Abstract
Birds are excellent vectors of allochthonous matter and energy due to their high mobility, with more intense flow when waterbirds congregate in breeding colonies, feeding in surrounding aquatic and terrestrial areas, and promoting nutritional pulses to nutrient-poor environments. In southern Brazil, a swamp forest on an estuarine island is used by waterbirds for breeding, providing an opportunity to investigate the potential effects of transport of matter between nutrient-rich environments. Soil, plants, invertebrates, and blood from terrestrial birds were collected and stable isotopes compared to similar organisms in a control site without heronries. Values of δ15N and δ13C from waterbirds were higher in the colony in comparison to the control site (spatial effect). The enrichment of 15N and 13C provided during the active colony period persisted after the breeding period, especially for δ15N, which was higher in all compartments (temporal effect). Moreover, the enrichment of 15N occurred along the entire trophic chain (vertical effect) in the colony environment, including different guilds of invertebrates and land birds. The enrichment in 13C seems to lose strength and was mostly explained by factors such as trophic guild rather than site, especially in birds. Bayesian mixture models with terrestrial vs. estuarine endpoints demonstrated that all organisms from both colony and control environments had assimilated estuarine matter. Finally, detritivorous invertebrates showed greater assimilation when compared to other guilds. This study demonstrates that adjacent nutrient-rich environments, such as palustrine forests and estuaries, are nutritionally enriched in several dimensions from nearby autochthonous subsidies that are maintained throughout the year.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mobile and migratory animals are good vectors of matter and energy between environments (Polis et al. 1997; Sanzone et al. 2003; Korobushkin 2014) because mobility is associated with transport capacity (Fariña et al. 2003). In addition to mobility, body size is an important factor for ecosystem functioning, because larger individuals tend to play a more substantial contribution for nutrient cycling (Vanni 2002; Norkko et al. 2013). Classic examples of flows between aquatic and terrestrial ecosystems are those promoted by bears, which transport salmon to forested riparian environments during migration from sea to rivers (Hilderbrand et al. 1999). Birds can also transport matter from their foraging areas in the sea, rivers, and lakes, moving part of the ingested or assimilated matter to colonies on land (Polis et al. 1997; Keatley et al. 2009; Gaiotto et al. 2022).
Waterbirds group together in colonies to increase protection from predators in specific locations where they can obtain food for themselves and their offspring in nearby areas (Frederick 2002; Keatley et al. 2009; Britto and Bugoni 2015). Pelecaniformes (herons, spoonbills, and ibises) depend on shallow aquatic environments for feeding and breeding (Frederick 2002). Foraging in areas adjacent to colonies results in a flow from the aquatic to the terrestrial environment mediated by waterbirds (Green and Elmberg 2014; Faria et al. 2016), with temporal and intense matter inputs, generating a nutritional pulse.
The intensity of matter and energy input, as well as spatial and temporal variation (Polis et al. 1997), can influence the receiving biological communities, as well as the water and soil (Polis et al. 1997; Keatley et al. 2009). The flow occurs primarily through guano deposition, which can have a positive and rapid impact on primary productivity and site diversity (Green and Elmberg 2014), both terrestrial and aquatic. This occurs because guano is rich in essential nutrients for producers, such as nitrogen and phosphorus, and can impact primary productivity and subsequently dissipate along the trophic chain (Polis et al. 1997; Kolb et al. 2010). In this way, this generates an increase in the nutritional quality of primary producers, which is transferred vertically along the trophic chain (Caut et al. 2012; Savage 2019; Gaiotto et al. 2022; Linhares and Bugoni 2023). In addition to guano, waterbirds also transfer matter and energy through nest-building material, feathers, eggs, regurgitated food, and carcasses (Polis et al. 1997; Caut et al. 2012). Thus, bird colonies alter the dynamics of the local trophic web, influencing the populations of animals feeding on allochthonous sources directly or indirectly (Sánchez-Piñero and Polis 2000; Caut et al. 2012). In this scenario, the transport of aquatic resources carried out by birds is a key process for the trophic webs of islands, and they are often able to maintain the biodiversity of these environments (Sánchez-Piñero and Polis 2000; Green and Elmberg 2014).
Analysis of fluxes of matter between environments elucidates questions about how organisms cope with the nutritional pulses (Correa and Winemiller 2014). In this context, stable isotope analysis (SIA) is a useful tool to track transported matter and provide information about its effects on the environment (Horn et al. 2019). Carbon isotopes (δ13C) allow for the tracking of the flow due to different values between C3 and C4 plants, remaining relatively similar along the trophic chain, while δ15N values increase predictably along the trophic chain, 2‰–5‰ per trophic level (Peterson and Fry 1987; Fry 2006). Therefore, it is possible to infer the horizontal flow or dissipation of matter through δ13C analysis whenever there are isotopic differences in the composition of primary producers and a vertical flow or dissipation of matter along the trophic chain. Several studies of plants, birds, rodents, arthropods, and lizards on marine islands occupied by bird colonies have demonstrated increased δ15N values from these groups compared to resident organisms from islands without colonies (e.g., Stapp et al. 1999; Stapp and Polis 2003; Caut et al. 2012).
In addition, strategies such as grouping organisms into trophic guilds, that is, groups that exploit the same class of resources (Root 1967), associated with isotopic analyses, allow verification of the use of resources according to the food preferences of consumer groups (Layman et al. 2012; Hamer et al. 2015; Navarro et al. 2021; Villegas et al. 2021). These studies have shown that transported allochthonous matter can be accessed by organisms living in the receiving environment (Savage 2019). Trophic subsidies are especially important when there is asymmetry in the productivity of environments, i.e., from nutrient-rich to nutrient-poor (Polis et al. 2004). There have been several studies on matter flux from marine islands to arid environments, which are typically poor environments and thus dependent on allochthonous subsidies of energy and matter from the sea (e.g., Stapp et al. 1999; Sánchez-Piñero and Polis 2000; Kolb et al. 2010; Savage 2019). However, studies on the subsides between environments with high productivity, such as swamp forest regions and estuarine aquatic environments are still lacking.
In this context, the present study aims to analyze patterns of matter dissipation, vertically and horizontally, mediated by waterbirds in a large colony and incorporated by organisms in the terrestrial environment on an estuarine island. Specifically, we hope to demonstrate that (i) colony-mediated transport of matter generates isotopic enrichment in resident organisms of the swamp environment due to inputs of nutrients from the estuary in the resident organisms of the swamp environment; (ii) soil, plants, invertebrates, and resident land birds of the colony environment will be enriched in 15N compared to the control environment; (iii) terrestrial enrichment mediated by colonial birds during the breeding period will be present in the colony environment even during the nonbreeding period; (iv) dissipation of estuarine matter obtained from foraging areas will reach higher trophic levels in the colony environment; and (v) assimilation of estuarine matter will occur only in the colony environment.
