial stage before slowing down. A smaller yet still statistically significant increase was also found with nitrogen source of KNO3, urea, NaNO3 for biomass productivity (repeated measures one-way ANOVA) compared to nitrogen sources of (NH4)2CO3, NH4NO3. After 8 days of cultivation, the biomass of S. rubescens under different nitrogen source conditions presented the following descending order: KNO3 > urea > NaNO3 > (NH4)2CO3 > NH4NO3.
Under equimolar concentrations of nitrogen, S. rubescens grew better in KNO3, NaNO3, and urea medium than in the other two media. The biomass of S. rubescens from KNO3 source was the highest compared with other treatment groups, with biomass and bioproductivity reaching 75.8 g/m2 and 9.5 g/(m2 day), respectively. After the same cultivation time, the algae biomass and bioproductivity in NaNO3, urea, (NH4)2CO3 as well as NH4NO3 media were 73.6 g/m2 and 9.2 g/(m2 day), 74.9 g/m2 and 9.4 g/(m2 day), 51.4 g/m2 and 6.4 g/(m2 day), and 31.5 g/m2 and 3.9 g/(m2 day), respectively. Microalgae were most likely to use ammonium nitrogen, but medium pH gradually decreases as a result of NH4 + usage, therefore inhibiting microalgal growth. Thus, high concentrations of ammonium salt retard the growth of microalgae. However, nitrates show no obvious inhibitory function on the growth of algal cells because the amount of NH3 reduced by the latter exhibits no proportional relation to amounts of nitrates in culture medium. Therefore, nitrates did not impede growth of S. rubescens. Only a few blue-green algae with self-capacity to fix nitrogen exist in nature. Other microalgae absorb a certain amount of nitrogen source to grow, and different types of algae require different kinds of nitrogen at different concentrations [18, 19]. Li et al. [18] found that Neochloris oleoabundans with nitrate grew faster and accumulated higher lipid than that with urea, but the cell grew poorly in medium with ammonium as the nitrogen source. Most studies showed that microalgal growth prioritizes the use of nitrate nitrogen [18, 20]. However, urea absorption and assimilation by S. rubescens were involved in algal growth. Scenedesmus rubescens can grow better in urea medium as nitrogen source because urea does not change acid–base reaction of culture medium and maintains pH stability [21]. Our test results were in accordance with previous studies. Overall consideration, this research has selected urea as optimal nitrogen source.
Influence of different nitrogen sources on lipid accumulation of Scenedesmus rubescens
According to the measurement of algal cell lipids in above section, lipid content in S. rubescens was measured under different nitrogen sources, and lipid productivity was calculated. Figure 3a shows the influence of KNO3, NaNO3, urea, (NH4)2CO3, and NH4NO3 on lipid accumulation of algal cells in attached cultivation of S. rubescens, whereas Fig. 3b displays lipid productivity of algal cells. Lipid content of S. rubescens in urea medium reached 31.1% with a bioproductivity of 2.9 g/(m2 day) with attached cultivation for 8 days. Lipid contents from KNO3, NaNO3, and (NH4)2CO3 media reached 26.8, 26.6, and 26.4%, respectively, and corresponding lipid productivities of 2.5, 2.4, and 1.7 g/(m2 day). Lipid content and productivity of S. rubescens in NH4NO3 medium were 21.7% and 0.8 g/(m2 day) respectively, and these values were considered relatively low. So, statistically significant increase of lipid accumulation was obtained with nitrogen source of urea compared to the other treatments.
