Abstract
The use of commercial-grade nutrients such as agricultural fertilizers is important for commercial microalgae cultivation, and this is particularly the case for biofuel production which is associated with low added value. Nitrogen is a very important macronutrient in microalgae cultivation, and ammonium sources are cheaper than nitrate sources. However, the growth response and cellular composition can be altered by the different nutrient sources. In the study reported here, we investigated the effects of different ammonium doses and commercial-grade macronutrients from agricultural fertilizers on the growth of Scenedesmus sp. BR003, a promising genus for biofuel production. Five growth media were developed using fertilizers and evaluated during Scenedesmus sp. cultivation under autotrophic conditions. The growth media differed in terms of their composition and concentration of macronutrients. We found that all commercial-grade media supported equal or higher cell concentrations, dry weight, water-soluble proteins, neutral carbohydrates, and total lipid production compared to the conventional BG11 medium. However, the commercial-grade growth medium with the highest ammonium content affected the coenobium pattern of Scenedesmus sp. BR003. Commercial-grade nutrient sources were a low-cost alternative to improve the growth of Scenedesmus sp. BR003. The different fertilizers also allowed for manipulation of microalgae chemical composition and phenotypic plasticity to target traits of commercial interest. Our results demonstrate the potential of using ammonium from agricultural fertilizers as a nitrogen source in combination with other commercial-grade macronutrients sources. In addition, this work demonstrates the ability of a robust Scenedesmus strain to grow in media of different compositions, even when a high dosage of ammonium was used.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Depletion of fossil fuel sources and the environmental issues associated with their use has motivated studies to identify alternative fuels, such as biodiesel produced from microalgae. In comparison with oleaginous plants, microalgae have the potential to achieve higher oil production (Moazami et al. 2012), lower water consumption (Wijffels and Barbosa 2010), and higher CO2 assimilation capacities, ultimately contributing to a reduction in greenhouse gas emissions (Wang et al. 2008). Moreover, microalgae may also be grown in areas that are unsuitable for agriculture (Williams and Laurens 2010), reducing the potential for conflict between the production of food and the production of biofuels.
Microalgae species with high lipid yield have been studied for biodiesel synthesis (Chen et al. 2012). However, in order to make microalgae biodiesel production commercially viable and competitive with fossil fuels, the costs associated with its production must be reduced (Campbell et al. 2011). A promising alternative to reduce the costs of microalgae cultivation is the replacement of high-cost analytical-grade nutrients with low-cost commercial grade fertilizers (Raoof et al. 2006). Indeed, the source of nutrients used in growth medium formulations represents 30–35% of the total material costs needed for biomass production (Molina Grima et al. 2003; Jaramillo et al. 2012). Since agricultural fertilizers are widely commercialized, generally available at low prices and have many alternative compositions, they could be used to prepare growth media for microalgae cultivation at lower cost. Previous studies have also demonstrated that growth media formulated from fertilizers can support algal biomass productions similar to those generated from media formulated with analytical-grade materials (Valenzuela-Espinoza et al. 2002; Raoof et al. 2006; Guzmán-Murillo et al. 2007).
Nitrogen (N) is a very important macronutrient in microalgae cultivation and, in general, fertilizers containing ammonium are cheaper than those using nitrate. However, ammonium is less tolerated by microalgae than nitrate, especially when free ammonia (NH3) is present. The concentration of NH3 is < 1% at a pH around 7, but it can achieve equilibrium with the ionic form (NH4 +) at pH 9.3 (Emerson et al. 1975). Maintaining a lower pH is critical to avoid ammonia stripping (Li et al. 2011) and growth inhibition effects (Azov and Goldman 1982). Commercial-grade ammonium has already been used in microalgae cultivation at low concentrations (5–300 mg L−1) in combination with a few (e.g. 2–3) other commercial-grade nutrient sources (Guzmán-Murillo et al. 2007). However, the development of a robust and low-cost microalgae cultivation system demands the substitution of all the analytical-grade macronutrients by commercial-grade nutrients. One of the challenges in the use of fertilizers for microalgae cultivation is the proper growth media formulation because the fertilizers are developed according to the nutritional demands of vascular plants.
The Scenedesmus genus is considered to be a promising microalga for biofuel production because this algal species has a high growth rate and lipid production, exhibits resistance to elevated carbon dioxide (CO2) and ammonium concentrations, and also has an adequate fatty acid profile for biodiesel synthesis (Gouveia and Oliveira 2009; Mandal and Mallick 2009; Yoo et al. 2010). Previous studies have shown the robustness of Scenedesmus strains to grow in the presence of different ammonium and nitrate sources, such as NH4CH3CO2, (NH4)2HPO4, (NH4)2CO3, NH4NO3, and NaNO3 (Chandra et al. 2016). However, knowledge of Scenedesmus cultivation using commercial-grade ammonium sources is important for further biotechnological applications. For example, the Scenedesmus genus has the ability to build colonies of four to eight cells as an anti-grazing response (Lürling 2011). This phenotype seems to be desirable in open cultivation systems, such as raceway ponds, but the colonial pattern of Scenedesmus can be affected by the ammonium concentration (Trainor and Roskosky 1967). Interestingly, there is a lack of studies in the recent literature reporting the use and dosage of ammonium-like fertilizers for Scenedesmus cultivation in large scale. Therefore, the aim of our study was to investigate the replacement of all analytical-grade macronutrients by commercial-grade nutrients from agricultural fertilizers on Scenedesmus sp. BR003 cultivation, by evaluating its growth, biomass composition, coenobia pattern, and cell dimensions.
