Abstract
How the resource use by consumers vary in different environments and time scales is one of the fundamental ecological questions. Replicated field studies are rare, however; so the extent to which nutrient use varies and why is uncertain. We studied an endangered tyrphobiotic species, the black bog ant (Formica picea), and its feeding preferences in temperate peatlands. We conducted a baiting experiment at three different sites with high nest densities, repeated over three years and three periods of growing season. Preferences for three main macronutrients (carbohydrates, proteins and lipids) were assessed. We hypothesised that if nutrient limitation plays a role, ants will have an increased need for proteins and lipids in early seasons when brood is raised, while carbohydrates use will increase in late seasons. We also expected that site identity would influence nutrient preferences, but not year. Our results supported the nutrient limitation hypothesis for proteins that were consumed more in the early season. In contrast, preference for carbohydrates was rather high and did not increase consistently through season. Although the occupancy of lipid baits was low overall, it increased at colder temperatures, in contrast to carbohydrate and protein baits. Nutrient preferences varied more among sites than years, with the lowest nutrient use observed in a diverse fen-meadow, consistent with the nutrient limitation hypothesis. Year affected ant abundance, but not bait occupancy. Our results suggest that black bog ants flexibly adapt their diet to environmental conditions and that an interplay between nutrient limitation and climate determines their feeding behaviour.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
How do resource pulses affect resource use in organisms is one of the fundamental research questions in ecology (Sutherland et al. 2013). While we have advanced in understanding of individual nutrient needs from laboratory-controlled experiments on model organisms (e.g. Lee et al. 2008; Jensen et al. 2012; Li et al. 2019), our knowledge on animal feeding ecology based on the field experiments are more limited (Boggs 2009). This is due to the most evidences from the wild based on snapshot studies, where replication over different temporal scales are rather rare (but see e.g. Cumming et al. 2013; Hou et al. 2021; De Cuyper et al. 2023). Such replicated studies are important as the nutrient preferences and behaviour of the species can change considerably through temporal scales, in particular in the seasonal ecosystems (Skinner 1980; De Cuyper et al. 2023; Zvereva and Kozlov 2023). Resource availability varies significantly not only temporarily, but between different habitats, affecting nutrient use by associated fauna (Remonti et al. 2011; Lasmar et al. 2021). The inclusion of both environmental history and temporal variability is therefore an important frontier for such studies (Thompson et al. 2001). Finally, although climatic differences affect the availability of resources throughout the seasons, increasing temperature also has a direct effect on the foraging activity of animals, especially ectotherms (Roslin et al. 2017; Twardochleb et al. 2020), thus affecting the resource consumptions. Yet previous field studies of nutrient preferences have rarely considered the effects of time, climate and habitat simultaneously.
The nutrient limitation hypothesis states that the growth of an organism is primarily determined by the nutrient that is in the shortest supply relative to the organism's requirements. This hypothesis, widely used in plant ecology, was originally known as Liebig´s Law of the Minimum (von Liebig 1840; Agren et al. 2012). A similar concept can be applied to the foraging of animals, which are thought to prefer resources enriched with the nutrient that is limited in the environment (Mayntz et al. 2005; Kaspari et al. 2012). The related hypothesis of optimal foraging states that animals make decisions about where and how to obtain food to maximise their energy gain (Stephens and Krebs 2019). As a result of the variable nutrient constrains at each trophic level, different feeding guilds have been shown to be limited by different macronutrients in the food web. For instance, herbivores tend to be more limited by sodium and proteins (Davidson 1997; Kaspari 2020), while predators by lipids and carbohydrates (Wilder et al. 2013; Toft et al. 2021). In omnivorous species, vertebrates have been shown in the wild to be able to balance their diets according to the most locally accessible resources (Remonti et al. 2011; De Cuyper et al. 2023), while terrestrial invertebrates are thought to be more nutrient-limited (Mayntz et al. 2005; Kaspari et al. 2012; Nielsen et al. 2022). Alternatively, an animal species might specialise in consuming certain types of resources and therefore prefer not the resources with nutrients that are scarcest, but those that fit best into its food niche (Machovsky-Capuska et al. 2016; Marsh et al. 2021). Both mechanisms, nutrient limitation and specialisation, can play a role, even within the same animal group, depending on scale and habitat considered. For example, canopy ants have been shown to prefer proteins over carbohydrates compared to ants from the lower forest strata in a rainforest in Panama and Borneo, supporting the nutrient limitation hypothesis (Kaspari and Yanoviak 2001; Law and Parr 2020). In contrast, a recent large-scale experiment on nutrient preference in Brazil has shown that predatory ant species on the ground prefer lipids, while herbivorous ants in trees prefer carbohydrates (Lasmar et al. 2023), in agreement rather with the resource specialisation hypothesis.
Nutrient limitation is considered particularly important in environments with extreme conditions and low net primary productivity (Fisher et al. 2012). One of these environments is peatlands, with high humidity, oxygen deficiency, slowed decomposition and increased accumulation of organic matter (Bridgham et al. 1996). The animals adapted to these specific conditions are tyrphobionts (Minayeva et al. 2008), which are mostly various insects and other invertebrates (Batzer and Wissinger 1996). As natural peatlands harbour unique and threatened fauna, most invertebrate studies have been conducted to assess their biodiversity and conservation (reviewed by Spitzer and Danks 2006; Similä and Aapala 2014). Yet the research on nutrient and food preferences by their invertebrates is scarce and limited to a few studies on bacteria and microfauna (Jassey et al. 2012; Krab et al. 2013; Mieczan et al. 2015). Moreover, these studies focused on nutrient addition experiments, rather than seasonality.
Ants are known for their ecological success, as they are found in almost all terrestrial habitats, where they use a wide variety of food sources (Lach et al. 2010). Central European ants are mostly omnivores, combining various strategies, mainly zoophagy and trophobiosis (Seifert 2018). However, the relative contribution of predation/scavenging and trophobiosis to feeding strategies may vary within and between ant species, and also between habitats (Fiedler et al. 2007; Guariento et al. 2021). Multiple studies showed that the ant communities are nutrient-limited using both observational and manipulative experiments (e.g. Hahn and Wheeler 2002; Kaspari et al. 2012; Lasmar et al. 2023). Although differences in nutrient requirements between ant species are relatively well known in Europe (Fiedler et al. 2007; Seifert 2018), replicated field studies examining differences in preferences for key three macronutrients across growing seasons are lacking to our knowledge. The existing studies focused on one food type (e.g. Lenoir 2002; Sipos and Kindlmann 2013) or conducted experiments with the laboratory colonies collected in different seasons (e.g. Cook et al. 2011); but see Judd (2005) for an exception and comparison between carbohydrate and protein diet. This is surprising given the wide variation in climatic conditions, vegetation growth and population cycles of ants and their prey during the season (Skinner 1980; Fellers 1989), including the peat bog habitats (Batzer and Wissinger 1996; Carscallen 2019). It is, therefore, important to understand the seasonal food-limitations, but no such study has been conducted yet in peatlands.
In temperate regions, only some ant species build nests in peatlands, especially in open bog areas, where the mosses can support rapid warming and brood development (Dlussky 2001; Seifert 2018). One of the few obligatory ant tyrphobionts in Central Europe is the black bog ant, Formica picea. The species is adapted to the peatlands because of its low super-cooling point and a long survival in the flooded soils (Bönner 1915; Seifert 2018). The black bog ant is considered a glacial relict in central Europe, where it is restricted to the remaining small bogs, and is therefore of high conservation interest (Mabelis and Chardon 2005). A recent molecular analysis of F. picea supported the single species concept for the European population (Zhigulskaya et al. 2022). Despite F. picea is a conspicuous species, its biology and ecology are still poorly understood. There are only observational notes on the food requirements, speculating on the black bog ant’ dependence on fungal hyphae and aphids in the nest and flower nectar (Seifert 2018 and references there). Although a search for interactions with aphids in a British peatland does not prove this to be a common case (Hopkins 1996).