Nitrogen source posed considerable influence on lipid accumulation and its components. Presumably, lipids in microalgae may significantly accumulate under nitrogen-deficient conditions and affect their growth, whereas sufficient nitrogen benefits microalgal cell growth and fission but not lipid accumulation [22]. Lipid production of unit volume was a basic condition for measuring the feasibility of microalgal biodiesel. Our research showed that S. rubescens grew better and accumulates more biomass in media containing urea, KNO3, or NaNO3. Correspondingly, the content of lipid that transformed from other components within cells had increased after the stable phase of S. rubescens cell growth [23]. Therefore, lipid accumulation was relatively high in algal cells growing in urea, KNO3, or NaNO3 medium. Rincon et al. [24] studied Chlorella vulgaris cultivation under different nitrogen sources and discovered that pH changes in culture medium primarily cause neutral lipid changes in algal cells rather than nitrogen sources, and that significant differences in lipid accumulation of algal cells with urea or ammonium salt possibly arise from pH changes in ammonium salt accumulation [24]. As an organic nitrogen source, urea posed a minor influence on medium pH during consumption, agreeing with conclusion of this research that lipid accumulation of S. rubescens was highest in medium containing urea as nitrogen source. Therefore, this research had selected urea as optimal nitrogen source for further studies given the influence of different nitrogen sources on lipid accumulation and algal cells growth.
Influence of different concentrations of nitrogen sources on attached growth and lipid accumulation of Scenedesmus rubescens
Influence of different concentrations of nitrogen sources on attached growth of Scenedesmus rubescens
Based on part of test results, urea was considered the best nitrogen source. Attached growth of S. rubescens improved with the highest lipid content of algal cells. However, the influence of different concentrations of nitrogen sources on algal growth did not reflect a fixed value, and optimal nitrogen source concentration for algal growth differed under different environments or different cultivation methods. Extremely high or extremely low nitrogen concentration could affect microalgal biomass. To further maximize concentration of nitrogen source in algal cultivation, this paper selected urea (0.53 g/L) as standard source and prepared urea at concentrations of 0.53, 0.40, 0.27, 0.11, 0.05, and 0.03 g/L to explore the optimal concentration needed for attached growth and algal cells lipid accumulation of S. rubescens.
Figure 4a showed the changes of S. rubescens biomass. By using different urea concentrations during cultivation, algae cells can slowly consume and utilize all urea, but growth conditions of algal cells differed for each urea concentration. S. rubescens grew best at a urea concentration of 0.53 g/L, with bioproductivity of 9.1 g/(m2 day). When urea concentrations were 0.40, 0.27, and 0.11 g/L, the biomass of S. rubescens reached 69.8, 70.3, and 52.1 g/m2, with corresponding bioproductivities of 8.7, 8.8, and 6.5 g/(m2 day). As illustrated in Fig. 4a, biomass of S. rubescens in 0.05 and 0.03 g/L of urea were significantly less from the third day compared with those grown in other concentrations because of nitrogen deficiency. After cultivation of 8 days, biomass and bioproductivity of algae grown in 0.05 g/L urea reached 44.7 g/m2 and 5.6 g/(m2 day), respectively. Algal cells grown in 0.03 g/L urea yielded the biomass of 40.7 g/m2 and bioproductivity of 5.1 g/(m2 day). Thus, nitrogen concentration was an important component that affects microalgal growth. As nitrogen source did not satisfy normal attached growth of S. rubescens under low urea concentrations, nitrogen limitation resulted in slow increase in algal cell biomass during the middle and late phases of cultivation.
Influence of different concentrations of nitrogen sources on algal cell lipids accumulation of Scenedesmus rubescens
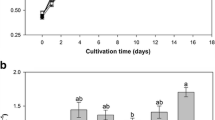
Figure 5 presented lipid accumulation with attached cultivation of S. rubescens under different urea concentrations. As shown in Fig. 5a, algal cells lipid content was of 36.9% and lipid productivity was of 1.9 g/(m2 day) with 0.03 g/L of urea. As urea concentration increased in medium, corresponding lipid contents of algal cells presented an inverse pattern, where lipid contents of algal cells totaled 34.2, 33.5, 33.4, 32.5, and 28.3% at 0.05, 0.11, 0.27, 0.40, and 0.53 g/L urea, respectively, with corresponding lipid productivities of 2.0, 2.8, 3.0, 2.8, and 2.6 g/(m2 day).
Nitrogen source presented considerable influence on lipid accumulation and its components. Presumably, lipid in microalgae may accumulate significantly under nitrogen-deficient conditions, whereas sufficient nitrogen conditions benefits growth and fission of microalgae cells but not lipid accumulation [22]. There was no observed significant difference among the three-nitrogen concentration of urea with concentration of 0.27, 0.40, and 0.53 g/L. Also, given the influence of different nitrogen sources and concentrations on attached growth and lipid accumulation of S. rubescens, this paper has selected urea at a concentration of 0.27 g/L as the optimal nitrogen source and concentration for attached cultivation of the algae.