Material and methods
Production of alternative growth media
Alternative growth media were formulated using seven different fertilizers selected according to the purity of the nutrient sources so as to provide all the macronutrients present in conventional BG11 medium. The compositions of the different water-soluble fertilizers were provided by the manufacturers and are presented in Table 1. The BG11 growth medium (Andersen 2005) was used as the control in the experiment.
Five different fertilizer-based growth media (B1, B2, B3, B4, and B5) were formulated as sources of N, phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), and iron (Fe) (Table 2). The ammonium concentrations in growth media B1, B2, B3, and B4 were 0.3189, 0.1063, 0.2472, and 0.0824 g L−1, respectively. The Ca source of growth media B3 and B4 contains nitrate; therefore, the ammonium dosage used in those growth media was slightly reduced to obtain the same dosage of total nitrogen (ammonium and nitrate) as in growth media B1 and B2, respectively. Growth media B1 and B3 were formulated to mimic the conventional medium, BG11, while B2 and B4 were developed to contain one-third of the N concentration of BG11. Growth medium B5 was formulated with a nitrate source (Ca nitrate) to compare the effect of different N sources in microalgae growth. Micronutrients were added from analytical-grade chemicals at equal concentrations to those of the BG11.
Strain and inocula production
The monospecific Scenedesmus sp. BR003 strain was obtained from the Collection of Cyanobacteria and Microalgae of the Plant Science Department, Universidade Federal de Viçosa (Minas Gerais, Brazil).
The strain was inoculated in 50-mL flasks containing BG11 medium at an initial pH 7.4 ± 0.1 and maintained under photoautotrophic growth conditions at a temperature of 25 ± 2 °C, 16/8 h photoperiod (light/dark cycle), and irradiance at bench height of 60 μmol photons m−2 s−1 provided by two 40-watt daylight fluorescent lamps. When the inoculum reached a concentration of between 106 and 107 cells mL−1, it was transferred to larger flasks, and the volume was made up to 2 L with BG11. The cultivation then received constant aeration by means of a diaphragm pump. The contents of each flask of cultivated algae were transferred to a 9-L flask when a concentration of 107 cells mL−1 was reached, and the volume was made up to 8 L with BG11; this culture was maintained for 16 days. A diaphragm pump was used to provide mixing for the cultivated algae in the flasks. All solutions and materials used to produce the inoculum and growth medium were sterilized by autoclaving.
Growth conditions and growth measurements
Cultivations were carried out under photoautotrophic growth conditions at a temperature of 30 ± 2 °C, a photoperiod of 16/8 h (light/dark cycle), and irradiance at bench height of 110 μmol photons m−2 s−1 provided by four 40-watt daylight fluorescent lamps. Although the temperature and light intensity were higher than that used for inocula production, the growth performance was not affected due to the culture density. The cultivations were carried out in 2-L flasks, with each flask containing 1.52 L of growth medium and 0.38 L of inoculum (20% v v−1 of inoculum). The cells from the inoculum were settled by centrifugation at 3000 g and washed twice to remove traces nutrients from the BG11 medium. The final cellular concentration of the Scenedesmus sp. BR003 inoculum was 2.17 × 107 cells mL−1. Culture mixing was carried out by injecting atmospheric air into each flask at 0.2 vvm (air volume per medium volume per minute), supplied by a 2-HP compressor. The air was enriched with 5% CO2 by volume and monitored daily by a CO2 analyzer (model GFM 130; Gas Data, Coventry, UK). The enriched air was filtered through a 0.22-μm filter to avoid contamination. All glassware and growth media were autoclaved except for the commercial ammonium-based fertilizers which were added after sterilization to avoid ammonia volatilization.
The pH of the growth media was adjusted daily and maintained at a pH of between 6.5 and 7.0 by the addition of 1 M NaOH or HCl solutions. Cultivations were carried out for 16 days, and a constant volume was maintained by the addition of sterile deionized water.
Growth curves were obtained by cell counting using an improved Neubauer hemocytometer (Laboroptik Ltd., Lancing, UK). Samples were collected in duplicate for each biological repetition, then fixed and diluted in 10% (v v−1) buffered neutral formalin/0.05 M potassium phosphate buffer (pH 6.8). All samples were conditioned in the dark at room temperature after the fixation step.
At the end of the experiment, 40 mL of each culture was collected and centrifuged at 18,500 g for 10 min at 25 °C (Heraeus Multifuge X1; Fisher Scientific GmbH, Schwerte, Germany). The pellet was washed twice and dried at 75 °C to achieve a constant dry weight for determining algal biomass production in the different growth media.
Algal biomass composition
The cultures were harvested on day 16 for determination of the protein, carbohydrate, lipid, and pigment (chlorophylls a and b and total carotenoids) content. Aliquots of the culture samples were harvested and either immediately used for pigment determination or maintained at −20 °C for quantification of first the total water-soluble protein content and then the total neutral carbohydrate content. Another part of the culture was centrifuged at 18,500 g for 10 min at 25 °C, washed twice, and lyophilized for total lipid quantification.