Materials and methods
Study area
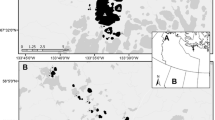
Marinheiros Island, Lagoa dos Patos estuary, southern Brazil (Fig. 1), is the largest island in the estuary and is composed of wetlands, shallow lakes, dunes, and swamp forests (Quintela et al. 2009). The climate is temperate subtropical, with minimum and maximum temperatures ranging from 9.5 to 27 °C, respectively (Vieira 1983). The highest precipitation occurs between July and September, with an annual average of 1522 mm (Vieira 1983). The colony of Pelecaniformes on Marinheiros Island is occupied by seven species of herons and egrets (Ardeidae): great egret Ardea alba, cocoi heron Ardea cocoi, cattle egret Bubulcus ibis, little blue egret Egretta caerulea, snowy egret Egretta thula, black-crowned night heron Nycticorax nycticorax, and yellow-crowned night heron Nyctanassa violacea, as well as by roseate spoonbill Platalea ajaja (Threskiornithidae) (Gianuca 2010). The occupation of the colony, except for A. cocoi that uses the site year-round, is seasonal, from late August to March (Gianuca 2010). The area is approximately 150 × 100 m, separated from the estuarine environment by an approximately 70 m wide strip of Scirpus giganteus (Gianuca 2010). The colony site is predominantly composed of tree species with a C3 photosynthetic cycle (Faria et al. 2016), such as the cockspur coral tree (Erythrina crista-galli), Brazilian pepper tree (Schinus terebinthifolius), and fig tree (Ficus cestrifolia).
Approximately 5 km northeast from the colony site, on the opposite coast of the island, a swamp forest without colony or waterbird roosts was chosen as a control site (Fig. 1) due to its floristic characteristics and similar proximity to the estuarine shoreline. This area is 600 × 200 m, separated from the lagoon by a marsh with a predominance of ferns Acrostichum danaeifolium and Cyperus spp. It also presents a predominance of C3 plant species, such as S. terebinthifolius, E. crista-galli, and large Ficus spp. The colony and control sites are isolated from each other, even for the most mobile organisms, such as land birds, as demonstrated by no recapture between sites during over 254 h of mist netting effort and individually tagged with metal rings provided by CEMAVE/ICMBio along 3 years.
Sampling
Sampling occurred in two summers and two winters in 2018 and 2019 at the end of each season, corresponding to periods with and without waterbird breeding, respectively (Supplementary Material Table S1). Soil collection occurred in triplicate in each of the study sites, randomly, during each sampling season. Soil samples were stored in plastic jars and frozen until preparation for isotopic analysis. Two to three buds of plants with a C3 photosynthetic cycle (A. danaeifolium, Crocosmia crocosmiiflora, Dioscorea sp., E. crista-galli, Heteranthera reniformis, and Hibiscus diversifolius), predominant in both environments, were randomly collected during each sampling period, stored in plastic bags, and kept frozen until identification and preparation for isotopic analysis. Plants were identified using guides (Sobral 2006; Sobral et al. 2012) and with support from experts from the Floristics Laboratory at Universidade Federal do Rio Grande—FURG.
Abundant invertebrates at both sampling sites were randomly collected individually with tweezers through an active search in soil and vegetation. After collection, they were stored in plastic jars and frozen until preparation for analysis. The invertebrates were identified with support of experts and identification keys: Armadillidium sp., Camponotus sp. ants, Lycosa sp., Scolopendridae, and Trichonephila clavipes spiders.
Approximately 30 individuals of various land bird species (Table S1) were also captured in each sampling site (colony and control) per season. Captures occurred using six mist nets, 30 mm mesh, and 12 m long. Mist netting was distributed homogenously between seasons (summer and winter), years (2018 and 2019), and sites (colony and control). After capture, birds were identified (Narosky and Yzurieta 2011; Timm and Timm 2016) and a few drops of blood (approximately, 0.2 ml) were collected from the brachial vein using a hypodermic syringe.
Stable isotope analysis (SIA)
Soil samples were oven-dried at 60 °C, ground with pestle and mortar, acidified with 10% hydrochloric acid (HCl) to remove carbonates, washed in distilled water, oven-dried for about 12 h, and weighed (2 mg) for stable isotope analysis (Kennedy et al. 2005; Levin and Currin 2012). Plant tissues were washed with distilled water, dried for 24 h, ground as described above, and weighed (3 mg). Whole bodies of the collected invertebrates were prepared for isotopic analysis. Since lipid-rich tissues are depleted in 13C, lipids were extracted from the samples in a Soxhlet apparatus for 6 h using petroleum ether as solvent (Bugoni et al. 2010). Samples were subsequently lyophilized for 24 h, homogenized, and weighed (1 mg) (Mancini and Bugoni 2014). Whole blood samples from land birds were also freeze-dried for 24 h and weighed (0.7 mg). In passerines, the estimated δ13C half-life for whole blood is around 2.5 weeks (Hahn et al. 2012), reflecting the diet assimilated during this period. All samples were packed in tin capsules for analysis in an isotope ratio mass spectrometer (IRMS) coupled to an elemental analyzer at the Integrated Analysis Center, Federal University of Rio Grande—FURG (CIA-FURG, Brazil). The internal standards of the laboratory (glutamic acid and caffeine) had a standard deviation of 0.1‰ for δ13C and 0.4‰ for δ15N.
The determination of the values was expressed by the δ notation, in parts per thousand (‰). The standards used were VPDB (Vienna Pee Dee Belemnite limestone) for carbon and AIR for nitrogen.
Data analysis
Isotopic variation
To assess isotopic variation in soil, producers (C3 plants), and consumers (invertebrates and land birds), we performed generalized linear models—GLMs (McCulloch and Searle 2000) with Gaussian distribution and identity family. We used values of δ15N and δ13C for all groups, except for plants, for which only δ15N values were considered, as plants obtain their carbon from atmospheric air (Table 1).
The factors used in all models were sites (2 levels—colony and control); season (2 levels—winter and summer); and year (2 levels—2018 and 2019). For the models using plants, the species factor was included (6 levels—A. danaeifolium, C. crocosmiiflora, Dioscorea sp., E. crista-galli, H. reniformis and H. diversifolius). For invertebrates, the guild factor was included (3 levels—carnivorous, omnivorous, and omnivorous–detritivorous). The determination of trophic guilds for invertebrates followed Oelbermann and Scheu (2002), Ronque (2013), and Montesanto and Cividini (2017).