Here we studied F. picea and its feeding preferences in temperate peatlands, where we conducted a bait experiment in three different sites with relatively high ant populations, repeated over three years and three periods of growing season. We consider the effects of time (season and year), climate (temperature) and habitat (site) effects simultaneously. In particular, we aim to assess, how the ant workers’ preferences for the three major macronutrients, carbohydrates, proteins, and lipids (Machovsky-Capuska et al. 2016), change in situ with season. We hypothesised that if nutrient limitation plays a role, workers will have an increased need for proteins and lipids in the early season when brood is being raised, while carbohydrate consumption will increase in the late season due to the expected increased need for rapid energy resources to sustain foraging (Csata and Dussutour 2019). We also expected that the variance in macronutrient use between the sites would be greater than between the years, as we sampled sites with different vegetation composition and nutrient deficiencies. Alternatively, if resource limitation does not play a role in F. picea, preference for each macronutrient would be expected to remain stable over time at all sites, with any fluctuations caused only by temperature, due to increased foraging activity on hot days.
Materials and methods
Studied sites and their characteristics
The data were collected from 2017 to 2019 in three peatlands in the Bohemian–Moravian Highlands, a low mountain range in the central part of the Czech Republic. The experiments were conducted from the end of June to the beginning of September, during the peak season of F. picea activity. We chose three peatland sites with different vegetation structure (a degraded fen-meadow, a natural fen-meadow and a peat bog), as we aimed to cover the variance of vegetation stands that ants colonise of different nutrient potentials (e.g. seeds, nectar, honeydew and prey; see the sites characteristics below). All three sites host a relatively high population of the black bog ants (Bezděčková et al. 2011) (Supporting information Fig. S1).
The first site Bílý Kámen (49 °25 '54.574 ''N, 15 °30 '20.016 ''E, 550 m a.s.l., 1.5 ha, Fig. S1a) is a degraded fen-meadow of a former occurrence of peatbog plants, such as Pedicularis sylvatica (see Růžička 1972), but is nowadays lacking these specialised herbs due to eutrophication and overgrown by nutrient-intensive plants. The meadow is surrounded by shrubs and trees (birch) on a tributary of the pond Dolňák. The site is dominated by Carex nigra, Cirsium palustre, Filipendula ulmaria, Angelica sylvestris, Urtica dioica, and Rubus spp. Margins are dominated by tall grasses Phragmites australis a Phalaris arundinacea. There is a lower diversity of herbs compared to the latter site (Jankov), as Bílý Kámen lacks large populations of early spring blooming and myrmecochorous plants. However, it is relatively rich in plant species that are known to provide nectar in the summer season (Kapyla 1978). Furthermore, one of the dominant plants, Ci. palustre, is known to provide myrmecochorous seeds in the late summer (Chytrý et al. 2021). Numerous populations of aphids (Brachycaudus cardui) on Ci. palustre are tended by F. picea at the site (Fig. S2a) and the workers attend also the flowers in F. ulmaria and A. sylvestris. We also observed the presence of psyllids at the site, which are known to benefit from attendance by ants (Novak 1994). The relatively rich local population of F. picea inhabited robust sedge tussocks in the eastern part of the site (Fig. S1ab).
The second site, Jankov (49 °24 '55.848 ''N, 15 °23 '51.528 ''E, 670 m a.s.l., 6.1 ha, Fig. S1c), is a complex of natural fen-meadows with plant communities typical for tall sedges and peatbogs with solitary birch trees. The site is a part of the natural reserve U Milíčovska. The sampled area is managed by mowing once a year for preservation of habitat mosaics, including the removals of invasive shrubs. The meadow is characterised by dominance of Carex diandra, Sphagnum spp., A. sylvestris, Ci. palustre and Lysimachia vulgaris, with presence of Dactylorhiza majalis, Eriophorum angustifolium, Drosera rotundifolia, Menyanthes trifoliata, Scorzonera humilis, Valeriana dioica (Čech et al. 2002). Our studied transect hosted a nectar-rich and relatively diverse plant communities including spring-blooming myrmecochorous species Comarum palustre, Myosotis sylvatica, Viola palustris and Pedicularis sylvatica (see Chytrý et al. 2021). Formica picea tends aphids (B. cardui) on Ci. palustre and the flowers of A. sylvestris at this site (Fig. S2). The relatively abundant local population of the ant species lived on a peat-meadow on the eastern part of the reserve (Fig. S1cd).
The last studied site, Radostín (49 °39 '24.795 ''N, 15 °53 '14.212 ''E, 620 m a.s.l., 3 ha, Fig. S1e), is a transitional peat bog, a part of the national natural reserve Radostínské rašeliniště in Žďárské vrchy Protected Landscape Area. The site is based at the former peat extraction location and it is characterized by secondary peat vegetation with a high proportion of Sphagnum and Polytrichum mosses, Vaccinium myrtillus, Eriophorum vaginatum and solitary birch and pine trees, adjacent to the coniferous forests (Čech et al. 2002). It is thus the least nutrient-rich site and limited in nectar-bearing plants. We also did not observe here any myrmecochorous herbs and any aphids on the dominant herbs. We recorded, however, aphids (Cinara spp.) on pines, visited occasionally by the ants. The relatively abundant local population of F. picea nested in moss cushions and blueberry bushes (Fig. S1f).
Experimental design
We used three major macronutrients, proteins, carbohydrates and lipids, as attractants to study feeding preferences of F. picea. Individual baits were placed inside glass vials of an internal diameter 2 cm and a length of 6 cm, each containing 2 ml of the attractant. We used three resources as proxy of each macronutrient that all contain a mixture of nutrients including vitamins rather than a single inert compound (e.g., sacharose as carbohydrate or glutamine as protein in some previous studies, see Spotti et al. 2015). We opted for this approach as we wanted to reflect the resources corresponding to the ants´ natural diet and food odour (i.e., prey, honeydew, plant oil), each containing predominantly one of the three macronutrients. As protein bait, we used a commercially available earthworm flour for fishing (www.chytiljsem.cz, ZZM/500) mixed in water (ratio 2:1) to achieve a mushy consistency and thereby avoid rapid removal of the bait. The freeze-dried earthworm product was used because such diet is free from added sugar and artificial flavourings and it contains > 50% proteins, less of lipids (10–20%) compared to insect food and relatively high energy (20 J/mg) (Sun and Jiang 2017; Kavle et al. 2023). Its complex polysaccharides in mucus although numerous (20% of dry weight) cannot be digested by ants unlike the simple carbohydrates (Mony et al. 2013). Flower meadow honey (www.medokomerc.cz) was used as a carbohydrate bait (13.9 J/mg, 81 g carbohydrates, 0.3 g proteins, no oils and fatty acids, per 100 ml). Poor rapeseed oil (Glencore Agriculture, www.viterra.cz/cs) was used as a lipid bait (34 J/mg, 92 g of lipids, including 7.4 g of saturated fatty acids, per 100 ml). We used rapeseed oil instead of olive oil that was used in other studies (Lasmar et al. 2023) since Brassicaceae are widespread in Czech Republic meadows, but Olea trees do not grow in the region. The oil was prevented from leaking with a piece of cotton wool placed in each vial, while protein and honey were of mushy consistency and so placed without it. The cotton soaked up the oil to ensure the accessibility for the ants. Since it is known that a different volume of food can influence the behaviour of individual workers (Mailleux et al. 2000), we used the same volume for all macronutrients, sufficient to attract many workers during the experiment. We have not standardised the weight of food per bait by its energy content (J/mg), as the above measures reflect the human perspective rather than that of the insects. Moreover, the possibly different size of the food among treatments and thus the empty space in the vials may have affect, how many workers can feed inside when the vial is collected. Thus, we regard same amount and volume to be here an important standardisation. Empty vials were used as a control (no attractant). The same brands were used for all the macronutrients over the sampled years.