Quantification of chlorophylls a and b and total carotenoid contents was carried out at according to methodology proposed in Griffiths et al. (2011). Absorbance of the methanolic extract was measured at wavelengths of 665, 652, and 470 nm in a microplate reader (Asys UVM 340; Biochrom Ltd., Cambridge, UK). The procedure was performed in the dark to avoid pigment oxidation. The equations proposed in Wellburn (1994) were used to calculate chlorophylls a and b and total carotenoid concentrations.
Extraction of total water-soluble proteins was carried out according to Meijer and Wijffels (1998), and quantification was performed by Lowry’s method, as adapted by Lucarini and Kilikian (1999). Bovine serum albumin (Sigma-Aldrich, St. Louis, MO) was used to prepare the standard curve in the range of 4–1200 μg mL−1 (R 2 = 0.9806). The extraction of intracellular carbohydrates was performed according to Teoh et al. (2005), and quantification was performed by the Dubois method, as adapted by Masuko et al. (2005), which uses dextrose (Sigma-Aldrich) to prepare the standard curve in the range of 4–250 μg mL−1 (R 2 = 0.9877). Samples were diluted with deionized water when necessary to bring the concentrations to within the range of the standard curves for the protein and sugar analysis.
Total lipid extraction was carried out using methanol and chloroform (Smedes and Thomasen 1996; Izard and Limberger 2003) and carried out by the sulfo-phospho-vanillin method (Izard and Limberger 2003). Corn oil was used to prepare the standard curve to a range of 80–800 μg mL−1 (R 2 = 0.96), as it has a fatty acid profile similar to that of microalgal oil, according to the method described in Cheng et al. (2011). All analyses were carried out in triplicate for each treatment.
Characterization of phenotypic plasticity
Photomicrographs were obtained using an inverted microscope (CKX41; Olympus Corp. Tokyo,, Japan) coupled with an image capture system (SC30; Olympus Corp.). Cellular measures and coenobia pattern analyses were carried out from photomicrographs using AxioVision 4.8 (Carl Zeiss Imaging Solutions, Carl Zeiss, Wetzlar, Germany). Cell lengths were obtained by measuring the distance of the longest axis of the cell. Cell widths were obtained by measuring the medium region, perpendicular to the longest cell axis. For all cellular measurements, 30 cells were randomly selected. Twenty cells were randomly counted to determine coenobium pattern analysis in the different growth media.
Statistical analysis
The experiment was performed in a completely randomized factorial delineation, where the cultivation of Scenedesmus sp. BR003 was evaluated in six growth media with three replicates, resulting in 18 experimental units. The results were submitted to analysis of variance (ANOVA), and means were compared by Tukey’s test at 5% significance level. ANOVA, Tukey’s test and the Pearson coefficient were carried out using SAS software version 9.2 (SAS Institute, Cary, NC). Except when specified otherwise, the results of this study are presented as mean ± standard deviation.
Results
Growth performance
Scenedesmus sp. BR003 was cultivated in different growth media composed of agricultural fertilizers as a low-cost alternative to the analytical-grade nutrients. Growth media B1, B3, and B5 were formulated to mimic the conventional BG11 cultivation medium, while B2 and B4 contained one-third of the N dosage of BG11 (Table 1). BG11 medium has a high concentration of N compared with the other growth media used in the cultivation of Scenedesmus, such as Bold’s Basal Medium (Gardner et al. 2011) and Daigo IMK (Matsunaga et al. 2009). Therefore, low N dosages were investigated to observe possible inhibitory effects of ammonium on the cultivation of microalgae. The other macronutrients (P, K, Ca, Mg, and Fe) used in this study were also from commercial-grade nutrients (Table 1).
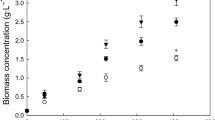
A typical growth profile without a lag phase was observed in the different growth media, indicating the robustness of Scenedesmus sp. BR003 to different nutritional conditions (Fig. 1a). The cultures reached the stationary phase between days 10 and 13 of cultivation for all media (Fig. 1a).
Growth curves (a) and dry weight (b) after 16 days of Scenedesmus sp. BR003 cultivation in conventional BG11 medium and in different fertilizer-based growth media (B1–B5). Solid circle BG11, open circle B1, inverted solid arrowhead B2, open triangle B3, solid square B4, open square B5. Data are the mean with the standard error (SE). Bars with different lowercase letters differ by Tukey’s test at the 5% significance level
Higher biomass (cell concentration and dry weight) production was achieved in the alternative media than in the BG11 medium. The cell concentrations of Scenedemus sp. BR003 were higher in all fertilizer-based growth media than in the BG11 medium (Fig. 1a). The growth media B1 (7.3 ± 0.9 × 107 cells mL−1) and B4 (7.1 ± 1.1 × 107 cells mL−1) showed the highest concentrations of cells on day 16 of cultivation (Fig. 1a). A higher dry weight was observed in B5 (1.7 ± 0.1 g L−1) compared to the growth in the BG11 (1.1 ± 0.01 g L−1) and B3 (1.3 ± 0.1 g L−1) media (Fig. 1b). Intermediate dry weight values were observed in growth media B1, B2, and B4 (Fig. 1b).
