Abstract
Nitrogen (N) resorption from senescing leaves enables plants to reuse N, making them less dependent on current N uptake from the environment, leading to higher fitness, particularly under low N supply. Species that form a symbiotic association with N2-fixing bacteria have not evolved proficient N resorption, i.e., they retain more N in the senesced leaves than non-N2-fixing species. However, the physiological mechanism underlying the difference is still unknown. Metabolic and structural protein contents in green and senesced leaves, as well as protein degradation during leaf senescence—a critical initial process for subsequent N resorption—were determined in four N2-fixing legumes and in four non-N2-fixers. The metabolic proteins were highly degraded in legumes and to a lesser extent in nonlegumes. Nonetheless, legumes retained more metabolic proteins in their senesced leaves than nonlegumes, because symbiotic N2 fixation improved the metabolic protein content in green leaves. Symbiotic N2 fixation did not change the structural protein content in green leaves. The structural proteins were moderately degraded in nonlegumes, and almost undegraded in legumes, and more structural proteins remained in the senesced leaves of legumes than in those of nonlegumes. The higher metabolic and structural protein contents in the senesced leaves of N2-fixing legumes properly explained the less proficient N resorption. This is an important step in unraveling molecular mechanisms of different N conservation strategies among plant functional types.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During leaf senescence, part of the leaf nitrogen (N) is resorbed, remobilized, and re-utilized for the growth of young tissues (Chapin 1980; Killingbeck 1986). Nitrogen resorption from senescing leaves makes plants less dependent on current N uptake from environments, thus, plays an essential role, particularly in N-poor environments. Nitrogen resorption has been quantified by two indices: N resorption efficiency (the proportion of green leaf N resorbed during senescence) and N resorption proficiency (the N content of fully senesced leaves). Nitrogen resorption proficiency was more responsive to alterations in N availability in the soil than N resorption efficiency: the lower the N availability in the soil, the lower the N content of senesced leaves and the lower the N loss through leaf fall (Aerts 1996; Killingbeck 1996; Ratnam et al. 2008; Lü et al. 2011). It may be a preferable index for testing hypotheses regarding the evolutionary significance of N resorption as natural selection acts on each green or senesced leaf N content but not on their proportion (Killingbeck 1996; Kobe et al. 2005; Drenovsky et al. 2013).
Killingbeck (1996) hypothesized that species that form a symbiotic association with N2-fixing bacteria had not evolved proficient N resorption if the energetic cost of resorbing N is higher than fixing atmospheric N. He revealed that various woody species that form an N2-fixing symbiosis with the actinomycete genus Frankia (i.e., actinorhizal plants) resorbed N less proficiently than those of non-N2-fixing woody species. Field studies that compared N2-fixers (actinorhizal plants or legumes) and sympatrically growing non-N2-fixers (Stewart et al. 2008; Oikawa et al. 2020), and data compilations (Vergutz et al. 2012; Drenovsky et al. 2019) also supported this hypothesis. However, the physiological mechanism underlying the difference in N resorption proficiency between N2-fixers and non-N2-fixers remains unknown.
Nitrogen is a significant component of all proteins (Chapin et al. 1986; Evans and Seemann 1989), and protein degradation is a critical initial process for subsequent N resorption (Stoddart and Thomas 1982; Noodén 1988; Aerts 1996). Metabolic proteins mainly consist of soluble enzymes in the chloroplast stroma and cytosol, and membrane-associated proteins (Evans and Seemann 1989), and are presumed to be largely degradable. Conversely, structural proteins are thought to be less degradable, because they associate with cell wall proteins, and are inaccessible and recalcitrant to proteolytic enzymes (Lamport 1966; Charles-Edwards et al. 1987; Niinemets and Tamm 2005; González-Zurdo et al. 2015). In temperate woody species, a leaf age-related variation in metabolic protein content was positively correlated with that in leaf N content, whereas no such correlation was observed between structural protein content and leaf N content (Takashima et al. 2004; Yasumura et al. 2006; Yasumura and Ishida 2011). This result indicates that the N resorption progress during leaf senescence is explained by metabolic protein degradation, but not by structural protein degradation. Then, we hypothesize that metabolic proteins are less degraded in N2-fixers than in non-N2-fixers, and that structural proteins would be less degradable in both N2-fixers and non-N2-fixers.