In each experiment, 60 vials were placed at each site in each sampling event: 15 baits of each of three nutrient types plus 15 controls (empty vials) in a single transect, and at 2 m distance between the neighboring baits. The individual four treatments were placed in a random order by pulling each vial out of a plastic bag and placing it on the ground with its rim open to be accessible to the ants. We placed the foods at random order and at this distance following other similar studies (e.g. Lasmar et al. 2021; Guariento et al. 2021), rather than placing all four treatments together, as we were interested in both visitation rates and ant abundances without possible confounding effects on one treatment on another. The vials were exposed for one hour. We then recorded the number of workers of F. picea and other ant species inside the vial in the field. The vials were sealed, the workers counted and then released unharmed after the experiment. The species identity was checked by a hand 30x-magnifier. The ants were identified using Czechowski et al. (2012) and Seifert (2018). The vials are referred to as baits including the controls for simplicity hereafter.
The experiment was repeated three times through the growing season at each of the three sites and 3 years (27 sampling events). Thus, we exposed a total of 1620 baits, 540 in each year in 2017, 2018 and 2019, respectively. The experiments were replicated at the turn of each month, June/July (early season: “1”), July/August (mid season: “2”) and August/September (late season: “3”), between 13:00 and 16:00 and on sunny or partly cloudy days. If a rain occurred during the experiment, we repeated it on another day.
Temperature data
We used two temperature measures to reflect different temporal scales. First, we measured the temperature on site when conducting each set of 60 baits (variable Site temperature hereafter) in the shade close to the ground using a thermometer at the start of each experiment (Hama 075298). Site temperature varied between 17 and 33 °C, depending on the site and the sampling period, see data in Bezděčková et al. (2024). Second, to account for possible effects of local temporal climatic trends prior to our experiments on F. picea nutrient preferences in each of the years, we calculated mean daily temperatures in each region over a 21-day period prior to each sampling event (Mean temperature hereafter). Data were obtained from publicly available records of the meteorological stations of the Czech Hydrometeorological Institute (CHMI) closest to the sampled sites: Hubenov (49°23'31.642"N, 15°28'2.015"E), ca. 5 km from Bílý Kámen and about 10 km from the Jankov site; and Vatín (49°31'29.621"N, 15°58'1.847"E), ca. 16 km from the Radostín site.
Data analysis
We used generalised linear models (GLMs) to test for the effects of environmental variables on the ant response. All plots visualising the responses of the ant variables were made using ‘ggplot2’ package and its extensions ‘ggeffects’, ‘ggpubr’ and ‘egg’ (Wickham 2016). All analyses were conducted in R version 4.2.2 (R Core Team 2022). The dataset and associated R script to reproduce all analyses are available in Bezděčková et al. (2024).
A balanced design of 1620 entries of ant abundances on the baits was used for the analyses over three seasons, three sites and 3 years. We recovered all but six vials, and we found no ants and no bait residue in 16 vials. Hence, 22 ant abundance entries of the 1620 (0.01%) were missing and were estimated. The lost vials were rare (0 to 2 per experimental series of 60 baits) and the eaten 16 baits were all in the protein and carbohydrate treatments, where they were likely consumed by the focal ant species due to their high abundance observed on those baits and events in the field. Therefore, instead of omitting these replicates, we used as conservative estimates either the mean ant abundance counted across all baits (for the lost vials) or the mean abundance counted across the baits visited by F. picea (for eaten baits); the means were calculated separately for each treatment and sampling event (i.e. for a set of the 15 baits).
We included only the activity data for the focal species F. picea as a response in our models, since this species occupied the vast majority of baits and encounters with other ant species were rare (all from the genus Myrmica; see Results). We used two response variables of F. picea activity at the baits, as an indication of the species' feeding preferences: (i) the number of workers observed in each bait (hereafter referred to as ant abundance) and (ii) the proportion of baits visited by workers (hereafter referred as bait occupancy). The first measure comprises the extent of preferences due to the presumably higher accumulation of workers at the preferred resource. In contrast, the latter measure of presence–absence data is more conservative when testing for differences in feeding preferences (i.e., baits with one or many workers are considered equivalent). Although the occupancy might be confounded more with bait distance from nest mounds which we did not measure, we do not expect this to be source of bias due to a random placement of all baits along transects of uniformly high foraging activity and nest density.
Since there were many zeros and the residuals were not normal (i.e., right skewed distribution of the data, with overdispersion and inhomogeneous variance in the tested groups), we used a negative binomial distribution with log link function for the ant abundance (‘glm.nb’ from R package ‘MASS’) and a binomial distribution with logit link function for the bait occupancy (‘glm’) (O'Hara and Kotze 2010; Ripley et al. 2022). To test for our hypotheses from the Introduction, for both response variables we ran a model with all four main effects of interest (variables Treatment, Season, Site, Year), including their first-order interactions. All four explanatory variables were scored as categorical factors. Analysis of deviance using likelihood ratio chi-square tests (χ2) of Type III was applied to test for significances of the individual model terms (‘Anova’ function from R package ‘car’, type = "III", p < 0.05). Furthermore, we calculated explanatory power of each model using pseudo-R2 of the explained deviance compared to null model (D2 hereafter). The inclusion of Myrmica spp. abundance as a covariate did not improve our model in terms of D2 (negative binomial model) or did not converge due to the rarity of Myrmica’ records (binomial model). Inclusion of Site temperature and Mean temperature variables also did not improve the models. Therefore, only the four categorical factors were retained in the final model. We performed posthoc Tukey’ tests for the main levels of each factor using the function ‘glth’ from the R package 'multicomp' with adjusted p values by the Holm method (Hothorn et al. 2008) and comparisons being averaged over interaction terms. The interaction effects were plotted for simplicity using the observed means with standard errors to assess between-group variance, without further post hoc comparisons. For abundance, scaling of values with the common logarithm was used to make it easier to read the observed values in the plots.
To test whether temperature itself had any effect on ant activity at baits, we run the corresponding GLM models ('glm.nb', 'glm' with same set up as above) on the ant abundance and occupancy, respectively, as response variables, using either Mean temperature or Site temperature as predictor. In addition, we run these models with both treatment and temperature effects and their interaction to determine, whether the ants use the different nutrients differently at different temperatures compared to the control across the whole experiment.
Results
Overall bait occupancy by the ants and the treatment effect
Across whole data series, 773 (48%) of the baits were visited by the black bog ant (F. picea), totalling 9978 ant workers. Only 38 baits (2%) were visited by other ant species, all from the genus Myrmica (two species, M. scabrinodis in 36 baits and M. ruginodis in two baits). We did not observe strong competition between the species at the bait resources, as the black bog ant co-occurred with Myrmica in 19 out of 38 cases. Regarding the overall use of resources by the black bog ant during the whole experiment, 375 carbohydrate baits (23%), 302 protein baits (19%), 73 oil baits (5%) and 23 controls (1%) were visited. We did not observe any other animals interfering with the black bog ants.
Nutrient preference varied greatly between all four treatments (χ2, p < 0.001 for Treatment effect in GLMs (Table 1), as well as in all post hoc comparisons (Table S1). Carbohydrates were most preferred in terms of number of workers on bait (mean number of individuals, standard deviation and maximum value: mean = 18, SD = 25.6, max = 110), proteins second most preferred (mean = 6.3, SD = 10.8, max = 100) and oil least preferred (mean = 0.3, SD = 0.9, max = 7) but still significantly more than the control (mean = 0.1, SD = 0.3, max = 3). These differences were similar for ant abundance and bait occupancy, although the difference between carbohydrate and protein treatment was less pronounced in the latter (Fig. S3).