For models using land birds, the guild factor (5 levels—frugivorous, granivorous, insectivorous, nectarivorous, and omnivorous) was included. The guilds of the land birds followed the classifications found in the bibliography (Supplementary Material Table S2). The groups consisted in frugivorous (feeding predominantly on fruits); granivorous (predominantly seeds); insectivorous (predominantly insects); omnivorous (feeding on fruits, arthropods and small vertebrates); and nectarivorous (feeding on nectar). Bird’s models also included feeding area, with four levels (factors): resident-restricted habitat (RRH); resident broad habitat (RBH); migrant-restricted habitat (MRH); and migrant broad area (MBH). For the categorization, we considered RRH as animals that live on the site throughout the year and are more restricted to the forest interior; RBH as animals that live on site throughout the year but are not restricted to the forest interior; MRH as animals that live only a period of the year on site (i.e., migratory) and are restricted to the forest interior; and MBH as animals that live a period of the year on site but are not restricted to the forest interior (i.e., migratory that also use open areas).
Several interactions among factors were tested, subsequently retaining the statistically significant interactions with the lowest AIC (Akaike information criterion) values of the model. The residuals of the GLMs were analyzed through an ANOVA to obtain the percentage of variation explained by each factor in the chosen model. Residuals were tested for normality, homoscedasticity, and independence, attending all assumptions for using a Gaussian distribution. The GLMs were carried out in R software version 4.1.2 (R Core Team 2022), using the packages “arm” version 1.11-2 (Gelman and Hill 2006), “lme4” version 1.1-27-2 (Bates et al. 2015), and “car” version 3.0-11 (Fox and Weisberg 2019). The p values were regarded as significant if < 0.05.
Estimation of estuarine and terrestrial contribution to consumers
The δ15N and δ13C values were used to estimate the estuarine and terrestrial contribution in primary and secondary consumers of the colony, as well as to determine the assimilation of allochthone matter by each trophic guild of birds and terrestrial invertebrates. The vertical dissipation, i.e., along trophic levels, determined by nitrogen increased isotopic values, was determined through Bayesian isotopic mixing models generated in R software using the “simmr” package version 0.4.5 (Parnell 2021). The source for the terrestrial environment was represented by the isotopic mean of the C3 photosynthetic pathway (leaf bud values) from the control environment for each season, due to the estuarine influence on the colony vegetation. As sources of nutrients mediated by colonial waterbirds and potentially representing estuarine baseline values, we used mean isotopic values from samples of particulate organic matter (POM) and sedimentary organic matter (SOM), which include photosynthesizing organisms such as phytoplankton and microphytobenthos, and green algae, through Ulva sp. and Rhizoclonium sp.
For the baseline for primary production determination, we used δ15N of primary producers analyzed prior to our sampling season. We followed this approach based on results of a previous study conducted at the same estuary (Lagoa dos Patos), in which a set of models using δ15N values along a period of 9 years indicated that the best temporal window to use when setting up a baseline for primary production is the season prior to the collection season (Possamai et al. 2021). Therefore, instead of using an interpolated approach, in which we would consider δ15N from the previous and the sampling seasons for primary producers, we used a delayed approach, in which we just considered 2017 and 2018 values, as they were the years prior to this study and accounted for substantial environmental variability (see supplementary material for details and Possamai et al. 2021). The values used to build the baseline (Supplementary Material Table S3) were obtained from the Brazilian Long-Term Ecological Research program (PELD-ELPA; www.peld.furg.br and supplementary material S2), which has been carried out at the Lagoa dos Patos estuary since 1998.
The guilds of land birds used in the mixture models were defined as those used for the GLMs. However, we only used insectivorous and omnivorous trophic guilds for the mixture models, because these are the groups present in both sites with sufficient individuals for the analysis. Mixture models of each trophic guild of invertebrates followed the same classification as GLMs. Because source values in 2018 and 2019 did not differ between seasons and between sites, consumers from the 2018 and 2019 seasons were grouped into a single year. The source–consumer trophic discrimination factors (TDFs) used for ΔC and ΔN were selected according to the diet and tissues of the consumers (Supplementary Material Table S4).
Results
Spatial dissipation
For δ15N values the input of matter into the colony environment from, or mediated by, waterbirds was demonstrated when compared to the control site for all ecosystem compartments, i.e., soil, plants, invertebrates, and land birds (Fig. 2). The variable “site” was significant, and explained 79%, 22%, 61%, and 15% of the data variation for models developed for soil, plant, invertebrate, and land birds, respectively (Table 1).
Stable isotope values of δ15N in soil (a), vegetation (b), invertebrates (c), and blood of land birds (d) from the colony and control environments during the summer and winter seasons. Values indicate the mean (central bars) and standard deviation (rectangles), with minimum and maximum values (outer lines)
For δ13C values, the factor “site” showed positive effects in the soil and land bird models. Despite this, there were negative effects in invertebrates for δ13C (Table 1). The ANOVA of these models indicated that the “colony” site explained the variation in δ13C values by 26% for soil. For land birds, the effect of site lost the significance indicated by the GLMs when analyzed by ANOVA (Supplementary Material Table S5). For invertebrates, the effect was negative, and although significant, “site” explained only 2.4% of the data variation (Supplementary Material Table S5). The results showed a smaller difference in carbon when compared to nitrogen, but that could still be noticed in the soil samples compared to the control site (Fig. 3).
Temporal dissipation
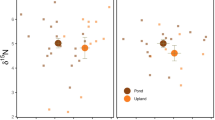
Temporal variation in δ15N values (with and without breeding waterbirds, i.e., summer vs. winter) occurred only for invertebrates, with the season factor “winter” indicating negative effects. The interaction between the factors “site” and “season” showed a positive effect on the colony in winter (Table 1). Despite this, the model showed no statistical significance for the seasonal effect and retained significance in the interaction, which explained only 2% of the variance in the data (Supplementary Material Table S5). This pattern was corroborated graphically (Fig. 4), as during winter the isotopic values of organisms in the colony environment maintained their positions at the top of the graph, thus indicating enrichment of 15N compared to organisms from the control site (Fig. 4b, d).
For δ13C values, the only group that showed a significant difference in GLMs was land birds, which showed negative effects for the factor “season” (Table 1). In addition, the interaction “site*season” showed positive effects: season explained 2.2% of the variation in the data, while the interaction site*season explained 6.1% (Supplementary Material Table S5), thus indicating the tendency of reducing δ13C values in the blood of land birds during winter in the colony site (Fig. 3).