Protein content in senesced leaves is determined by its degradability and initial content in green leaves. In a field study conducted at the Australian National Botanic Gardens, the green leaf N contents in RuBisCO and that in cell wall proteins were higher in two Hardenbergia species (legume vines) than in sympatrically growing nonlegume trees and shrubs, while those in two Acacia species (legume tree and shrub) were comparable to nonlegumes (Harrison et al. 2009). In an experiment with potted plants that investigated N allocation within a green leaf, the N allocated to photosynthetic apparatus (e.g., RuBisCO) and cell walls were lower in two legume trees than in two nonlegume trees (Tang et al. 2019). An experiment comparing a nodulating soybean cultivar to its non-nodulating isogenic line showed that the RuBisCO and chlorophyll contents were generally higher in the former than in the latter, but the differences varied widely among experimental conditions (Maekawa and Kokubun 2005). One may expect that legumes have higher protein contents than nonlegumes as it is well known that green leaf N content is higher in legumes than in nonlegumes (e.g., Adams et al. 2016; Wolf et al. 2017), but there are very few data available to compare protein contents in green leaves of legumes and nonlegumes, and not enough to generalize differences between groups.
In this study, metabolic and structural protein contents in green and senesced leaves, and protein degradation during leaf senescence were determined in four N2-fixing legumes Amorpha fruticosa L., Lespedeza bicolor Turcz., Pueraria lobata (Willd.) Ohwi, and Wisteria floribunda (Willd.) DC., and four non-N2-fixers Acer crataegifolium Siebold et Zucc., Akebia trifoliata (Thunb.) Koidz., Carpinus tschonoskii Maxim., and Cerasus jamasakura (Siebold ex Koidz.) H. Ohba (Table 1). These are common, widespread woody species sympatrically growing in the temperate region of Japan. The following hypotheses were tested: (1) protein content in senesced leaves is higher in legumes than in nonlegumes, following the pattern of senesced leaf N content. (2) The higher protein content in senesced leaves of legumes is attributable to the lower degradability of metabolic proteins. (3) Degradability of structural proteins is low in both N2-fixers and non-N2-fixers and is not different between groups; thus it does not account for the difference in protein content in senesced leaves between groups. (4) Both metabolic and structural protein contents in green leaves do not show consistent differences between groups.
Materials and methods
Sampling of green and senesced leaves
Sampling was conducted at forest edges in a secondary deciduous forest-residential zone and a nearby road verge vegetation (36°44′ N, 140°37′ E), Mito, Ibaraki, Japan (Table 1). Well sunlit, green leaves (1–5 leaves per individual) were sampled from 5 adult individuals per species except for C. tschonoskii (four individuals) between 13 Jun and 15 Jul 2019. Four of the six species are of succeeding type of leaf production, and the remaining two species are intermediate between flush type and succeeding type (sensu Kikuzawa 1983); in intermediate type, several leaves emerge almost simultaneously in early spring (April), followed by the continuous emergence of the remaining leaves. Their leaf lifespans are relatively short and leaf fall occurs continuously from July to November (personal observations). Hence, it would be reasonable to assume that the leaves reach maturity in June—July. For P. lobata, the vines were intertwined with each other at the sampling site, and identification of individuals was impossible. Thus, the samplings were conducted for five shoots that were distant from each other. For all species, care was taken to select fully expanded fresh leaves that did not show symptoms of senescence, damage by herbivores, or any disease. The leaves were put into polyethylene bags and kept cool until they were brought to the laboratory. Immediately, 2–30 disks (0.5-cm2 each) were punched from each sampled leaf (totally, 20–30 disks per individual were obtained). Four discs were stored at − 80 °C for protein analysis for each individual. Other disks were dried at 70 °C for at least 48 h and used to determine the leaf mass per unit area (LMA) and N content. Nitrogen content was determined using an N/C analyzer (Sumigraph NC-95A; Sumika-Bunseki, Osaka, Japan).