Variance in individual nutrient use across seasons, sites and years
The abundance of F. picea workers on baits was significantly influenced by all four factors, as all first-order interaction effects were significant (p < 0.05) except ‘season:site’, and ‘season:year’. Furthermore, conditional effects of Treatment and Site, that is the separate effect of each of the two from all the others, were also highly significant (see Table 1 for the significance of individual model terms). Whole abundance model explained 70% of the deviance (D2 = 0.7). Bait occupancy was similarly affected by these factors, except for the insignificant effects that included Year (whole model D2 = 0.5). Therefore, we only present the overall response across all years pooled for bait occupancy (Fig. 1 and Figs S3–S5) unlike for ant abundance (Fig. 2). In post hoc comparisons of the other factors than Treatment, ants accumulated on average in significantly higher abundances at baits in the mid season (summer; 2) than in the early season (late spring; 1) and the late season (3) (Fig. 1 and Table S1). Furthermore, all sites differed from each other in overall nutrient requirements, which is reflected in both ant abundance (Table S1) and bait occupancy (Table S2). Only the effect of Year was not significant in the post-hoc comparisons.
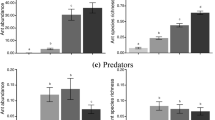
Black bog ant preferences for individual nutrients (oil, carbohydrate and protein baits) compared to control (empty vial) over three seasons from early (1) to late (3). Data are presented as the observed means of a worker abundances on the baits and b proportional visits to the baits. The horizontal bars denote the standard errors of the mean. Note the common logarithmic scale is used for the worker abundance. Seasons 1: late spring; 2: summer, 3: late summer (see Methods for more)
Black bog ant preferences for individual nutrients (oil, carbohydrate and protein baits) compared to control (empty vial) over three seasons, three years (2017–2019) and three sites (indicated by top bar of the chart). Data are presented as the observed means of worker abundances on the baits. The horizontal bars denote the standard errors of the mean. For the significance of the explanatory variables including their first-order interaction, see Table 1. Note that the common logarithmic scale is used for the ant abundance. Seasons 1: late spring; 2: summer, 3: late summer (see Methods for more details). Sites: Bílý Kámen––degraded fen-meadow. Jankov––natural fen-meadow. Radostín––peat bog
The three nutrients were preferred differently in each of the three seasons (‘Treatment: Season’ interaction: abundance model χ2 = 22.66, p = 0.001; occupancy model χ2 = 26.68, p < 0.001) (Fig. 1 and Table 1). The preference for protein decreased sharply from the early to the late season, especially in worker abundances (Fig. 1). Occupancies of protein and carbohydrate baits were similarly high in early season (Fig. 1b), while worker abundances were lower on protein than carbohydrate baits through all seasons (Fig. 1a). Preferences for carbohydrate varied less across seasons compared to protein treatment, but were highest in the mid season in both ant abundance and occupancy (Fig. 1). Preferences for oil and ant occurrence in controls were relatively stable throughout the period, with the exception of a slight increase in lipid bait occupancy in the early season (Fig. 1).
Individual nutrient preferences also varied greatly between sites, as reflected by both ant abundance and bait occupancy response plotted per site and per season, with additional differences in abundance response observed also between the years (Figs. 2, S4, S5). The overall preference for proteins versus carbohydrates was highest in the degraded fen-meadow (Bílý Kámen): the ratio of protein versus carbohydrate use decreased relatively less through season in that site and protein was more preferred than carbohydrate in spring in the first two years but third year 2019 (Fig. 2adg). In contrast, the peat bog site (Radostín) yielded the highest and relatively most stable preferences for carbohydrate from all sites through temporal scales (Figs. 2 and S5), with the exception of a decline in carbohydrate use in 2019 early season (Fig. 2i). On average, preferences were lowest for all three nutrients in the natural fen-meadow (Jankov; Figs. 2 and S4). At this site, the protein use declined most significantly through season from all sites, while relatively lower use of carbohydrates was observed in the early season except 2019 (Fig. 2beh, Fig S5b). Oil preference was relatively stable across all time scales and all sites in terms of ant abundance (Fig. 2), but was lower in the natural fen-meadow (Jankov) and decreased through the season in the peat bog (Radostín) in terms of bait occupancy (Figs. S4, S5). The controls were usually ignored by the ants, apart a still negligible but increased abundance and occupancy in the earlier seasons in the degraded fen-meadow (Bílý Kámen; Figs. 2adg, S5a).
Predictions of nutrient use with varying temperature
The abundance of ants at the baits was neither influenced by the temperature measured at the time of each experiment (Site temperature, glm.nb: D2 = 0.001, p = 0.27) nor by the three-week daily temperature before the experiment (Mean temperature, glm: D2 = 0.002, p = 0.14). Bait occupancy also did not change with Site temperature (glm.nb: D2 = 0.001, p = 0.13) and Mean temperature (glm, D2 = 0.001, p = 0.29). However, when the Site temperature effects are considered together with the effect of Treatment (glm: D2 = 0.46), there is a significant interaction between the two variables on the bait occupancy (χ2 = 14.43, p = 0.002) (Table S3). This effect reflected the varying changes in the three nutrient uses with Site temperature compared to the control: ant presence in the control vials increased towards higher temperatures, as expected for ectotherms. However, this positive relationship with control hold only for carbohydrates and proteins, whilst occupancy rates of lipid baits were higher at colder temperatures (Fig. 3). Such interaction effects were, however, not significant in the case of Mean and Site temperature and ant abundance, neither of Mean temperature and bait occupancy (χ2, p > 0.05).
Variation of the mean occupancy of the baits by the black bog ant workers with the site temperature measured at the time of experiment. The trends in ant preferences for individual nutrients (oil, carbohydrate and protein) compared to control (empty vial) are predicted across the temperature gradient using a generalized linear model. See the Results and Table S3 for details of the model. Coloured areas around the trend lines denote to 95% confidence intervals from the model
Discussion
General macronutrient preferences and support for nutrient limitation in the black bog ant
Our results of an experiment on the preference for the three most important macronutrients by a numerically dominant ant species in temperate peatlands, Formica picea, support the nutrient limitation hypothesis for proteins and carbohydrates, as they were in high demand during whole our experiment. Many other studies on ants (e.g. Kaspari and Yanoviak 2001; Hahn and Wheeler 2002; Law and Parr 2020; Guariento et al. 2021) and also on other ecologically important ground-dwelling invertebrates, such as ground beetles, spiders and harvestmen (Mayntz et al. 2005; Nielsen et al. 2022), interpreted increased use of a macronutrient in the environment through nutrient limitation. However, to our knowledge, our study is the first to disentangle variance in preferences for three key macronutrients by an invertebrate species across different temporal scales (i.e. season and year), taking into account temperature and site effects.
The highest preference for carbohydrates, followed by proteins (or amino acid), and the lowest preference for lipids is consistent with other studies that have investigated nutrient preference in European temperate ant communities (Spotti et al. 2015; Guariento et al. 2018, 2021), pointing to the highest limitation of workers and colonies by carbohydrates (Domisch et al. 2009; Feldhaar 2014). This contrasts with tropical communities, where the preference for lipids is comparable to that of carbohydrates (Moses et al. 2023), perhaps due to a higher occurrence of predatory and scavenging species (Lasmar et al. 2023). Although lipids have higher energy content than other two macronutrients, we speculate that perhaps only certain tropical species can digest this food effectively unlike the ants of Formica genus, which may explain the low preferences. Of all three macronutrients, the temporal limitation of nutrient supply is most pronounced for proteins, whose consumption declined significantly throughout the growing season and at most sites and in most years of our study. The differences between sites are also consistent with nutrient limitation, as the lowest demand for macronutrients was found at the most resource-rich site (see below for further discussion of seasonal and site effects). In contrast, neutral preferences or specialisations on single macronutrient do not seem to play an important role in our model species. This suggests that the black bog ant behaves as an omnivore, similar to the other species of the Formica genus that are known to feed on diverse resources (Skinner 1980; Domisch et al. 2009), and is able to adapt its diet to various environmental conditions.