Dissipation along the trophic chain (vertical)
Soil, vegetation, and invertebrates showed positive effects on δ15N values in the colony site (Table 1). The variance on the data for the vegetation model with δ15N values was explicated by “species” (32.2%) and “site” (22.3%) factors (Supplementary Material Table S5). Land birds showed positive effects, but despite this, the group of migratory birds with a wide habitat (MBH) had negative effects on values. The data variance was explicated in 14.8% by “site” and in 3.3% by “habitat” used by birds (Supplementary Material Table S5). The positive effects on the vegetation occurred for both Dioscorea sp. and C. crocosmiiflora, indicating that both herbaceous plants and trees responded positively to nitrogen enrichment from allochthonous sources in the colony site.
For carbon isotopes, the colony site showed positive effects for soil values and negative effects for invertebrate values. The omnivorous–detritivorous invertebrate guild had positive effects on values (Table 1). Colony soil values explicated 26.8% of the variance in δ13C values. For invertebrates, the factor “site” explicated 2.4% of the data variance, while “guild” explained 22% of the variations in δ13C values (Table 1). Moreover, there was a positive effect on the mean δ13C values for birds from the colony compared to the control site. However, similar to the results found for δ15N, there was a negative effect on migratory birds that do not exclusively use the colony forest for feeding (MBH). The ANOVA indicated explicabilities of 3.2% and 8.1% for the factors “habitat” and “guild”, respectively (Supplementary Material Table S5). The factor “site” did not vary significantly.
Bayesian mixture models
Bayesian mixture models with two end points—terrestrial and estuarine—showed that all consumers, in both control and colony environments, had estuarine isotopic signatures in their tissues or from other sources with values similar to those found in the nearby estuarine waters (Fig. 5; Supplementary Material Table S6). The higher probability of assimilation of estuarine matter into the tissues of invertebrates in the colony and control environments, respectively, was 31.2% ± 2.2% and 37.4% ± 5.1% (omnivorous) (Fig. 5a), 48.3% ± 4.1% and 44.2% ± 2.8% (omnivorous–detritivorous) (Fig. 5b), and 35.2% ± 4.4% and 43% ± 11.5% (carnivorous) (Fig. 5c). For land birds, the higher probabilities of assimilation of estuarine matter in the colony and control environments were 29.6% ± 2.8% and 35.2% ± 3.4% (omnivorous) (Fig. 5d) and 25.1% ± 4.0% and 34.3% ± 3.2% (insectivorous), respectively (Fig. 5e). Thus, the omnivorous–detritivorous invertebrate group showed a predominance of estuarine matter compared to the control environment in both seasons (Fig. 5b). Furthermore, during the summer season, omnivorous birds showed a higher probability of assimilating estuarine matter than the control (Fig. 5d). The other groups (omnivorous and carnivorous invertebrates and insectivorous birds) demonstrated a higher likelihood of assimilation of estuarine matter in the control environment than in the colony environment in both seasons (Fig. 5a, c, e).
Discussion
Nutrients transported by herons and spoonbills support the environment and the entire trophic web where they breed. This conclusion was based on analyses in several dimensions—spatial, temporal, and vertical—and in different compartments of the swamp forest ecosystems of the colony compared to a control non-colony site. This study demonstrates that the colony environment is enriched in 15N and 13C compared to the control environment, although this occurs to a lesser degree for 13C (spatial dissipation). Resources remain in the environment even after the waterbird-breeding season, demonstrating that the nutrient load deposited during spring–summer is a key resource for the trophic web of the swamp forest long afterward (temporal dissipation). In addition, a vertical analysis (along the trophic chain) showed that estuarine subsidies reach higher trophic levels. However, there are other important factors, such as the influence of trophic guild and feeding habitat. Omnivorous–detritivorous invertebrates, for example, tended to assimilate estuarine matter more frequently than other invertebrate groups. These results may indicate that, because of their detritivorous habit of consuming matter available on the soil, they directly access the matter transported to the colony by waterbirds. This increases their assimilation of estuarine matter in comparison to other invertebrate guilds. Furthermore, migratory birds using broad habitats (MBH) showed lower isotopic values and lower assimilation of estuarine matter than other groups of land birds. This indicates that, because they are migratory and not limited to the colony forest, they use less enriched food resources. Therefore, their tissues were less isotopically enriched in comparison to other bird groups in the colony forest.
Spatial dissipation
The δ15N and δ13C values in the organisms and soil of the colony environment were higher than those of the control environment. This indicates that the presence of waterbirds isotopically enriches the colony forest. Furthermore, because predatory waterbirds occupy high trophic levels, they have excreta enriched in 15N (Caut et al. 2012). Thus, guano and deposited nitrogen-enriched matter are accessed by organisms at low trophic levels in the terrestrial environment surrounding the colony (Gaiotto et al. 2022; Linhares and Bugoni 2023), generating an upward enrichment cascade across all higher trophic levels. The nitrogen isotope analysis showed that waterbird colony promoted 15N enrichment, as demonstrated by the strong effect on the GLM with δ15N values (Table 1; Supplementary Material Table S5). Similarly, δ15N in soil, vegetation, invertebrates, and land birds in the colony environment showed higher values than those in the control site. Other studies conducted in oligotrophic receptor environments show that bird colonies, through the deposition of guano and matter, tend to enrich these environments (Polis et al. 1997; Kolb et al. 2010; Caut et al. 2012; Savage 2019). In the present study, it was shown that enrichment also occurs in a productive receptor environment, such as swamp forests. This is likely due to the intensity of loading, with recurrent breeding at the same site, mediated by large-sized and numerous waterbirds. Our results suggest enrichment by guano, tissues of waterbirds, and their prey, from estuarine areas, or nutrients with high δ15N values, into the swamp forest, and from soil to land birds (Fig. 2). The isotopic nitrogen values of the organisms were higher in the colony when compared to the control environment, as shown in Fig. 4a, c, reinforcing the enrichment by 15N in this environment. Despite this, our study indicates that this deposition is widely distributed throughout the colony area, as everything from soil to vertebrates appears enriched. This spatial distribution in the colony environment occurs through biotic and abiotic vectors (Green and Elmberg 2014; Schindler and Smits 2016; Griffiths et al. 2018; Gaiotto et al. 2020), with water-saturated soil likely playing a key role, similar to water runoff on oligotrophic tropical islands (e.g., Sánchez-Piñero and Polis 2000; Gaiotto et al. 2022).