Leaves that were discolored (often brown, yellow, and red) and detached easily from plants by gentle flicking were defined as ‘fully senescent.’ Leaves showing any symptom of shrinkage and damage by herbivory or other factors, or those that had fallen on the ground were avoided. Following the sampling procedure used for green leaves, 20–30 disks per individual were obtained from the same individuals between July and November. As noted above, the leaf lifespan of the study species is relatively short, with earlier emerging leaves being abscised in early summer (July). Hence, the sampling times are never too early. For each individual, four disks were stored at – 80 °C for protein analysis, and other disks were dried at 70 °C for at least 48 h to determine the LMA and N content.
During leaf senescence, leaf N and leaf mass can decrease. This makes quantifying N resorption ability using mass-based leaf N content challenging (van Heerwaarden et al. 2003; Mediavilla et al. 2014). Therefore, leaf N content was corrected by multiplying it with the mass loss correcting factor (m, van Heerwaarden et al. 2003; Vergutz et al. 2012; Oikawa et al. 2020):
For each species, m was calculated using the average dry mass of green leaves (n = 5 or n = 4) and the average dry mass of fully senesced leaves (n = 5 or n = 4).
Protein measurements
Protein content in green and senesced leaves was determined according to Takashima et al. (2004). Leaf protein was divided into three fractions: water-soluble, sodium dodecyl sulfate (SDS, a detergent)-soluble, and SDS-insoluble fraction. In this study, the sum of water-soluble and SDS-soluble fraction was considered the metabolic proteins following Yasumura et al. (2006). The SDS-insoluble fraction was assumed to represent structural proteins.
Frozen disks were powdered in liquid N in a mortar, and homogenized with 1.0-ml 100 mM Bicine-NaOH buffer (pH 8.0, containing 0.4-M sorbitol, 2-mM MgCl2, 10-mM NaCl, 5-mM iodo-acetate, 10-mM dithiothreitol, 1-mM phenylmethyl sulfonyl fluoride, and 1% (w/v) polyvinylpolypyrroridone [PVPP]). The homogenate was collected in a tube. The mortar was washed with 0.5-ml buffer, which was added to the homogenate; this was repeated twice. The homogenate was vortexed, centrifuged at 15,000×g for 30 min in a refrigerated microcentrifuge (SS-1500X, Sakuma, Tokyo, Japan), and the supernatant was carefully collected. The pellet was homogenized with 0.5-ml buffer again, centrifuged at 15,000×g for 15 min, and the supernatant was collected; this was repeated twice. The supernatant collected through these processes was used to determine the water-soluble fraction. The pellet was homogenized with 1-ml Bicine-NaOH buffer containing 3% (w/v) SDS. The homogenate was centrifuged at 10,000×g for 10 min, and the supernatant was collected. The pellet was homogenized with 0.5 ml buffer with 3% (w/v) SDS, centrifuged at 10,000×g for 10 min, and the supernatant was collected; this was repeated four times for green leaves and three times for senesced leaves. The supernatant collected through these processes was used to determine the SDS-soluble fraction. Water-soluble fraction and SDS-soluble fraction were precipitated with 10% (v/v) and 15% (v/v) trichloroacetic acid, respectively, and were centrifuged at 10,000×g for ten minutes, and the supernatant was discarded. For other frozen disks, proteins were divided into the three fractions using soluble polyvinylpyrrolidone instead of PVPP. The other processes are the same as when using PVPP. After removing the water-soluble fraction and the SDS-soluble fraction, the remaining pellet was collected and washed with ethanol and used to determine the SDS-insoluble fraction. Protein content in each fraction was determined by ninhydrin after hydrolysis to amino acids with a procedure described by McGrath (1972). For hydrolysis, 0.1 g-Ba(OH)2∙8H2O plus 0.2-mL distilled water was applied on the pellets in each tube and followed by autoclave treatment (121 °C, 0.12 MPa for 20 min). The calibration curve was established using bovine serum albumin (r2 = 0.98–0.99). Protein content in senesced leaves of each species was corrected by multiplying the respective m (Eq. 1). As well as N contents in senesced leaves, protein contents in senesced leaves are apparently higher in species that loses more dry matter from the senescing leaf, even if the amount of protein remaining in a senesced leaf is the same as other species. To minimize the effect of dry mass loss on interspecific comparisons of the leaf protein that is lost with senesced leaves, protein content in senesced leaves was multiplied by m.