Seasonal effects
We expected F. picea to have an increased need for proteins and lipids in the early season when brood is being reared, but carbohydrate requirements to increase in the late season due to the higher energy needs by foragers when resources become scarcer (Judd 2005; Csata and Dussutour 2019). The results confirmed our expectations regarding proteins, as ant activity on the earthworm baits decreased significantly in the late season in all but one of the nine sampling events. This effect was quite strong in ant abundance and also detectable in bait occupancy, although F. picea are able to raise two brood generations during the growing season (Dlussky 2001). The increased protein requirements of ants in the early season may have important consequences for prey populations, as invertebrate biomass peaks in summer (Hallmann et al. 2017), but predation pressure on arthropods by both vertebrates and invertebrates is highest in spring (Cardoso et al. 2007; Dunn et al. 2011). For example, if we consider this result as an indicator of predation pressure, it decreased on average by 70% (ant abundance) and 20% (proportion of baits visited) between June and August, that is, in only two months. This variance could even increase between early spring and late summer, although this would be difficult to verify due to the disruptive effect of the cold spring climate, which we avoided. However, a limitation of our experiment is that we cannot distinguish whether our protein source reflects predation or scavenging behaviour.
Although the increased use of proteins in the early season by ants was an expected outcome, evidences of this phenomenon from the field experiments are rather rare, which led us to design this study. Most of previous studies on the seasonal effects of food preferences, including proteins and carbohydrates, have been limited to (sub)tropical regions and invasive ants (e.g. Stein et al. 1990; Abbott et al. 2014). Those studies demonstrated an increased use of carbohydrates vs. protein diet in the wet (colder) season. This is consistent with a recent study by Guariento et al. (2018), which found increased carbohydrate use by Formica lemani at colder (high) elevations than at warmer elevations in the Alps. Nevertheless, protein preferences did not change with elevation and seasonal effects were not considered in that study. Other temperate studies that have sampled during the growing season either have focused on observing natural food items carried to the nests or used indirect measurements of diet shifts via the ratios of natural stable isotopes of the wood ants (Formica rufa group) (e.g. Skinner 1980; Iakovlev et al. 2017). These wood ant´ studies found different patterns, ranging from rather minor changes over the growing season (Iakovlev et al. 2017) to increased use of most resources in the early season (Horstmann 1972; Skinner 1980) or increased use of all resources in hot summers when workers are most active (Domisch et al. 2009). To our knowledge, there are only a few temperate studies that demonstrated the increased use of experimentally offered protein-rich resources in the early season, as in our study: two on the wood ants from Scandinavia (Lenoir 2002; Zvereva and Kozlov 2023) and one on a Pheidole species from North America (Judd 2005). In contrast, similar studies from forests in the Czech Republic showed no such seasonal trend in predation by ants (Sipos et al. 2013; Sipos and Kindlmann 2013), possibly due to sampling of other ant taxa. Taken together, our and previous studies suggest that the extend of nutritional requirements for protein-rich resources in the early season may depend on habitat and ant species.
In contrast to the patterns of protein use, the seasonal changes in carbohydrates and lipids were less clear. There was some evidence of increased carbohydrate use in the mid season (July), but it was not strong and inconsistent. Contrary to our expectation of their increased use through season, no such trend was observed in F. picea. This contrasts with the predatory wasps where the carbohydrate preference increases in the late season (Hoshikawa 1981). Overall, the workers seem to have a high demand for carbohydrates throughout the growing season, while the preference for lipids is negligible (but see also the discussion on temperature effects below).
Site effects
Although most of the explained variance in nutrient preferences by our models can be attributed to treatment and season effects, we observed also differences among the sites. This was expected, as the three selected sites differed in terms of vegetation structure and diversity. The differences between sites are consistent with the nutrient limitation hypothesis, as the lowest total ant abundance and bait visitation rates for all macronutrients were observed in the natural fen-meadow (Jankov), the site with the greatest diversity of herbs and the presence of both aphids and myrmecochorous plants, the resources that potentially provide altogether all the macronutrients (Skinner 1980; Konecna et al. 2018). Although we did not measure food availability quantitatively, other studies have shown that more diverse and productive grasslands support higher diversity and abundance of arthropods and their food resources (Haddad et al. 2001; Fernandez-Tizon et al. 2020). It is, therefore, to be expected that this site will elicit the least response from the ants.
In accordance with nutrient limitation, we would then expect the highest nutrient requirements in the least diverse site, the peat bog (Radostín) that corresponds the most to nutrient-poor peatlands. Indeed, the preference for carbohydrates was highest at this site. Proteins however, were not most required there, but in the degraded fen-meadow (Bílý Kámen). While the increased carbohydrate use in the peat bog can be explained by the lower occurrence of nectar-rich plants and the absence (or rarity) of aphids in the low vegetation in this habitat (Hopkins 1996 and our observations), the unexpectedly increased protein consumption in the degraded fen-medow is more difficult to interpret. This site still hosts large nests of F. picea under tussocks of Carex nigra, but it supports also a number of competitive plants (Rubus spp., Filipendula ulmaria), so the vegetation is more uniform and quite high. We suspect that the degraded fen-meadow had less of protein in the form of suitable prey for ants due to the dominance of hard-shelled beetles and bugs, while honeydew-providing insects (aphids, whiteflies) were also relatively abundant there (personal observations). As F. picea is a tyrphobiotic species, it is likely that it consumes various dipterans to obtain proteins, which are common rather in peat bogs, especially in the early season (Batzer and Wissinger 1996; Carscallen 2019), or it may consume hyphae growing under Sphagnum nests (Skwarra 1929). This may explain the unexpectedly higher demands for protein in the degraded fen-meadow compared to the peat-bog. Direct evidence for these resources as part of the F. picea´ diet is lacking and more data on its natural food are needed. Nevertheless, our study provides the first evidence that F. picea tends commonly herds of aphids on the common plants not restricted to peat bogs, such as thistles and birches. This ant thus does not differ from other ant species in this behaviour (Stadler and Dixon 1998; Blackman and Eastop 2006), although the aphid honeydew resource is used less in peat-bogs (Hopkins 1996 and our observations).
Year and temperature effects
Changes in the number of workers observed at the baits may reflect not only nutrient requirements, but also temperature fluctuations, since ants are ectothermic and their foraging activity and speed increases with temperature (Cerda et al. 1998). This was not the case in our study, as we did not find a strong positive influence of temperatures on nutrient preferences. As we hypothesized, the interannual differences were then smaller than between sites. Moreover, they only affected ant abundance, not visitation rates. This suggests a relative robustness of the above patterns across different years and temperatures. We assume that the fact that we conducted our experiment between late June and August and on dry days contributed to the weak temperature effects. Our results therefore likely reflect actual behavioural preferences for the macronutrients offered, not a simple variance in ant activity due to fluctuation in temperatures. Nevertheless, we assume that part of the interannual variance can be explained by climatic differences between the three years preceding the period in which we conducted our experiment: for instance, a colder period in May 2019 compared to the other 2 years (Figure S6) could contribute to the decrease in ant activity on carbohydrate baits in the early season in the peat bog, i.e., site with the coldest microclimate (Bezděčková et al. 2024), but to an opposite effect on these baits and period due to delayed flowering of meadow plants at the other two sites.
Interestingly, although overall preference for lipids was low, they were visited by the ants more frequently under colder site temperatures, in contrast to the other two macronutrients and also to the control. This means that some of the individual workers were more attracted to oils on the colder days. However, their preference did not trigger responses from the whole colony, as we observed only one to three workers on oil baits in most cases unlike on the other macronutrients. Individual-based decisions by ant foragers are an important topic in behavioural studies, including ant feeding ecology (Czaczkes and Ratnieks 2013; Feldhaar 2014). Although recent laboratory experiments have shown that an individual worker acts rather as a unit within a colony, with positive feedback from other colony members to share and balance nutrient requirements within the colony (Hakala et al. 2021), we speculate that oils are only demanded by certain individuals of the black bog ant when the climate is colder. Indeed, it has been shown in laboratory studies in other ants and insects that a more lipid-rich diet and a higher tissue fat content help to cope with colder temperatures (e.g. Heinze et al. 2003; Li et al. 2019). It remains to be clarified whether this is the case for F. picea, as preferences for oils were low and we did not track the behaviour and the fat content of individual workers in this field study.