Algal biomass composition
The different media also resulted in a different cellular composition of Senedesmus sp. BR003. A higher level of protein was achieved in growth medium B1 (558.4 ± 23.1 mg L−1) than in BG11 (469.6 ± 26.3 mg L−1) and B2 (487.2 ± 12.9 mg L−1) (Fig. 2a). The highest level of total neutral carbohydrates (287.1 ± 55.1 mg L−1) was observed in cells grown in B2 medium, and it was significantly greater than the levels in cells grown in B1 (196.0 ± 33.3 mg L−1) and B4 (168.1 ± 36.5 mg L−1) media.
Contents of total water-soluble proteins (a), total neutral carbohydrates (b), total lipids (c), and pigments (d) in Scenedesmus sp. BR003 cultivated in different fertilizer-based growth media on the day 16 of cultivation. Pigments in d: chlorophyll a (black bar), chlorophyll b (dark-gray bar), total carotenoids (light-gray bar), chlorophyll a/b ratio (white bar). Data are the mean with the SE. Bars with different lowercase letters differ by Tukey’s test at the 5% significance level
A more expressive difference was observed for lipid production by Scenedesmus sp. BR003. Higher total lipid concentrations were obtained for all fertilizer-based growth media in comparison to the BG11 (Fig. 2c). This difference was statistically significant in the case of B4 (267.1 ± 64.1 mg L−1) and B5 (271.5 ± 17.6 mg L−1) media (Fig. 2c), with these growth media showing an increase of > 70% in total lipid production compared to the BG11 medium.
The different growth media also changed the level microalgal chlorophyll a, but no differences were observed for chlorophyll b, the chlorophyll a/b ratio, and carotenoid concentrations (Fig. 2d). A significantly higher production of chlorophyll a was observed in growth medium B1 (30.4 ± 4.6 mg L−1) compared to B3 (22.5 ± 1.3 mg L−1) (Fig. 2d).
Cell dimensions and phenotypic plasticity
Differences in cell length were observed for Scenedesmus sp. BR003 cultivated in the different growth media, but no differences were observed in cell width (Fig. 3a). Greater cell length was observed in growth medium BG11 (11.0 ± 0.6 μm) than in B1 (9.3 ± 0.3 μm) and B3 (8.9 ± 0.6 μm) media.
Cellular dimensions (a) and proportion of individual cells and coenobia with different quantities of Scenedesmus sp. BR003 cells in different growth media (b), on day 16. a Cellular dimensions: length (black bar), width (shaded bar). bNumber of cells: 1 (black shading), 2 (dark-gray shading), 3 (intermediate gray shading), 4 (light-gray shading), 5 (white, no shading). Data are the mean with the SE. Bars with different lowercase letters differ by Tukey’s test at the 5% significance level
The use of alternative growth media also influenced coenobium formation (Figs. 3b and 4). A lower number of cells per coenobium was observed in growth media B1 and B3, while coenobia containing four cells were more abundant in the BG11, B4, and B5 media. Coenobia containing eight cells were observed only in B4 medium. There were no differences between growth media in terms of coenobia formation containing two and three cells.
Discussion
Growth performance
The results of this study show that the substitution of all analytical-grade macronutrients by commercial-grade nutrients is a feasible and low-cost alternative to the cultivation of the genus Scenedesmus. Scenedesmus sp. BR003 cultivated in the fertilizer-based growth media, even those media formulated with ammonium, presented a similar or higher dry weight and final cell number as Scenedesmus sp. BR003 cultivated in the analytical-grade growth medium BG11.
The fertilizer-based media did not promote any detectable negative effects on Scenedesmus sp. BR003 growth, as indicated by the absence of an adaptation phase at the beginning of the cultivation period for all growth media tested (Fig. 1a). The growth rate remained elevated (log phase) up to day 10 of cultivation for all growth media, but the cultivations were maintained until day 16 due the tendency of microalgae to accumulate lipids in the stationary phase (Hu et al. 2008). The fast cell division rates observed during the first days of cultivation were probably due to the optimal growth conditions because at the beginning of cultivation, the biomass concentration is low, and there are no limitation in nutrients and light (Griffiths et al. 2011). A fast growth rate was also observed in a previous study using growth medium BG11 containing different N sources (nitrate, ammonium, and urea; Xin et al. 2010).
The dry weight results (Fig. 1b) corroborate the final cell concentration measurements (Fig. 1a). The increase in the number of cells showed a positive Pearson coefficient with the dry weight (ρ = 0.639). However, the length and width of the cells showed no correlation with the increasing dry weight (ρ = − 0.224 and − 0.14, respectively). The cell dimensions were slightly affected by the different growth media (Fig. 3a). The dry weight concentrations obtained in growth media B1, B2, B4, and B5, were similar to the values reported in the literature under similar cultivation conditions using growth medium N11 (Mandal and Mallick 2009) and a modified formulation of the BG11 medium (Tang et al. 2011). To the contrary, a lower biomass production was observed during the cultivation of S. obtusus using the Bold’s Basal Medium (Chandra et al. 2016).
In an earlier study, Scenedesmus genus presented a higher initial growth rate when cultivated in ammonium compared to other N sources, but ammonium assimilation by the microalgae ultimately acidified the culture and affected the final biomass production (Xin et al. 2010). In our study, we carried out a pH correction daily to avoid excessive acidification (fertilizers with ammonium) or alkalinization (fertilizers with nitrate) of the cultures.