The proportion of metabolic and structural proteins degraded during leaf senescence (PD,MET and PD,STR, respectively) was calculated as:
where [PMET,SE] is the metabolic protein content in fully senesced leaf, [PMET,GR] is the metabolic protein content in green leaf, [PSTR,SE] is the structural protein content in fully senesced leaf, and [PSTR,GR] is the structural protein content in green leaf.
Nitrogen content in each protein fraction was estimated assuming that leaf proteins have a 16% N content (Field and Mooney 1986; Takashima et al. 2004; Yasumura et al. 2006, 2007). Nitrogen content of non-protein nitrogenous compounds such as amino acids and inorganic N was estimated by subtracting N content in metabolic and structural proteins from the total leaf N content.
Statistics
Smirnov–Grubbs test was used to eliminate outlier data for leaf N content, leaf protein content, and leaf protein proportion degraded during leaf senescence. The following data were detected as outliers and excluded from the subsequent data analyses: [PSTR,SE], one individual of W. floribunda; PD,MET, one individual of W. floribunda; PD,MET, one individual of A. crataegifolium; PD,STR, one individual of A. fruticosa. The mean leaf N content of each group (legume or nonlegume) was calculated as the sum of the mean leaf N content of four species in each group, divided by the number of species. The mean leaf protein content ([PMET,GR], [PMET,SE], [PSTR,GR], and [PSTR,SE]) of each group was calculated as the sum of the mean leaf protein content of four species in each group, divided by the number of species. The mean proportion of leaf protein degraded (PD,MET and PD,STR) of each group was calculated as the sum of the mean proportion of leaf protein degraded of four species in each group, divided by the number of species.
Welch’s t test was used to compare leaf N content, the green leaf N proportion resorbed during senescence, leaf protein content, leaf protein degradability, and leaf N content of non-protein nitrogenous compounds between legumes and nonlegumes. Pearson correlation and standardized major axis (SMA) regression were examined to analyze the relationship between leaf N and leaf protein content. All statistical analyses were conducted using an open-source statistical software R, version 3.5.1 (R Development Core Team 2018). Smirnov–Grubbs test was conducted using the ‘outliers’ package (Komsta 2011), and SMA regression was conducted using the ‘lmodel2’ package of R (Legendre 2014).
Results
LMA decreased during leaf senescence in both legumes and nonlegumes (Table 2; for species-specific data, see Supplementary Information 1). Leaf mass loss during senescence was 28% in both groups, i.e., the mass loss correcting factor (m, Eq. 1) was 0.72. Leaf N content also decreased during leaf senescence in both groups (Table 2). The percentage difference in leaf N between green and senesced leaves (i.e., N resorption efficiency) was not different between groups (t = − 0.693, P = 0.519). Both N content in green leaves and N content in senesced leaves were higher in legumes than in nonlegumes (t = 18.924, P < 0.001 and t = 8.047, P < 0.001, respectively), in agreement with the previous observation on 50 species that included the 8 species studied here (Oikawa et al. 2020).
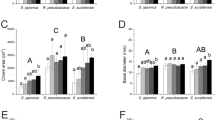
In green leaves, metabolic protein content ([PMET,GR]) was significantly higher in legumes than in nonlegumes (t = 5.736, P = 0.001; Fig. 1a), whereas no significant difference in structural protein content ([PSTR,GR]) was observed between groups (t = − 0.705, P = 0.507; Fig. 1b). In senesced leaves, metabolic protein content ([PMET,SE]) was marginally higher in legumes than in nonlegumes (t = 1.960, P = 0.098; Fig. 1c), and structural protein content ([PSTR,SE]) was also marginally higher in legumes than in nonlegumes (t = 1.974, P = 0.096; Fig. 1d). The metabolic protein degradability (PD,MET) was slightly but significantly higher in legumes (87%) than in nonlegumes (80%) (t = 2.510, P = 0.046; Fig. 2a). Structural proteins were degraded up to 43% in nonlegumes, but very little was degraded in legumes (5%); it varied widely among species, and significant difference in the mean values between groups could not be detected (t = − 1.475, P = 0.191; Fig. 2b). In legumes, some individuals showed negative degradability as the [PSTR,SE] was higher than the [PSTR,GR]; this might be due partly to the difficulty in determining the timing of sampling for green leaves (see “Discussion”).