Limitations of this study
Although we interpret our results on the seasonal and site-dependent changes in macronutrient preferences in the black bog ants due to nutrient limitation as the most likely driver, our study is constrained by the lack of quantitative background data on nutrient availability at our sites at the time of sampling. To test for the direct effects of nutrient availability (and hence limitation) in the ant populations, we would need gather temporal quantitative data on the peat-bog ants' natural diet (which, as explained above, is unknown) and the natural availability of these food resources over the sampled seasons and years. The lack of such data is a common limitation in similar ant feeding preference’ studies, which typically lack quantitative data on the changes of prey, nectar and honeydew over time in the sampled sites and times, as those are challenging and time-consuming to measure. Instead, ours and the previous studies used artificial resources (baits) and their visitation rates as surrogates for the nutrients predicted to be at the highest demand (see Lasmar et al. 2021 and Moses et al., 2023 for the other limitations of the approach). Unlike for pollinators, frugivores and specialised predators, mapping a single natural resource is not effective for ants that are often omnivorous. Here, since we worked with the protected ant species and two of our three sites were nature reserves, we could not investigate arthropod availability using methods that would require destructive sampling, neither open the nests to measure brood-development over time. Nevertheless, higher demands for proteins in early season and the overall lowest demands for macronutrients observed in the most diverse meadow site agree with expectations of the nutrient limitation hypothesis. Future studies should, where possible, also link the use of experimentally provided resources with quantitative natural food availability measured at the sampled sites.
Data availability
All data and the associated R code to reproduce the analyses and figures are fully available in Zenodo depository at https://doi.org/10.5281/zenodo.10955836. (Bezděčková et al. 2024).
References
Abbott KL, Green PT, O’Dowd DJ (2014) Seasonal shifts in macronutrient preferences in supercolonies of the invasive yellow crazy ant Anoplolepis gracilipes (Smith, 1857) (Hymenoptera: Formicidae) on Christmas Island, Indian Ocean. Austral Entomol 53:337–346. https://doi.org/10.1111/aen.12081
Agren GI, Wetterstedt JAM, Billberger MFK (2012) Nutrient limitation on terrestrial plant growth––modeling the interaction between nitrogen and phosphorus. New Phytol 194:953–960. https://doi.org/10.1111/j.1469-8137.2012.04116.x
Batzer DP, Wissinger SA (1996) Ecology of insect communities in nontidal wetlands. Annu Rev Entomol 41:75–100. https://doi.org/10.1146/annurev.en.41.010196.000451
Bezděčková K, Bezděčka P (2011) Endangered non-forest Formica. Formica picea, Formica exsecta, Formica foreli and Formica pressilabris [In Czech and English]. Muzeum Vysočiny Jihlava, Czech Republic.
Bezděčková K, Bezděčka P, Fibich P, Klimeš P (2024) Data and code: Different feeding preferences for macronutrients across seasons and sites indicate temporal and spatial nutrient limitation in the black bog ant (1.0). Zenodo. https://doi.org/10.5281/zenodo.10955836
Blackman RL, Eastop VF (2006) Aphids on the world’s herbaceous plants and shrubs. Wiley, Chichester
Boggs CL (2009) Understanding insect life histories and senescence through a resource allocation lens. Funct Ecol 23:27–37. https://doi.org/10.1111/j.1365-2435.2009.01527.x
Bönner W (1915) Die Überwintering von Formica fusca picea und andere biologische Beobachtungen. Biologisches Centralblatt 35:65–77
Bridgham SD, Pastor J, Janssens JA, Chapin C, Malterer TJ (1996) Multiple limiting gradients in peatlands: A call for a new paradigm. Wetlands 16:45–65. https://doi.org/10.1007/bf03160645
Cardoso P, Silva I, De Oliveira NG, Serrano ARM (2007) Seasonality of spiders (Araneae) in Mediterranean ecosystems and its implications in the optimum sampling period. Ecol Entomol 32:516–526. https://doi.org/10.1111/j.1365-2311.2007.00894.x
Carscallen G (2019) Arthropod Diversity in Contrasting Ontario Peatlands. Electronic Thesis and Dissertation Repository. 6703. The University of Western Ontario. https://ir.lib.uwo.ca/etd/6703/
Čech L, Šumpich J, Zabloudil V (2002) Jihlavsko. In: Mackovčin P, Sedláček M (eds) Chráněná území ČR, svazek VII. Praha, AOPK ČR a Ekocentrum Brno, pp 237–429
Cerda X, Retana J, Cros S (1998) Critical thermal limits in Mediterranean ant species: trade-off between mortality risk and foraging performance. Funct Ecol 12:45–55. https://doi.org/10.1046/j.1365-2435.1998.00160.x
Chytrý M et al (2021) Pladias database of the Czech flora and vegetation. Preslia 93(1):1–87
Cook SC, Eubanks MD, Gold RE, Behmer ST (2011) Seasonality directs contrasting food collection behavior and nutrient regulation strategies in ants. PLoS ONE. https://doi.org/10.1371/journal.pone.0025407
Csata E, Dussutour A (2019) Nutrient regulation in ants (Hymenoptera: Formicidae): a review. Myrmecol News 29:111–124
Cumming GS, Ndlovu M, Mutumi GL, Hockey PAR (2013) Responses of an African wading bird community to resource pulses are related to foraging guild and food-web position. Freshw Biol 58:79–87. https://doi.org/10.1111/fwb.12040
Czaczkes TJ, Ratnieks FLW (2013) Cooperative transport in ants (Hymenoptera: Formicidae) and elsewhere. Myrmecol News 18:1–11
Czechowski W, Radchenko A, Czechowska W, Vepsäläinen K (2012) The ants of Poland with reference to the myrmecofauna of Europe. Natura optima dux Foundation, Poland
Davidson DW (1997) The role of resource imbalances in the evolutionary ecology of tropical arboreal ants. Biol J Linnean Soc 61:153–181. https://doi.org/10.1006/bijl.1996.0128
De Cuyper A et al (2023) Nutrient intake and its possible drivers in free-ranging European brown bears (Ursus arctos arctos). Ecol Evol 13:15. https://doi.org/10.1002/ece3.10156
Dlussky GM (2001) Season dynamic of brood development of Formica candida colonies from isolated bog population. In: Ants and forest protection: materials of the 11th All-Russian Myrmecological Symposium, Perm, pp 69–71 [Conference proceedings in Russian with abstract in English]. Permsky gosudarstvennyj universitet, Perm.