Although the use of carbon isotopes as a marker of matter flux in complex environments, such as estuaries, can be inefficient (e.g., Spano et al. 2014), our results indicate that this is a useful tool for understanding the functioning of estuarine environments, confirming previous findings (e.g., Domingos and Lana 2017; Mont’Alverne et al. 2016). The constant input of terrestrial matter from C3 plants that flows into the water may reduce δ13C values (Spano et al. 2014). Moreover, in estuarine aquatic environments, the presence of C4 plants (phanerogams, algae in tidal flats) contributes to the enrichment of 13C, resulting in significantly higher mean values in the estuary (Garcia et al. 2007; Pereyra et al. 2016). Therefore, the significant differences in δ13C values found in the colony soil in comparison to the control demonstrate the importance of carbon as a natural marker, complementary to the nitrogen isotope. The results also demonstrate that it is possible to detect 13C enrichment generated by the transport of estuarine matter into terrestrial environments, even when the receiving environment is highly productive, as in swamp forests. Furthermore, regular heron breeding at the site, year after year, can lead to saturation of the environment, making it well marked isotopically.
Temporal dissipation
Soil, invertebrates, plants, and terrestrial birds maintained or increased values of δ15N even during the winter without the presence of the colonial waterbirds. This indicates that the pulse is so intense that the enrichment of the environment persists in the soil and vegetation. Similar studies on marine islands also indicate that once the vegetation is isotopically enriched, the marine signature persists in terrestrial consumers, even during periods without the presence of the colonial seabirds (Stapp and Polis 2003; Caut et al. 2012; McLoughlin et al. 2016).
For δ13C values, only land birds had a negative effect during the winter, although the effect shown was smaller than that shown for δ15N. It is expected that with the pause in the matter input pulse, the enrichment will not reach higher trophic level organisms, such as birds, due to the loss of trophic signal. In addition, other factors are involved in differences found between groups, such as the difference in tissue turnover related to each animal group, the guild, and the position in the trophic chain (Boecklen et al. 2011). However, for both isotopes, there is an indication that outside of reproductive periods, the soil and vegetation allow organisms in the environment to continue utilizing matter of allochthonous origin. Other studies have indicated that guano carried by birds has the ability to enrich producers in the environment (Shatova et al. 2017; Savage 2019). However, plants obtain their carbon from atmospheric air, and therefore 13C enrichment does not affect producers but rather organisms that feed on the carcasses of herons that die in the colony and the food scraps transported from the estuary. The δ13C values of these organisms are derived from their feeding and reflect the values at the base of the trophic chain (Craig 1953; DeNiro and Epstein 1981). Therefore, since vegetation carbon values are independent of allochthonous input, carbon enrichment ultimately has a limited influence on the trophic web. Even though, the colony shows higher isotopic carbon values in the winter compared to the control site at the same season, demonstrating the persistence of temporal enrichment by waterbirds.
Furthermore, our results demonstrated the depletion of 13C in invertebrates in 2018 compared to 2019. This may indicate that matter dissipation during the winter of 2019 may have been influenced by El Niño, when longer and intense periods of precipitation occur (Odebrecht et al. 2017). Periods of inundation caused by rainfall may aid in the dispersal of colony-deposited matter, such as guano, leading to its dissipation further into the environment in rainy years. Other studies have also demonstrated that some of the nutrient distribution in the environment is driven during periods of flooding and precipitation (Caut et al. 2012; Schindler and Smits 2016; Li et al. 2017; Gaiotto et al. 2022). This may indicate that abiotic factors such as rainfall and inundation periods, in addition to transport by biotic vectors, also contribute to the temporal dissipation of transported nutrients, similar to oligotrophic environments (Gaiotto et al. 2022).
Vertical dissipation and trophic guilds
Estuarine-derived nutrients were detected in the soil and tissues of all groups of organisms analyzed in the colony, and 15N enrichment was higher in the colony than in the control site. This indicates a link between the bird colony and the local trophic web, with the latter being dependent on allochthonous nutrient inputs mediated by waterbirds. Both herbaceous plants and trees were enriched by the input of estuarine matter, with no significant differences between them. Only the fern group showed no significant enrichment in the colony environment compared to the control. However, this group of plants was collected at the edges of the colony region, which may indicate that as the distance from the core region where a higher concentration of nests occurs, the enrichment loses intensity. Similarly, other studies have also shown that distance from the source of matter, whether rivers, ocean, or colonies, is important in regard to the dissipation of the isotopic signature (Caut et al. 2012; Korobushkin 2014; Gaiotto et al. 2022).
In primary consumers, the average enrichment was maintained compared to soil and vegetation. However, when separated into trophic guilds, we observed differences in the assimilation of estuarine matter. The omnivorous invertebrates (ants) of the genus Camponotus, which feed predominantly on plant matter, invertebrates, and fruits (Ronque 2013), showed lower levels of estuarine matter assimilation during winter (Fig. 5a). A similar pattern occurred with carnivorous invertebrates (Fig. 5c), which do not feed directly on producers but have isotopic signatures of their prey (DeNiro and Epstein 1981). In the guild of omnivorous–detritivorous (Armadillidium, common pill woodlouses), the pattern also emerged in the colony but not in the control environment, where there was an increased probability of assimilation during winter. Another difference found between invertebrate groups and the omnivorous–detritivorous guild was the higher probability of assimilation of estuarine matter in both seasons in the colony in comparison to the control (Fig. 5b). These results suggest that omnivorous–detritivorous common pill woodlice feed on sources other than matter from local producers, such as dead nestlings and food scraps carried by waterbirds (Markwell and Daugherty 2002).
Land birds at higher trophic levels in the colony environment had 15N enrichment in their blood. Besides that, the results suggest that a dissipation of nitrogen upward in the trophic chain (vertical dissipation) is also occurring, which was observed in insects and resident birds. This is the same pattern of dissipation found in marine islands (e.g., Gaiotto et al. 2022), which indicates that the sources of nitrogen throughout the trophic web is likely due to the input of guano and other waterbirds-origin material.
Similar to results found for δ15N values, the allochthonous carbon load seems to dissipate along the trophic web, but in the group of land birds, other factors seem to have a greater influence. Except for omnivorous birds during winter, the assimilation in insectivorous (both seasons) and omnivorous (summer) bird guilds had a higher probability and assimilation of estuarine matter in the control environment compared to the colony (Fig. 5d, e). These results can potentially be explained by the fact that the forest boundaries of the control environment are closer to the estuary than the colony environment. Previous studies have pointed out that emerging invertebrates in aquatic environments are important sources for terrestrial consumers (Nakano and Murakami 2001; Schindler and Smits 2016; Recalde et al. 2021). Therefore, we suggest that even in the control site not receiving any heron-mediated estuarine matter input, resident animal groups utilize external food resources associated with the estuarine environment found at the forest margins. Besides that, migratory birds, with a broad foraging habitat (MBH) and large movements, i.e., not feeding exclusively within the colony forest, tended to be less enriched than photophobic, forest-restricted bird groups. This group had lower 13C and 15N enrichment, suggesting that the length of time organisms had been present in the environment also influences the level of enrichment in tissues, due to the shorter exposure time to enriched foods from the colony site (DeNiro and Epstein 1978; Zupcic-Moore et al. 2017).