a Metabolic protein content and b structural protein content in green leaves ([PMET,GR] and [PSTR,GR], respectively), and c metabolic protein content and d structural protein content in fully senesced leaves ( [PMET,SE] and [PSTR,SE], respectively) of legumes (black bars) and nonlegumes (white bars). Means + 1 SE (n = 4) are shown. Significant (P < 0.05) and marginally significant (0.05 < P < 0.10) differences between means within each leaf age are denoted by asterisks and bracketed asterisks, respectively
In green leaves, the N content in protein fractions accounted for 65.1 and 64.1% of the total leaf N content in legumes and nonlegumes, respectively (Fig. 3a). One-third of the leaf N (35% in legumes and 36% in nonlegumes) was in non-protein nitrogenous compounds. Non-protein N content in green leaves was higher in legumes than in nonlegumes (t = 7.715, P = 0.002). In senesced leaves, the N content in protein fractions accounted for 46.5% and 67.3% of the total leaf N content in legumes and nonlegumes, respectively (Fig. 3b). The remainder (55% in legumes and 33% in nonlegumes) of the leaf N was present in non-protein nitrogenous compounds. Non-protein N content in senesced leaves was higher in legumes than in nonlegumes (t = 3.610, P = 0.015).
Nitrogen (N) partitioning in a green and b fully senesced leaves of legumes and nonlegumes. Proteins are assumed to have a N content of 16%. The numbers next to the bar indicate the percentage of the N content in each component (metabolic protein N, structural protein N, or non-protein N) to the total leaf N content
Leaf age-related variation in leaf N content and that in metabolic protein content correlated linearly with each other in both legumes and nonlegumes (Fig. 4a). Leaf N content and structural protein content also correlated positively with each other in nonlegumes, but the coefficient of determination was very low (Fig. 4b). In legumes, the relationship was not statistically significant. Leaf N and the total protein content (metabolic protein content + structural protein content) also correlated positively with each other in both groups (legumes; r2 = 0.947, P < 0.001, nonlegumes; r2 = 0.914, P < 0.001).
Standardized major axis (SMA) regressions of a metabolic protein content versus leaf nitrogen (N) content, and b structural protein content versus leaf N content for legumes (circles) and nonlegumes (triangles). Symbols: closed = green leaves; open = fully senesced leaves. Each symbol represents the data from each individual of eight species (4–5 individuals per species). Regression relationships: (a) legumes: leaf N content = 7.98 + 221.60 metabolic protein content (r2 = 0.933, P < 0.001); nonlegumes: leaf N content = 2.48 + 289.76 metabolic protein content (r2 = 0.750, P < 0.001); (b) legumes: leaf N content = 23.72 + 230.68 structural protein content (r2 = 0.014, P = 0.473); nonlegumes: leaf N content = 5.72 + 443.15 structural protein content (r2 = 0.364, P < 0.001). Significant regressions are indicated by continuous lines (legumes) and dashed lines (nonlegumes)
Discussion
This study was, to our knowledge, the first to analyze physiological mechanisms underlying the difference in N resorption proficiency between legumes and nonlegumes. It was found that legumes retained more metabolic and structural proteins in their senesced leaves than in those of nonlegumes (Fig. 1c, d), but the determination mechanism was different between metabolic and structural proteins. The higher metabolic protein content in senesced leaves of legumes resulted from the higher metabolic protein content in green leaves (Fig. 1a): contrary to the hypothesis, its degradability was higher in legumes than in nonlegumes (Fig. 2a). Meanwhile, the differences between groups in structural protein content in senesced leaves was attributed to differences in its degradability (5% in legumes and 43% in nonlegumes; Fig. 2b). Although the between-group difference in degradability was not statistically significant, the higher structural protein content in senesced leaves of legumes is logically attributed to their lower degradability, as the structural protein content in green leaves of legumes was comparable to that of nonlegumes (Fig. 1b).