The considerable reduction of the ammonium dosage in growth media B2 and B4 to one-third that in growth media B1–B3 had little effect on the growth pattern and biomass production when compared with treatments B1 and B3 (Fig. 1a, b). Those results clearly indicate that the B2 and B4 media had sufficient N to support growth of the microalgae in the conditions used in this study. Reduction and optimization of the N dosage is important for the commercial culture of microalgae because N is the main nutrient in many growth media. In addition, ammonium sources are cheaper than nitrate sources; consequently, growth media B2 and B4 containing ammonium fit the industrial demand for low-cost growth medium better than the growth medium B5 containing nitrate (Table 2). In another study, a reduction in the ammonium dosage (N starvation condition) resulted in a lower biomass production by Scenedesmus rubescens cultivated in raceway ponds (Lin and Lin 2011).
Algal biomass composition
Proteins, carbohydrates and lipids are considered to be the three main biochemical components in microalgae cells. Protein production by Scenedesmus sp. BR003 was marginally affected by the different media (Fig. 2a). The quantity of N provided was sufficient to enable protein synthesis and microalgal growth, even in the low-N growth media B2 and B4 (Fig. 2a). Cultivation of Scenedesmus at different temperatures (i.e. 10–30 °C) also showed that temperature had minimal effects on protein accumulation (Lu et al. 2017). Scenedesmus is a protein-rich genus, and proteins correspond to 30–50% of the biomass (Teuling et al. 2017). Protein levels were higher than carbohydrate and lipid levels (Fig. 2a–c). These results strongly suggest that Scenedesmus genus is a robust and promising alternative for the production of proteins and amino acids using inorganic N sources. However, despite Scenedesmus being considered a potential food supplement, this genus is not yet produced on a commercial scale (Kumar et al. 2015).
Lipid production was greatly affected by the composition of the growth media compared to the other cellular components. There was a higher production of lipids when Scenedesmus sp. BR003 was grown in growth media B4 and B5 than when grown in the BG11 medium (Fig. 2c). Previous work has demonstrated that alterations in cultivation conditions, such as different N sources (Chandra et al. 2016), pH (Gardner et al. 2011), and P (Mandal and Mallick 2009) and CO2 concentrations (Ho et al. 2010) increase lipid accumulation by Scenedesmus strains. Our results show that growth medium B4 is a promising alternative for microalgae cultivation as a raw material for biodiesel production. Lipid levels were similar or higher than previous lipid production obtained from the cultivation of Scenedesmus using analytical-grade nutrients and similar growth conditions (Ho et al. 2010; Griffiths et al. 2011; Tang et al. 2011; Chandra et al. 2016). The Scenedemus lipid profile has already been described in detail in previous studies, with the main fatty acids being palmitic acid (16:0), oleic acid (18:1), and linolenic acid (18:3) (Gouveia and Oliveira 2009; Mandal and Mallick 2009; Yoo et al. 2010). The fatty acid profile of the Scenedesmus genus is more suited for biodiesel synthesis than those of other microalgae (Gouveia and Oliveira 2009). In other studies in which fertilizers were used, little or no differences were reported in the microalgae cellular contents (Valenzuela-Espinoza et al. 2002; Pacheco-Vega and Sánchez-Saavedra 2009).
N starvation conditions have also been used to induce lipid synthesis in Scenedesmus (Griffiths et al. 2011; Lin and Lin 2011; Ho et al. 2012). Upon depletion of the N in the growth medium, microalgae cease to synthesize nitrogen-rich molecules (e.g., proteins and nucleic acids) but continue to synthesize lipid and carbohydrates (Williams and Laurens 2010). The growth media with low-N concentrations used in this study, B2 and B4, did not differ from the other media in terms of biomass and lipid production (Figs. 1a, b, 2c) and also did not result in N-starvation conditions, as observed in the aforementioned studies. Growth media B2 and B4, containing one-third of the N dosage of the BG11 medium, were formulated to evaluate possible inhibitory or negative effects of the fertilizers on the cultivation of Scenedesmus sp. BR003. Therefore, the higher levels of lipids in the fertilizer-based growth media (Fig. 3c) are due to the higher total biomass production rather than changes in cell composition by N-starvation conditions. The cultivation of microalgae under N-starvation conditions triggers lipid accumulation, but total biomass production is greatly affected (Mandal and Mallick 2009).
Carbohydrates were present at lower levels than proteins and lipids when the Scenedesmus sp. BR003 was cultivated in growth medium B4 (Fig. 2b, c). The main sugars present in Scenedesmus are glucose and mannose (Teuling et al. 2017), and such hexoses can be easily fermented in bioethanol. Previous studies also showed that the carbohydrates are present at lower levels compared with proteins and lipids (Chandra et al. 2016; Teuling et al. 2017).