Conclusion
This study demonstrated that waterbird colonies located on estuarine islands are able to isotopically enrich the environment in which they are established, even though fluxes occur among two productive environments, i.e., estuarine waters and swamp forests. The 13C and 15N enrichment was substantial in the colony environment compared to the control, even long after the waterbird-breeding period. This pattern suggests that even in swamp forest environments, recognized as rich in organic matter and high primary productivity levels, the matter transported by birds and nutrient load is maintained throughout the year. However, further studies are necessary to understand the benefits of the estuarine matter assimilated to demographic parameters, physiology, and health of organisms in the environment. We conclude that the colony is an important source of nutrients, energy, and matter, and that it is able to support the environment throughout the year, contributing to ecological processes in swamp forests. Thus, environmental impacts that lead waterbirds to stop using the environment for breeding could cause a reduction in the availability of resources, generating impacts on the population of organisms that are already familiar with the pulses of nutrients from the estuary.
Data availability
All data produced from this study are provided in this manuscript.
Code availability
Not applicable.
References
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Boecklen WJ, Yarnes CT, Cook BA, James AC (2011) On the use of stable isotopes in trophic ecology. Annu Rev Ecol Syst 42:411–440. https://doi.org/10.1146/annurev-ecolsys-102209-144726
Britto VO, Bugoni L (2015) The contrasting feeding ecology of great egrets and roseate spoonbills in limnetic and estuarine colonies. Hydrobiologia 744:187–210. https://doi.org/10.1007/s10750-014-2076-1
Bugoni L, McGill RAR, Furness RW (2010) The importance of pelagic longline fishery discards for a seabird community determined through stable isotope analysis. J Exp Mar Biol Ecol 291:190–200. https://doi.org/10.1016/j.jembe.2010.06.027
Caut S, Angulo E, Pisanu B, Ruffino L, Faulquier L, Lorvelec O, Chapuis J, Pascal M, Vidal E, Courchamp F (2012) Seabird modulations of isotopic nitrogen on islands. PLoS ONE 7:e39125. https://doi.org/10.1371/journal.pone.0039125
Correa SB, Winemiller KO (2014) Niche partitioning among frugivorous fishes in response to fluctuating resources in the Amazonian floodplain forest. Ecology 95:210–224. https://doi.org/10.1890/13-0393.1
Craig H (1953) The geochemistry of the stable carbon isotopes. Geochim Cosmochim Acta 3:53–92. https://doi.org/10.1016/0016-7037(53)90001-5
DeNiro MJ, Epstein S (1981) Influence of diet on the distribution of nitrogen isotopes in animals. Geochim Cosmochim Acta 45:341–351. https://doi.org/10.1016/0016-7037(81)90244-1
Domingos AM, Lana PC (2017) Detecting multiple states of trophic connectivity between mangroves and salt marshes. Ecosystems 20:1179–1189. https://doi.org/10.1007/s10021-016-0101-0
Faria FA, Silva-Costa A, Gianuca D, Bugoni L (2016) Cocoi heron (Ardea cocoi) connects estuarine, coastal, limnetic and terrestrial environments: an assessment based on conventional dietary and stable isotope analysis. Est Coast 39:1271–1281. https://doi.org/10.1007/s12237-016-0073-5
Fariña JM, Salazar S, Wallem KP, Witman JD, Ellis JC (2003) Nutrient exchanges between marine and terrestrial ecosystems: the case of the Galapagos sea lion Zalophus wollebaecki. J Anim Ecol 72:873–887. https://doi.org/10.1046/j.1365-2656.2003.00760.x
Fox J, Weisberg S (2019) An R companion to applied regression, 3rd edn. Sage, Thousand Oaks
Frederick PC (2002) Wading birds in the marine environment. In: Schreiber EA, Burger J (eds) Biology of marine birds. CRC Press, Boca Raton, pp 618–655
Fry B (2006) Stable isotope ecology, 1st edn. Springer, New York
Gaiotto JV, Abrahão CR, Dias RA, Bugoni L (2020) Diet of invasive cats, rats and tegu lizards reveals impact over threatened species in a tropical island. Perspect Ecol Conserv 18:294–303. https://doi.org/10.1016/j.pecon.2020.09.005
Gaiotto JV, Nunes GT, Bugoni L (2022) Dissipation of seabird-derived nutrients in a terrestrial insular trophic web. Austral Ecol 47:1037–1048. https://doi.org/10.1111/aec.13196
Garcia AM, Hoeinghaus DJ, Vieira JP, Winemiller KO (2007) Isotopic variation of fishes in freshwater and estuarine zones of a large subtropical coastal lagoon. Estuar Coast Shelf Sci 73:399–408. https://doi.org/10.1016/j.ecss.2007.02.003
Gelman A, Hill J (2006) Data analysis using regression and multilevel/hierarchical models. Cambridge University Press, Cambridge. https://doi.org/10.1017/CBO9780511790942
Gianuca D (2010) Ecologia reprodutiva de oito espécies de Ciconiiformes em uma colônia no estuário da Lagoa dos Patos. MSc. Dissertation, Instituto de Oceanografia, Universidade Federal do Rio Grande–FURG, Rio Grande
Green AJ, Elmberg J (2014) Ecosystem services provided by waterbirds. Biol Rev 89:105–122. https://doi.org/10.1111/brv.12045
Griffiths HM, Ashton LA, Walker AE, Hasan F, Evans TA, Eggleton P, Parr CL (2018) Ants are the major agents of resource removal from tropical rainforests. J Anim Ecol 87:293–300. https://doi.org/10.1111/1365-2656.12728
Hahn S, Hoye BJ, Korthals H, Klaassen M (2012) From food to offspring down: tissue-specific discrimination and turn-over of stable isotopes in herbivorous waterbirds and other avian foraging guilds. PLoS ONE 7:e30242. https://doi.org/10.1371/journal.pone.0030242
Hamer KC, Newton RJ, Edwards FA, Benedick S, Bottrell SH, Edwards DP (2015) Impacts of selective logging on insectivorous birds in Borneo: the importance of trophic position, body size and foraging height. Biol Conserv 188:82–88. https://doi.org/10.1016/j.biocon.2014.09.026