Domisch T et al (2009) Foraging activity and dietary spectrum of wood ants and their role in nutrient fluxes in boreal forests. Ecol Entomol 34:369–377. https://doi.org/10.1111/j.1365-2311.2009.01086.x
Dunn PO, Winkler DW, Whittingham LA, Hannon SJ, Robertson RJ (2011) A test of the mismatch hypothesis: how is timing of reproduction related to food abundance in an aerial insectivore? Ecology 92:450–461. https://doi.org/10.1890/10-0478.1
Feldhaar H (2014) Ant nutritional ecology: linking the nutritional niche plasticity on individual and colony-level to community ecology. Curr Opin Insect Sci 5:25–30. https://doi.org/10.1016/j.cois.2014.09.007
Fellers JH (1989) Daily and seasonal activity in woodland ants. Oecologia 78:69–76. https://doi.org/10.1007/BF00377199
Fernandez-Tizon M, Emmenegger T, Perner J, Hahn S (2020) Arthropod biomass increase in spring correlates with NDVI in grassland habitat. Sci Nat. https://doi.org/10.1007/s00114-020-01698-7
Fiedler K, Kuhlmann F, Schlick-Steiner BC, Steiner FM, Gebauer G (2007) Stable N-isotope signatures of central European ants––assessing positions in a trophic gradient. Insectes Soc 54:393–402. https://doi.org/10.1007/s00040-007-0959-0
Fisher JB, Badgley G, Blyth E (2012) Global nutrient limitation in terrestrial vegetation. Glob Biogeochem Cycle 26:9. https://doi.org/10.1029/2011gb004252
Guariento E, Martini J, Fiedler K (2018) Bait visitation by Formica lemani (Hymenoptera: Fomicidae) indicates shortage of carbohydrates in alpine grasslands. Eur J Entomol 115:217–222. https://doi.org/10.14411/eje.2018.020
Guariento E, Wanek W, Fiedler K (2021) Consistent shift in nutritional ecology of ants reveals trophic flexibility across alpine tree-line ecotones. Ecol Entomol 46:1082–1092. https://doi.org/10.1111/een.13052
Haddad NM, Tilman D, Haarstad J, Ritchie M, Knops JMH (2001) Contrasting effects of plant richness and composition on insect communities: a field experiment. Am Nat 158:17–35. https://doi.org/10.1086/320866
Hahn DA, Wheeler DE (2002) Seasonal foraging activity and bait preferences of ants on Barro Colorado Island, Panama. Biotropica 34:348–356
Hakala SM, Meurville MP, Stumpe M, LeBoeuf AC (2021) Biomarkers in a socially exchanged/fluid reflect colony maturity, behavior, and distributed metabolism. Elife. https://doi.org/10.7554/eLife.74005
Hallmann CA et al (2017) More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE. https://doi.org/10.1371/journal.pone.0185809
Heinze J, Foitzik S, Fischer B, Wanke T, Kipyatkov VE (2003) The significance of latitudinal variation in body size in a holarctic ant, Leptothorax acervorum. Ecography 26:349–355. https://doi.org/10.1034/j.1600-0587.2003.03478.x
Hopkins GW (1996) A local hoverfly and a rare aphid tended by an endangered ant on cottongrass. In: Morgan IK (ed) Newsletter No 33. Dyfed invertebrate group, Cairns, Australia
Horstmann K (1972) Investigations on food consumption of red wood ants (Formica polyctena Foerster) in oak forest: effect of season and supply of food. Oecologia 8(4):371–390. [In German with English abstract] https://doi.org/10.1007/bf00367539
Hoshikawa T (1981) Some colony factors influencing the hunting activity of Polistes chinensis antennalis Perez (Hymenoptera, Vespidae). Appl Entomol Zool 16:395–405. https://doi.org/10.1303/aez.16.395
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363. https://doi.org/10.1002/bimj.200810425
Hou R et al (2021) The geometry of resource constraint: an empirical study of the golden snub-nosed monkey. J Anim Ecol 90:751–765. https://doi.org/10.1111/1365-2656.13408
Iakovlev IK, Novgorodova TA, Tiunov AV, Reznikova ZI (2017) Trophic position and seasonal changes in the diet of the red wood ant Formica aquilonia as indicated by stable isotope analysis. Ecol Entomol 42:263–272. https://doi.org/10.1111/een.12384
Jassey VEJ, Shimano S, Dupuy C, Toussaint ML, Gilbert D (2012) Characterizing the feeding habits of the testate Amoebae Hyalosphenia papilio and Nebela tincta along a narrow “Fen-Bog” gradient using digestive vacuole content and C-13 and N-15 isotopic analyses. Protist 163:451–464. https://doi.org/10.1016/j.protis.2011.07.006
Jensen K et al (2012) Optimal foraging for specific nutrients in predatory beetles. Proc R Soc Lond Ser B-Biol Sci 279:2212–2218. https://doi.org/10.1098/rspb.2011.2410
Judd TM (2005) The effects of water, season, and colony composition on foraging preferences of Pheidole ceres (Hymenoptera : Formicidae). J Insect Behav 18:781–803. https://doi.org/10.1007/s10905-005-8740-6
Kapyla M (1978) Amount and type of nectar sugar in some wild flowers in Finland. Ann Bot Fenn 15:85–88
Kaspari M (2020) The seventh macronutrient: how sodium shortfall ramifies through populations, food webs and ecosystems. Ecol Lett 23:1153–1168. https://doi.org/10.1111/ele.13517
Kaspari M, Yanoviak SP (2001) Bait use in tropical litter and canopy ants - evidence of differences in nutrient limitation. Biotropica 33:207–211. https://doi.org/10.1111/j.1744-7429.2001.tb00172.x
Kaspari M, Donoso D, Lucas JA, Zumbusch T, Kay AD (2012) Using nutritional ecology to predict community structure: a field test in Neotropical ants. Ecosphere 3:15. https://doi.org/10.1890/es12-00136.1
Kavle RR, Nolan PJ, Carne A, Agyei D, Morton JD, Bekhit AEA (2023) Earth Worming An Evaluation of Earthworm (Eisenia andrei) as an Alternative Food Source. Foods. https://doi.org/10.3390/foods12101948
Konecna M, Moos M, Zahradnickova H, Simek P, Leps J (2018) Tasty rewards for ants: differences in elaiosome and seed metabolite profiles are consistent across species and reflect taxonomic relatedness. Oecologia 188:753–764. https://doi.org/10.1007/s00442-018-4254-8
Krab EJ, Berg MP, Aerts R, van Logtestijn RSP, Cornelissen JHC (2013) Vascular plant litter input in subarctic peat bogs changes collembola diets and decomposition patterns. Soil Biol Biochem 63:106–115. https://doi.org/10.1016/j.soilbio.2013.03.032
Lach L, Parr LC, Abbott KL (2010) Ant Ecology. Oxford University Press Inc., New York
Lasmar CJ, Bishop TR, Parr CL, Queiroz ACM, Schmidt FA, Ribas CR (2021) Geographical variation in ant foraging activity and resource use is driven by climate and net primary productivity. J Biogeogr 48:1448–1459. https://doi.org/10.1111/jbi.14089
Lasmar CJ et al (2023) Testing the context dependence of ant nutrient preference across habitat strata and trophic levels in Neotropical biomes. Ecology 104:e3975. https://doi.org/10.1002/ecy.3975
Law SJ, Parr C (2020) Numerically dominant species drive patterns in resource use along a vertical gradient in tropical ant assemblages. Biotropica 52:101–112. https://doi.org/10.1111/btp.12743
Lee KP et al (2008) Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc Natl Acad Sci U S A 105:2498–2503. https://doi.org/10.1073/pnas.0710787105
Lenoir L (2002) Can wood ants distinguish between good and bad food patches on the forest floor? Eur J Soil Biol 38:97–102. https://doi.org/10.1016/s1164-5563(01)01113-x
Li XP et al (2019) Effects of two natural diets on the response of the predator Arma chinensis (Hemiptera: Pentatomidae: Asopinae) to cold storage. Appl Ecol Environ Res 17:15329–15347. https://doi.org/10.15666/aeer/1706_1532915347
Mabelis AA, Chardon JP (2005) Survival of the black bog ant (Formica trankaucasica Nasanov) in relation to the fragmentation of its habitat. J Insect Conser 9(2):95–108. https://doi.org/10.1007/s10841-004-5987-8
Machovsky-Capuska GE, Senior AM, Simpson SJ, Raubenheimer D (2016) The multidimensional nutritional niche. Trends Ecol Evol 31:355–365. https://doi.org/10.1016/j.tree.2016.02.009