Pigments were also present at lower levels, in contrast with proteins and lipids (Fig. 2a–d). The levels of chlorophylls a and b and carotenoids were slightly affected by the different growth media and N sources (Fig. 2d). These results are similar to those observed in the cultivation of S. obtusus with NaNO3, (NH4)2HPO4 and NH4NO3; however, in that study the use of NH4CH3CO2, as N and C source resulted in a higher production of biomass, lipids, chlorophyll, and carotenoids (Chandra et al. 2016). A study by Lin et al. (2012) showed that the production of chlorophyll a by S. rubescens increased when chelated Fe was used instead of a non-chelated Fe source. The same study showed that a high dosage of Fe affected biomass production, and as a consequence, a lower chlorophyll a level was observed (Lin et al. 2012). The minimal difference in chlorophyll a observed in our study can also be partially correlated to the biomass production (ρ = 0.575) (Figs. 1b, 2d). However, the same dosage of chelated Fe was used in all the growth media (Table 1), suggesting that other nutrients sources affected the biomass and chlorophyll a production.
Cell dimensions and phenotypic plasticity
The different growth media also affected the cell dimensions and the phenotypic plasticity of Scenedesmus sp. BR003. Cells with a small length were observed in those growth media with a higher concentration of ammonium-based fertilizers, i.e., B1 and B3 media (Fig. 3a). In contrast, an inverse relation between cell length and P concentration has previously been observed in the cultivation of Scenedesmus obliquus (Chen et al. 2011). However, a clear negative effect of P on cell length was not observed in our study. Growth media B3 and B4 contained a level of P that is 66- and 22-fold higher, respectively, than that in BG11, while cells of a similar mean length were still observed in the BG11 and B4 growth medium (Fig. 3a). Growth media B3 and B4 were formulated with ammonium monophosphate as the main source of N and P (Table 1). Therefore, the inevitable high dosage of P was due to the high demand for N from the ammonium monophosphate to mimic the BG11 medium. Griffiths et al. (2011) did not observe any differences in cell length when Scenedesmus sp. was cultivated at different doses of NO3. The NO3 growth media used in our study (BG11 and B5) resulted in a similar cell length, reinforcing the notion that the high ammonium levels of growth media B1 and B3 affected cell length (Fig. 3a).
The genus Scenedesmus is characterized by coenobia formation of four to eight or even more cells; however, it is possible to observe unicellular forms under certain conditions (Peña-Castro et al. 2004). Herbivorous zooplankton are considered one of the main selective forces for inducing the formation of larger colonies, as larger colonies are considered to be a defense form against these predators (Lürling 2011). Cultivation conditions also can influence coenobium formation, as demonstrated in our study and other studies (Trainor and Roskosky 1967; Peña-Castro et al. 2004; Liu et al. 2010). In our study, the growth media with higher concentrations of ammonium, B1 and B3 (Table 2), greatly affected coenobium patterns of Scenedesmus sp. BR003 (Figs. 3b, 4) and led to an increased numbers of individual cells. Those results corroborate previous studies in demonstrating that higher numbers of individual Scenedesmus cells are produced in the presence of ammonium (Trainor and Roskosky 1967) and suggest an inhibitory effect of this N source. The high number of individual cells observed in growth media B1 and B3 is not desirable because colonies with more Scenedesmus cells are easier to consolidate (Lürling 2003), facilitating biomass recovery. This process of biomass recovery represents a significant cost in biotechnological applications of microalgae (Molina Grima et al. 2003). Therefore, optimization of the ammonium concentration in B4 medium resulted in a lower load of fertilizer and avoided a culture with a high number of individual cells (Fig. 3).
Conclusions
In conclusion, in this study tested five novel fertilizer-based growth media and observed that these presented equal or better results for the cultivation of Scenedesmus sp. BR003 than those obtained with the conventional BG11 medium, while showing the effect of different ammonium doses on the phenotypic plasticity. The different compositions and concentrations of fertilizers also led to alterations in the biochemical composition of the microalgae. Very noteworthy were increases in lipid production of over 70% when fertilizer-based media were used. The replacement of nitrate by low-cost ammonium fertilizers is therefore possible, and it is a promising alternative for the commercial cultivation of Scenedesmus strains. Growth media B4, containing ammonium, presented more lipid production and a larger number of coenobia. The latter is an optimal condition for final biomass harvesting and may increase the resistance to zooplanktonic predation, which can occur in open cultivation systems. A lower initial concentration of ammonium allowed for a reduction in the nutrient concentration and also resulted in a cultivation system that was more adequate for harvest due the higher number of coenobia. The results of this study reinforce the viability of fertilizers as a source of macronutrients and emphasize the need for further studies aiming to better understand the growth of microalgae in media containing agricultural fertilizers.