Influence of NFTs on attached growth of Scenedesmus rubescens
According to the test, urea served as optimal nitrogen source and concentration at 0.27 g/L. Different NFTs were set in a cultivation period of 8 days. As shown in Table 1, under NFT4 and NFT6 conditions, the biomass of S. rubescens reached high levels at 75.3 and 73.8 g/m2, respectively. Under NFT2 condition, S. rubescens biomass was significantly decrease and was only of 55.4 g/m2. The bioproductivities under NFT2, NFT4, and NFT6 were 6.9, 9.4, and 9.2 g/(m2 day), respectively. This paper studied attached cultivation of S. rubescens under 0.27 g/L urea and showed that NFTs of 4 days before cultivation was optimal within 8 days of cultivation period. With the comparison between Fig. 4 and Table 1, bioproductivity of S. rubescens under 0.27 g/L urea in 8 days of total nitrogen cultivation was lower than the values under NFTs of 4 and 6 days. Some feeding strategies have been reported for fed-batch microalgal cultivation to improve biomass productivity and efficiency of carbon dioxide fixation. Soletto et al. [25] used several fed-batch protocols of urea addition to enhance biomass growth during cultivation of Spirulina platensis. Our results are consistent with the previous research. When a certain amount of urea exceeds or goes beyond that of batch mode, fed-batch mode can enhance biomass concentration. Based on economic efficiency of large-scale cultivation of S. rubescens, short NFTs can simultaneously reduce costs and provide means for industrial algae cultivation.
Conclusions
Growth rate and lipid accumulation of S. rubescens were strongly related to the nitrogen source and concentration. Scenedesmus rubescens can grow well by adhering to the cellulose acetate membrane under five kinds of nitrogen source media, namely, KNO3, urea, NaNO3, (NH4)2CO3, and NH4NO3. Among the nitrogen sources with equimolar concentrations, urea medium produced the highest lipid content and lipid productivity of algal cells at 31.1% and 2.9 g/m2 day, respectively. Various levels of initial urea concentration were used to investigate its effects on cell growth and lipid contents. When the concentration of urea was of 0.27 g/L, bioproductivity and lipid productivity from attached growth of S. rubescens reached the highest at 8.8 g/(m2 day) and 3.0 g/(m2 day), respectively. Attached cultivation of S. rubescens was optimal with urea as nitrogen source at a concentration of 0.27 g/L, whereas algal cell bioproductivity was the highest under the NFT4 model. Different NFTs not only reduced the concentration required of nitrogen, but also improved bioproductivity of microalgal cultivation.
References
Christi Y (2007) Biodiesel from microalgae. J Biotechnol Adv 25(3):294–306
Borowitzka MA, Moheimani NR (2013) Sustainable biofuels from algae. Mitig Adapt Strateg Glob Change 18:13–25
Fields MW, Hise A, Lohman EJ, Bell T, Gardner RD, Corredor L, Moll K, Peyton BM, Characklis GW, Gerlach R (2014) Sources and resources: importance of nutrients, resource allocation, and ecology in microalgal cultivation for lipid accumulation. Appl Microbiol Biotechnol 98:4805–4816
Benvenuti G, Bosma R, Cuaresma M, Janssen M, Barbosa MJ, Wijffels RH (2015) Selecting microalgae with high lipid productivity and photosynthetic activity under nitrogen starvation. J Appl Phycol 27:1425–1431
Wijffels RH, Barbosa MJ (2010) An outlook on microalgal biofuels. Science 329:796–799
Davis R, Aden A, Pienkos PT (2011) Techno-economic analysis of autotrophic microalgae for fuel production. Appl Energy 88:3524–3531
Campbell PK, Beer T, Batten D (2010) Life cycle assessment of biodiesel production from microalgae in ponds. Bioresour Technol 102:50–56
Batan L, Quinn J, Willson B, Bradley T (2010) Net energy and greenhouse gas emission evaluation of biodiesel derived from microalgae. Environ Sci Technol 44:7975–7980