Hilderbrand GV, Hanley TA, Robbins CT, Schwartz CC (1999) Role of brown bears (Ursus arctos) in the flow of marine nitrogen into a terrestrial ecosystem. Oecologia 121:546–550. https://doi.org/10.1007/s004420050961
Horn S, De la Vega C, Asmus R, Schwemmer P, Enners L, Garthe S, Haslob H, Binder K, Asmus H (2019) Impact of birds on intertidal food webs assessed with ecological network analysis. Estuar Coast Shelf Sci 219:107–119. https://doi.org/10.1016/j.ecss.2019.01.023
Keatley BE, Douglas MSV, Blais JM, Mallory ML, Smol JP (2009) Impacts of seabird-derived nutrients on water quality and diatom assemblages from Cape Vera, Devon Island, Canadian High Arctic. Hydrobiologia 691:191–205. https://doi.org/10.1007/s10750-008-9670-z
Kennedy P, Kennedy H, Papadimitriou S (2005) The effect of acidification on the determination of organic carbon, total nitrogen and their stable isotopic composition in algae and marine sediment. Rapid Commun Mass Spectrom 19:1063–1068. https://doi.org/10.1002/rcm.1889
Kolb GS, Jerling L, Hambäck PA (2010) The impact of cormorants on plant-arthropod food webs on their nesting islands. Ecosystems 13:353–366. https://doi.org/10.1007/s10021-010-9323-8
Korobushkin DI (2014) Role of allochthonous carbon in the energy of terrestrial invertebrate communities at different distances from the Black Sea and a freshwater lake (isotopic evidence). Russ J Ecol 45:223–230. https://doi.org/10.1134/s1067413614030060
Layman CA, Araujo MS, Boucek R, Hammerschlag-Peyer CM, Harrison E, Jud ZR, Matich P, Rosenblatt AE, Vaudo JJ, Yeager LA, Post DM, Bearhop S (2012) Applying stable isotopes to examine food-web structure: an overview of analytical tools. Biol Rev 87:545–562. https://doi.org/10.1111/j.1469-185x.2011.00208.x
Levin LA, Currin C (2012) Stable isotope protocols: sampling and sample processing. Scripps Institution of Oceanography, San Diego
Li J, Gilhooly WP, Okin GS, Blackwell J (2017) Abiotic processes are insufficient for fertile island development: a 10-year artificial shrub experiment in a desert grassland. Geophys Res Lett 44:2245–2253. https://doi.org/10.1002/2016GL072068
Linhares BA, Bugoni L (2023) Seabirds subsidize terrestrial food webs and coral reefs in a tropical rat-invaded archipelago. Ecol Appl 33:e2733. https://doi.org/10.1002/eap.2733
Mancini PL, Bugoni L (2014) Resources partitioning by seabirds and their relationship with other consumers at and around a small tropical archipelago. ICES J Mar Sci 71:2599–2607. https://doi.org/10.1093/icesjms/fsu105
Markwell TJ, Daugherty CH (2002) Invertebrate and lizard abundance is greater on seabird-inhabited islands than on seabird-free islands in the Marlborough Sounds, New Zealand. Ecoscience 9:293–299. https://doi.org/10.2307/42901404
McCulloch CE, Searle SR (2000) Generalized, linear, and mixed models. Wiley Series in Probability and Statistics, 1st edn. Wiley, New York
McLoughlin PD, Lysak K, Debeffe L, Perry T, Hobson K (2016) Density-dependent resource selection by a terrestrial herbivore in response to sea-to-land nutrient transfer by seals. Ecology 97:1929–1937. https://doi.org/10.1002/ecy.1451
Mont’Alverne R, Pereyra PER, Garcia AM (2016) Trophic segregation of a fish assemblage along lateral depth gradients in a subtropical coastal lagoon revealed by stable isotope analyses. J Fish Biol 89:770–792. https://doi.org/10.1111/jfb.12903
Montesanto G, Cividini S (2017) A crossover design to assess feeding preferences in terrestrial isopods: a case study in a Mediterranean species. Biologia 72:194–203. https://doi.org/10.1515/biolog-2017-0020
Nakano S, Murakami M (2001) Reciprocal subsidies: dynamic interdependence between terrestrial and aquatic food webs. Proc Natl Acad Sci USA 98:166–170. https://doi.org/10.1073/pnas.98.1.166
Narosky T, Yzurieta D (2011) Birds of Argentina and Uruguay: a field guide, 16th edn. Vazquez Mazzini Editores, Buenos Aires
Navarro AB, Magioli M, Bogoni JA, Moreira MZ, Silveira LF, Alexandrino ER, Luz DTA, Pizo MA, Silva WR, Oliveira VC, Donatelli RJ, Christianini AV, Piratelli AJ, Ferraz KMPMB (2021) Human-modified landscapes narrow the isotopic niche of Neotropical birds. Oecologia 196:171–184. https://doi.org/10.1007/s00442-021-04908-9
Norkko A, Villnas A, Norkko J, Valanko S, Pilditch C (2013) Size matters: implications of the loss of large individuals for ecosystem function. Sci Rep 3:2646. https://doi.org/10.1038/srep02646
Odebrecht C, Secchi ER, Abreu PC, Muelbert JH, Uiblein F (2017) Biota of the Patos Lagoon estuary and adjacent marine coast: long-term changes induced by natural and human-related factors. Mar Biol Res 13:3–8. https://doi.org/10.1080/17451000.2016.1258714
Oelbermann K, Scheu S (2002) Stable isotope enrichment (δ13C) in a generalist predator (Pardosa lugubris, Araneae: Lycosidae): effects of prey quality. Oecologia 130:337–344. https://doi.org/10.1007/S004420100813
Parnell AC (2021) Simmr: a stable isotope mixing model. R Package version 0.4.5. https://doi.org/10.1371/journal.pone.0009672
Pereyra PER, Mont’Alverne R, Garcia AM (2016) Carbon primary sources and estuarine habitat use by two congeneric ariid catfishes in a subtropical coastal lagoon. Zoologia 33:e20150075. https://doi.org/10.1590/s1984-4689zool-20150075
Peterson BJ, Fry B (1987) Stable isotopes in ecosystem studies. Annu Rev Ecol Syst 18:293–320. https://doi.org/10.1146/annurev.es.18.110187
Polis GA, Anderson WB, Holt RD (1997) Toward an integration of landscape and food web ecology: the dynamics of spatially subsidized food webs. Annu Rev Ecol Syst 28:289–316. https://doi.org/10.1146/annurev.ecolsys.28.1.289
Polis GA, Power ME, Huxel GR (2004) Food webs at the landscape level, 1st edn. University of Chicago Press, Chicago
Possamai B, Hoeinghaus DJ, Garcia AM (2021) Shifting baselines: integrating ecological and isotopic time lags improves trophic position estimates in aquatic consumers. Mar Ecol Prog Ser 666:19–30. https://doi.org/10.3354/meps13682