References
Andersen RA (2005) Algal culturing techniques, 1st edn. Elsevier Inc., Amsterdam
Azov Y, Goldman JC (1982) Free ammonia inhibition of algal photosynthesis in intensive cultures. Appl Environ Microbiol 43:735–739
Campbell PK, Beer T, Batten D (2011) Life cycle assessment of biodiesel production from microalgae in ponds. Bioresour Technol 102:50–56. https://doi.org/10.1016/j.biortech.2010.06.048
Chandra TS, Deepak RS, Maneesh Kumar M et al (2016) Evaluation of indigenous fresh water microalga Scenedesmus obtusus for feed and fuel applications: effect of carbon dioxide, light and nutrient sources on growth and biochemical characteristics. Bioresour Technol 207:430–439. https://doi.org/10.1016/j.biortech.2016.01.044
Chen M, Li J, Dai X et al (2011) Effect of phosphorus and temperature on chlorophyll a contents and cell sizes of Scenedesmus obliquus and Microcystis aeruginosa. Limnology 12:187–192. https://doi.org/10.1007/s10201-010-0336-y
Chen YH, Huang BY, Chiang TH, Tang TC (2012) Fuel properties of microalgae (Chlorella protothecoides) oil biodiesel and its blends with petroleum diesel. Fuel 94:270–273. https://doi.org/10.1016/j.fuel.2011.11.031
Cheng YS, Zheng Y, VanderGheynst JS (2011) Rapid quantitative analysis of lipids using a colorimetric method in a microplate format. Lipids 46:95–103. https://doi.org/10.1007/s11745-010-3494-0
Emerson K, Russo RC, Lund RE, Thurston RV (1975) Aqueous ammonia equilibrium calculations: effect of pH and temperature. J Fish Res Board Can 32:2379–2383. https://doi.org/10.1139/f75-274
Gardner R, Peters P, Peyton B, Cooksey KE (2011) Medium pH and nitrate concentration effects on accumulation of triacylglycerol in two members of the chlorophyta. J Appl Phycol 23:1005–1016. https://doi.org/10.1007/s10811-010-9633-4
Gouveia L, Oliveira AC (2009) Microalgae as a raw material for biofuels production. J Ind Microbiol Biotechnol 36:269–274. https://doi.org/10.1007/s10295-008-0495-6
Griffiths MJ, Garcin C, van Hille RP, Harrison STL (2011) Interference by pigment in the estimation of microalgal biomass concentration by optical density. J Microbiol Methods 85:119–123. https://doi.org/10.1016/j.mimet.2011.02.005
Guzmán-Murillo MA, López-Bolaños CC, Ledesma-Verdejo T et al (2007) Effects of fertilizer-based culture media on the production of exocellular polysaccharides and cellular superoxide dismutase by Phaeodactylum tricornutum (Bohlin). J Appl Phycol 19:33–41. https://doi.org/10.1007/s10811-006-9108-9
Ho SH, Chen WM, Chang JS (2010) Scenedesmus obliquus CNW-N as a potential candidate for CO2 mitigation and biodiesel production. Bioresour Technol 101:8725–8730. https://doi.org/10.1016/j.biortech.2010.06.112
Ho SH, Chen CY, Chang JS (2012) Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW-N. Bioresour Technol 113:244–252. https://doi.org/10.1016/j.biortech.2011.11.133
Hu Q, Sommerfeld M, Jarvis E et al (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–639. https://doi.org/10.1111/j.1365-313X.2008.03492.x
Izard J, Limberger RJ (2003) Rapid screening method for quantitation of bacterial cell lipids from whole cells. J Microbiol Methods 55:411–418. https://doi.org/10.1016/S0167-7012(03)00193-3
Jaramillo JJ, Naranjo JM, Cardona CA (2012) Growth and oil extraction from Chlorella vulgaris: a techno-economic and environmental assessment. Ind Eng Chem Res 51:10503–10508. https://doi.org/10.1021/ie300207x
Kumar SA, Magnusson M, Ward LC et al (2015) A green algae mixture of Scenedesmus and Schroederiella attenuates obesity-linked metabolic syndrome in rats. Nutrients 7:2771–2787. https://doi.org/10.3390/nu7042771
Li Y, Chen Y-F, Chen P et al (2011) Characterization of a microalga Chlorella sp. well adapted to highly concentrated municipal wastewater for nutrient removal and biodiesel production. Bioresour Technol 102:5138–5144. https://doi.org/10.1016/j.biortech.2011.01.091
Lin Q, Lin J (2011) Effects of nitrogen source and concentration on biomass and oil production of a Scenedesmus rubescens like microalga. Bioresour Technol 102:1615–1621. https://doi.org/10.1016/j.biortech.2010.09.008
Lin Q, Gu N, Lin J (2012) Effect of ferric ion on nitrogen consumption, biomass and oil accumulation of a Scenedesmus rubescens-like microalga. Bioresour Technol 112:242–247. https://doi.org/10.1016/j.biortech.2012.02.097
Liu Y, Wang W, Zhang M et al (2010) PSII-efficiency, polysaccharide production, and phenotypic plasticity of Scenedesmus obliquus in response to changes in metabolic carbon flux. Biochem Syst Ecol 38:292–299. https://doi.org/10.1016/j.bse.2010.02.003
Lu Q, Li J, Wang J et al (2017) Exploration of a mechanism for the production of highly unsaturated fatty acids in Scenedesmus sp. at low temperature grown on oil crop residue based medium. Bioresour Technol 244:542–551. https://doi.org/10.1016/j.biortech.2017.08.005
Lucarini AC, Kilikian BV (1999) Comparative study of Lowry and Bradford methods: interfering substances. Biotechnol Tech 13:149–154. https://doi.org/10.1023/A:1008995609027