Pate R, Klise G, Wu B (2011) Resource demand implications for U.S. algae biofuels production scale-up. Appl Energy 88:3377–3388
Ozkan A, Kinney K, Katz L, Berberoglu H (2012) Reduction of water and energy requirement of algae cultivation using an algae biofilm photobioreactor. Bioresour Technol 114:542–548
Uduman N, Qi Y, Danquah M, Forde G, Hoadley A (2010) Dewatering of microalgal cultures: a major bottleneck to algae-based fuels. J Renew Sustain Energy 2:23–571
Brennan L, Owende P (2010) Biofuels from microalgae—a review of technologies for production, processing and extractions of biofuels and co-products. Renew Sustain Energy Rev 14:557–577
Mcmillan JD, Chen-Glasser M, Laurens LML (2017) A perspective on renewable bioenergy from photosynthetic algae as feedstock for biofuels and bioproducts.Algae Res 24:261–264
Liu T, Wang J, Hu Q, Cheng P, Ji B, Liu J, Chen Y, Zhang W, Chen X, Chen L, Gao L, Ji C, Wang H (2013) Attached cultivation technology of microalgae for cost-affordable biomass feedstock production. Bioresour Technol 127:216–222
Wang J, Liu J, Liu T (2015) The difference in effective light penetration may explain the superiority in photosynthetic efficiency of attached cultivation over the conventional open pond for microalgae. Biotechnol Biofuels 8:1–12
Cheng P, Ji B, Gao L, Zhang W, Wang J, Liu T (2013) The growth, lipid and hydrocarbon production of Botryococcus braunii with attached cultivation. Bioresour Technol 138:95–100
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Li Y, Horsman M, Wang B, Wu N, Lan CQ (2008) Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl Microbiol Biotechnol 81:629–636
Liu ZY, Wang GC, Zhou BC (2008) Effect of iron on growth and lipid accumulation in Chlorella vulgaris. Bioresour Technol 99:4717–4722
Arumugam M, Agarwal A, Arya MC, Ahmed Z (2013) Influence of nitrogen sources on biomass productivity of microalgae Scenedesmus bijugatus. Bioresour Technol 131:246–249
Hsieh CH, Wu WT (2009) Cultivation of microalgae for oil production with a cultivation strategy of urea limitation. Bioresour Technol 100:3921–3926
Lin Q, Lin J (2011) Effects of nitrogen resource and concentration on biomass and oil production of a Scenedesmus rubescens like microalga. Bioresour Technol 102(2):1615–1621
Takagi M, Watanabe K, Yamaberi K, Yoshida T (2000) Limited feeding of potassium nitrate for intracellular lipid and triglyceride accumulation of Nannochloris sp. UTEX LB1999. Appl Microbiol Biot 54(1):112–117
Rincon SM, Romero HM, Aframehr WM, Beyenal H (2017) Biomass production in Chlorella vulgaris biofilm cultivated under mixotrophic growth conditions. Algal Res 26:153–160
Soletto D, Binaghi L, Lodi A, Carvalho JCM, Converti A (2005) Batch and fedbatch cultivation of Spirulina platensis using ammonium sulphate and urea as nitrogen sources. Aquaculture 243:217–224
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31560724), the Natural Science Foundation of Jiangxi Province (20171BAB214014), China Postdoctoral Science Foundation (2017T100583, 2016M600616), Natural Science and Technology Major Special Program of China (2014ZX07104-005-02) and Collaborative Innovation Center for Major Ecological Security Issues of Jiangxi Province and Monitoring Implementation (JXS-EW-00).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that there is no competing interest.
Rights and permissions
About this article
Cite this article
Cheng, P., Wang, Y., Osei-Wusu, D. et al. Development of nitrogen supply strategy for Scenedesmus rubescens attached cultivation toward growth and lipid accumulation. Bioprocess Biosyst Eng 41, 435–442 (2018). https://doi.org/10.1007/s00449-017-1877-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-017-1877-9