Quintela FM, Neves LFM, Medvedovisky IG, Santos MB, Oliveira MCLM, Figueiredo MRC (2009) Relação dos anfíbios da Ilha dos Marinheiros, estuário da Lagoa dos Patos, Rio Grande do Sul, Brasil. Rev Bras Biocienc 4849:231–233
R Core Team (2022) R: a language and environment for statistical computing. R Foundation for Statistical Computing. https://www.r-project.org/
Recalde FC, Breviglieri CPB, Kersch-Becker M, Romero GQ (2021) Contribution of emergent aquatic insects to the trophic variation of tropical birds and bats. Food Webs 29:e00209. https://doi.org/10.1016/j.fooweb.2021.e00209
Ronque MUV (2013) História natural, comportamento e ecologia de Camponotus rufipes e Camponotus renggeri (Formicidae: Formicinae): um estudo comparativo em vegetação do Cerrado. MSc. Dissertation, Instituto de Biologia, Universidade Estadual de Campinas, Campinas, São Paulo
Root RB (1967) The niche exploitation pattern of the blue-gray gnatcatcher. Ecol Monogr 37:317–350. https://doi.org/10.2307/1942327
Sánchez-Piñero F, Polis GA (2000) Bottom-up dynamics of allochthonous input: direct and indirect effects of seabirds on islands. Ecology 81:3117–3132. https://doi.org/10.2307/177405
Sanzone DM, Meyer JL, Marti E, Gardiner EP, Tank JL, Grimm NB (2003) Carbon and nitrogen transfer from a desert stream to riparian predators. Oecologia 134:238–250. https://doi.org/10.1007/s00442-002-1113-3
Savage C (2019) Seabird nutrients are assimilated by corals and enhance coral growth rates. Sci Rep 9:4284. https://doi.org/10.1038/s41598-019-41030-6
Schindler DE, Smits AP (2016) Subsidies of aquatic resources in terrestrial ecosystems. Ecosystems 20:78–93. https://doi.org/10.1007/s10021-016-0050-7
Shatova OA, Wing SR, Hoffmann LJ, Wing SR, Gault-Ringold M (2017) Phytoplankton community structure is influenced by seabird guano enrichment in the Southern Ocean. Estuar Coast Shelf Sci 191:125–135. https://doi.org/10.1016/j.ecss.2017.04.021
Sobral M (2006) Flora arbórea e arborescente do Rio Grande do Sul, Brasil, 2nd edn. Novo Ambiente, São Carlos, Rima
Sobral M, Jarenkow JA, Brack P, Irgang B, Larocca J, Rodrigues RS, Souza VC, Lorenzi H (2012) Botânica sistemática: guia ilustrado para identificação das famílias de fanerógamas nativas e exóticas no Brasil, baseado em APG III, 3rd edn. Nova Odessa, São Paulo
Spano S, Belem AL, Doria RN, Zucchi MR, Souza JRB, Costa AB, Lentini CAD, Azevedo AEG (2014) Application of organic carbon and nitrogen stable isotope and C/N ratios as source indicators of organic matter of Nova Viçosa-Caravelas estuarine complex, southern Bahia, Brazil. Braz J Geol 44:13–21. https://doi.org/10.5327/z2317-4889201400010003
Stapp P, Polis GA (2003) Influence of pulsed resources and marine subsidies on insular rodent populations. Oikos 102:111–123. https://doi.org/10.1034/j.16000706.2003.12445.x
Stapp P, Polis GA, Sánchez-Piñero F (1999) Stable isotopes reveal strong marine and El Niño effects on island food webs. Nature 401:467–469. https://doi.org/10.1038/46769
Timm CD, Timm VF (2016) Aves do extremo sul do Brasil: guia de identificação. Useb, Pelotas
Vanni MJ (2002) Nutrient cycling by animals in freshwater ecosystems. Annu Rev Ecol Syst 33:341–370. https://doi.org/10.1146/annurev.ecolsys.33.010802.150519
Vieira EF (1983) Rio Grande: geografia física, humana e econômica, 1st edn. Sagra, Porto Alegre
Villegas M, Soos C, Jiménez-Uzcátegui G, Matan S, Hobson KA (2021) Isotopic niche segregation among Darwin’s finches on Santa Cruz Island, Galápagos. Diversity 13:147. https://doi.org/10.3390/d13040147
Zupcic-Moore JR, Ruiz-Cooley RI, Paliza O, Koch PL, McCarthy MD (2017) Using stable isotopes to investigate foraging variation and habitat use of sperm whales from northern Peru. Mar Ecol Prog Ser 579:201–212. https://doi.org/10.3354/meps12281
Acknowledgements
Special thanks are due to the Floristic Laboratory staff at Universidade Federal do Rio Grande—FURG, for identifying the plant species collected. We also thank Aline Barbosa and Saulo Pino for their help in preparing maps. We would also like to thank Márcio Repenning for his help in classifying the range of habitats used by the terrestrial birds collected and Amanda Travessas, Fernanda Valls, Francisco Eliseu, Juliana Gaiotto, Larissa Alvariz and Leonardo Soares for their help in field collection. The authors are grateful to Alexandre M. Garcia and Fátima C. Recalde for comments and careful reading of a previous version.
Funding
This study was funded by the National Council for Scientific and Technological Development (CNPq) (Universal No. 422759/2016-3 granted to LB). In addition, it was also financially supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) through a MSc Fellowship granted to FCS (process numbers: 88882.443875/2019-01). L. Bugoni is a research fellow from CNPq (grant 311409/2018-0).
Author information
Authors and Affiliations
Contributions
FCS and LB formulated the idea and developed the methodology. FAF and CTB provided methods adjustment advice. FCS, LB, FAF, CTB, and CNF carried out the fieldwork. FCS, LB, and FAF analyzed the data and CTB and CNF offered statistical advice. FCS wrote the manuscript. CTB, CNF, FAF, and LB reviewed and provided contributions to the content and offered editorial advice.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional and national guidelines for animal care and use were followed. Necessary licenses were obtained from the Ethical Committee on Animal Use (CEUA P020/2020) and biological sampling (SISBIO 50810-9) for this research.
Additional information
Communicated by Seth Newsome.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Caseiro-Silva, F., Faria, F., Barreto, C. et al. Colonial waterbirds provide persistent subsidies to swamp forests along an estuarine island food chain. Oecologia 202, 113–127 (2023). https://doi.org/10.1007/s00442-023-05377-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-023-05377-y