Lürling M (2003) Phenotypic plasticity in the green algae Desmodesmus and Scenedesmus with special reference to the induction of defensive morphology. Ann Limnol Int J Limnol 39:85–101. https://doi.org/10.1051/limn/2003014
Lürling M (2011) Metribuzin impairs the unicell-colony transformation in the green alga Scenedesmus obliquus. Chemosphere 82:411–417. https://doi.org/10.1016/j.chemosphere.2010.09.070
Mandal S, Mallick N (2009) Microalga Scenedesmus obliquus as a potential source for biodiesel production. Appl Microbiol Biotechnol 84:281–291. https://doi.org/10.1007/s00253-009-1935-6
Masuko T, Minami A, Iwasaki N et al (2005) Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Anal Biochem 339:69–72. https://doi.org/10.1016/j.ab.2004.12.001
Matsunaga T, Matsumoto M, Maeda Y et al (2009) Characterization of marine microalga, Scenedesmus sp. strain JPCC GA0024 toward biofuel production. Biotechnol Lett 31:1367–1372. https://doi.org/10.1007/s10529-009-0029-y
Meijer EA, Wijffels RH (1998) Development of a fast, reproducible and effective method for the extraction and quantification of proteins of micro-algae. Biotechnol Tech 12:353–358. https://doi.org/10.1023/A:1008814128995
Moazami N, Ashori A, Ranjbar R et al (2012) Large-scale biodiesel production using microalgae biomass of Nannochloropsis. Biomass Bioenergy 39:449–453. https://doi.org/10.1016/j.biombioe.2012.01.046
Molina Grima E, Belarbi EH, Acién Fernández FG et al (2003) Recovery of microalgal biomass and metabolites: process options and economics. Biotechnol Adv 20:491–515. https://doi.org/10.1016/S0734-9750(02)00050-2
Pacheco-Vega JM, Sánchez-Saavedra M (2009) The biochemical composition of Chaetoceros muelleri (Lemmermann grown) with an agricultural fertilizer. J World Aquacult Soc 40:556–560. https://doi.org/10.1111/j.1749-7345.2009.00276.x
Peña-Castro JM, Martínez-Jerónimo F, Esparza-García F, Cañizares-Villanueva RO (2004) Phenotypic plasticity in Scenedesmus incrassatulus (Chlorophyceae) in response to heavy metals stress. Chemosphere 57:1629–1636. https://doi.org/10.1016/j.chemosphere.2004.06.041
Raoof B, Kaushik BD, Prasanna R (2006) Formulation of a low-cost medium for mass production of Spirulina. Biomass Bioenergy 30:537–542. https://doi.org/10.1016/j.biombioe.2005.09.006
Smedes F, Thomasen TK (1996) Evaluation of the Bligh and Dyer lipid determination method. Mar Pollut Bull 32:681–688. https://doi.org/10.1016/0025-326X(96)00079-3
Tang D, Han W, Li P et al (2011) CO2 biofixation and fatty acid composition of Scenedesmus obliquus and Chlorella pyrenoidosa in response to different CO2 levels. Bioresour Technol 102:3071–3076. https://doi.org/10.1016/j.biortech.2010.10.047
Teoh M, Chu W, Marchant H, Phang S (2005) Influence of culture temperature on the growth, biochemical composition and fatty acid profiles of six Antarctic microalgae. J Appl Phycol 2:421–430. https://doi.org/10.1007/s10811-005-5502-y
Teuling E, Wierenga PA, Schrama JW, Gruppen H (2017) Comparison of protein extracts from various unicellular green sources. J Agric Food Chem 65:7989–8002. https://doi.org/10.1021/acs.jafc.7b01788
Trainor FR, Roskosky FG (1967) Control of unicell formation in a soil Scenedesmus. Can J Bot 45:1657–1664. https://doi.org/10.1139/b67-172
Valenzuela-Espinoza E, Millán-Núñez R, Núñez-Cebrero F et al (2002) Protein, carbohydrate, lipid and chlorophyll a content in Isochrysis aff. galbana (clone T-Iso) cultured with a low cost alternative to the f/2 medium. Aquac Eng 25:207–216. https://doi.org/10.1016/S0144-8609(01)00084-X
Wang B, Li Y, Wu N, Lan CQ (2008) CO2 bio-mitigation using microalgae. Appl Microbiol Biotechnol 79:707–718. https://doi.org/10.1007/s00253-008-1518-y
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313. https://doi.org/10.1016/S0176-1617(11)81192-2
Wijffels RH, Barbosa MJ (2010) An outlook on microalgal biofuels. Science 329(80):796–799. https://doi.org/10.1126/science.1189003
Williams PJLB, Laurens LML (2010) Microalgae as biodiesel & biomass feedstocks: review & analysis of the biochemistry, energetics & economics. Energy Environ Sci 3:554. https://doi.org/10.1039/b924978h
Xin L, Hong-ying H, Ke G, Jia Y (2010) Growth and nutrient removal properties of a freshwater microalga Scenedesmus sp. LX1 under different kinds of nitrogen sources. Ecol Eng 36:379–381. https://doi.org/10.1016/j.ecoleng.2009.11.003
Yoo C, Jun S-Y, Lee J-Y et al (2010) Selection of microalgae for lipid production under high levels carbon dioxide. Bioresour Technol 101:S71–S74. https://doi.org/10.1016/j.biortech.2009.03.030
Acknowledgements
The authors thank the founding agency Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) for grants, and Pretóleo Brasileiro S/A (Petrobrás) for research support.
This study is dedicated to the memory of Professors Rosane Maria de Aguiar Euclydes, Ph.D. and Marcelo Pereira Coelho, D.Sc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soares, J., Kriiger Loterio, R., Rosa, R.M. et al. Scenedesmus sp. cultivation using commercial-grade ammonium sources. Ann Microbiol 68, 35–45 (2018). https://doi.org/10.1007/s13213-017-1315-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-017-1315-x