Mailleux AC, Deneubourg JL, Detrain C (2000) How do ants assess food volume? Anim Behav 59:1061–1069. https://doi.org/10.1006/anbe.2000.1396
Marsh KJ, Blyton MDJ, Foley WJ, Moore BD (2021) Fundamental dietary specialisation explains differential use of resources within a koala population. Oecologia 196:795–803. https://doi.org/10.1007/s00442-021-04962-3
Mayntz D, Raubenheimer D, Salomon M, Toft S, Simpson SJ (2005) Nutrient-specific foraging in invertebrate predators. Science 307:111–113. https://doi.org/10.1126/science.1105493
Mieczan T, Adamczuk M, Pawlik-Skowronska B, Toporowska M (2015) Eutrophication of peatbogs: consequences of P and N enrichment for microbial and metazoan communities in mesocosm experiments. Aquat Microb Ecol 74:121–141. https://doi.org/10.3354/ame01727
Minayeva T et al (2008) Peatlands and biodiversity. Assessment on Peatlands, Biodiversity and Climate Change. Global Environment Centre, Kuala Lumpur & Wetlands International, pp 60–98
Mony R, Dejean A, Bilong CFB, Kenne M, Rouland-Lefèvre C (2013) Melissotarsus ants are likely able to digest plant polysaccharides. C R Biol 336:500–504. https://doi.org/10.1016/j.crvi.2013.08.003
Moses J et al (2023) Nutrient use by tropical ant communities varies among three extensive elevational gradients: a cross-continental comparison. Glob Ecol Biogeogr 32(12):2212–2229. https://doi.org/10.1111/geb.13757
Nielsen SMB, Bilde T, Toft S (2022) Macronutrient niches and field limitation in a woodland assemblage of harvestmen. J Anim Ecol 91:593–603. https://doi.org/10.1111/1365-2656.13649
Novak H (1994) The influence of ant attendance on larval parasitism in hawthorn psyllids (Homoptera, Psyllidae). Oecologia 99:72–78. https://doi.org/10.1007/bf00317085
O’Hara RB, Kotze DJ (2010) Do not log-transform count data. Methods Ecol Evol 1:118–122. https://doi.org/10.1111/j.2041-210X.2010.00021.x
R Core Team (2022) R: A Language and Environment for Statistical Computing. Ver. 4.2.2. R Foundation for Statistical Computing, Vienna, Austria
Remonti L, Balestrieri A, Prigioni C (2011) Percentage of protein, lipids, and carbohydrates in the diet of badger (Meles meles) populations across Europe. Ecol Res 26:487–495. https://doi.org/10.1007/s11284-011-0804-9
Ripley B, Venables B, Bates DM, Hornik K, Gebhardt A, Firth D (2022) Package “MASS”: support functions and datasets for venables and ripley’s MASS. R Package Version 7:3–29
Roslin T et al (2017) Higher predation risk for insect prey at low latitudes and elevations. Science 356:742–744. https://doi.org/10.1126/science.aaj1631
Růžička I (1972) Příspěvek ke květeně Českomoravské vysočiny II. [A contribution to the flora of the Bohemian-Moravian Highlands]. [In Czech]. Vlastivědný sborník Vysočiny. Oddíl věd přírodních: 53–65.
Seifert B (2018) The ants of central and north Europe. Lutra Germany
Similä M, Aapala K (2014) Ecological restoration in drained peatlands: best practices from Finland. Metsähallitus, Natural Heritage Services
Sipos J, Kindlmann P (2013) Effect of the canopy complexity of trees on the rate of predation of insects. J Appl Entomol 137:445–451. https://doi.org/10.1111/jen.12015
Sipos J, Drozdova M, Drozd P (2013) Assessment of trends in predation pressure on insects across temperate forest microhabitats. Agric Entomol 15:255–261. https://doi.org/10.1111/afe.12012
Skinner GJ (1980) The feeding-habits of the wood-ant, Formica-rufa (hymenoptera, formicidae), in limestone woodland in Northwest England. J Anim Ecol 49:417–433. https://doi.org/10.2307/4255
Skwarra E (1929) Formica fusca-picea Nyl. als Moorameise. Zool Anz 82:46–55
Spitzer K, Danks HV (2006) Insect biodiversity of boreal peat bogs. Annu Rev Entomol 51:137–161. https://doi.org/10.1146/annurev.ento.51.110104.151036
Spotti FA, Castracani C, Grasso DA, Mori A (2015) Daily activity patterns and food preferences in an alpine ant community. Ethol Ecol Evol 27:306–324. https://doi.org/10.1080/03949370.2014.947634
Stadler B, Dixon AFG (1998) Costs of ant attendance for aphids. J Anim Ecol 67:454–459. https://doi.org/10.1046/j.1365-2656.1998.00209.x
Stein MB, Thorvilson HG, Johnson JW (1990) Seasonal-changes in bait preference by red imported fire ant, Solenopsis invicta (Hymenoptera, Formicidae). Fla Entomol 73:117–123. https://doi.org/10.2307/3495334
Stephens DW, Krebs JR (2019) Foraging Theory: Monographs in Behavior and Ecology. Princeton University Press, Princeton
Sun Z, Jiang H (2017) Nutritive Evaluation of Earthworms as Human Food. In: Mikkola H (ed) Future foods. INTECH, Croatia, pp 127–142
Sutherland WJ et al (2013) Identification of 100 fundamental ecological questions. J Ecol 101:58–67. https://doi.org/10.1111/1365-2745.12025
Thompson JN et al (2001) Frontiers of ecology. Bioscience 51:15–24. https://doi.org/10.1641/0006-3568(2001)051[0015:foe]2.0.co;2
Toft S et al (2021) Contrasting patterns of food and macronutrient limitation in the field among co-existing omnivorous carnivores. Ecol Entomol 46:898–909. https://doi.org/10.1111/een.13026
Twardochleb LA, Treakle TC, Zarnetske PL (2020) Foraging strategy mediates ectotherm predator-prey responses to climate warming. Ecology. https://doi.org/10.1002/ecy.3146
von Liebig J (1840) Die organische Chemie in ihrer Anwendung auf Agricultur und Physiologie. Friedrich Vieweg und Sohn Publ, Co, Braunschweig, Germany
Wickham H (2016) ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag, New York
Wilder SM, Norris M, Lee RW, Raubenheimer D, Simpson SJ (2013) Arthropod food webs become increasingly lipid-limited at higher trophic levels. Ecol Lett 16:895–902. https://doi.org/10.1111/ele.12116
Zhigulskaya ZA, Shekhovtsov SV, Poluboyarova TV, Berman DI (2022) Formica picea and F candida (Hymenoptera: Formicidae): Synonyms or two species? Divers-Basel. https://doi.org/10.3390/d14080613
Zvereva EL, Kozlov MV (2023) Predation risk estimated on live and artificial insect prey follows different patterns. Ecology 104:13. https://doi.org/10.1002/ecy.3943
Acknowledgements
We would like to thank our organisations, the Muzeum Vysočiny Jihlava and the Biology Centre of CAS, for their support in carrying out this research. We thank Lena Dušátková for discussing the feeding ecology of ants and Magdaléna Tichá and Alžběta Ruschková for technical support. Jiří Juřička is acknowledged for consulting with us the vegetation characteristics of the sites.
Funding
The study was supported by Interreg V-A, Project ATCZ214.
Author information
Authors and Affiliations
Contributions
KB and PB conceived the study, performed the experiments and collected the data. KB and PK assembled the dataset for analyses. PF and PK conceived the analyses. PF and PK wrote the code and analysed the data. KB, PB and PK formulated hypotheses and interpreted the results. PK led writing of the manuscript, with KB contributing significantly to the methods. All authors have contributed critically to the drafts and gave final approval for publication of the submitted manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
For this type of study, ethics approval was not required.
Additional information
Communicated by Sylvain Pincebourde.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bezděčková, K., Bezděčka, P., Fibich, P. et al. Different feeding preferences for macronutrients across seasons and sites indicate temporal and spatial nutrient limitation in the black bog ant. Oecologia 204, 959–973 (2024). https://doi.org/10.1007/s00442-024-05545-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-024-05545-8