Metabolic protein was highly degradable in both groups (Fig. 2a), and correlated strongly with leaf N content (Fig. 4a), indicating that it contributed significantly to N resorption during leaf senescence, regardless of the presence or absence of symbiotic N2 fixation. Previous works also found high degradabilities of metabolic proteins (90–97%) and strong correlations between metabolic protein and leaf N content in nonlegume woody species (Yasumura et al. 2006; Yasumura and Ishida 2011). The results from this study, together with these previous works, suggest that the energetic cost of resorbing N in metabolic proteins is significantly lower than that of fixing atmospheric N2, and the absorbing mineral N in soil. Furthermore, the fact that the metabolic protein degradability was slightly higher in legumes than in nonlegumes (Fig. 2a) may suggest that the cost of absorbing mineral N in soil is less than the cost of fixing atmospheric N2. Despite the high degradability, more metabolic proteins remained in senesced leaves in legumes than in nonlegumes, because the initial content in green leaves was higher. The higher initial content of metabolic proteins would be due to the high N availability associated with symbiotic N2 fixation (e.g., Adams et al. 2016; Wolf et al. 2017). In other studies, however, protein content in green leaves was not necessarily higher in legumes than in nonlegumes (Harrison et al. 2009; Tang et al. 2019). These discrepancies could result from differences in resource limitation in each study. For example, symbiotic N2 fixation could be strongly limited by phosphorus and water availabilities of the habitats (Vitousek et al. 2002). More comparative studies are needed before more definite conclusions can be made.
Interestingly, structural proteins degraded little in legumes and contributed little to N resorption (Figs. 2b, 4b). In nonlegumes, the structural protein was partially degraded, and it was weakly but significantly correlated with leaf N content, suggesting that it made a moderate contribution to N resorption. Thus, we suspect that the energetic cost of resorbing N in structural proteins is higher than that of fixing atmospheric N2, but is lower than that of absorbing mineral N in soil. It should be noted that the structural protein contents observed in this study are slightly higher than the leaf N allocation to cell walls compiled by Onoda et al. (2017). We found that the percentage of green leaf N allocated to structural proteins was 7% in legumes and 18% in nonlegumes (Fig. 3a), while the leaf N allocation to cell walls reported by Onoda et al. (2017) ranged from 1 to 14% when the LMA was between 30 and 51 g m−2 (the LMA values are those observed for our study species; Table S1). They stated that the leaf N allocation to cell walls varies widely among different protein assays. Of the data they compiled, Takashima et al. (2004), using the same protein assay as ours, showed the green leaf N allocated to structural proteins of 4–16% for evergreen and deciduous oak species. Yasumura et al. (2006) also obtained a value of 6% for a deciduous tree. The green leaf N allocated to structural proteins shown by these studies are very close to our results. We also quantified amino acid N contents in the SDS-insoluble fractions for half of the study species, and found that the decrease in amino acid N content during leaf senescence was smaller in legumes than in nonlegumes (Supplementary Information 2), consistent with the results obtained with ninhydrin-based assay. In addition, the SDS-insoluble fractions may have contained N other than cell wall N.
Note also that the calculated degradability was negative in a few individuals of legume species, because [PSTR,SE] was higher than [PSTR,GR]. When leaf lifespan is relatively short, and leaf protein content increases rapidly with leaf development and decreases shortly after attaining a maximum, as observed in some woody species (Yasumura et al. 2006; Yasumura and Ishida 2011), it is difficult to determine the highest content and obtained samples may exhibit slightly lower contents than the peak. Meanwhile, such rapid changes in leaf protein content were not observed in some species such as Polygonum cuspidatum (Onoda et al. 2004) and Glycine max subsp. soja (Marin Tanaka and Shimpei Oikawa, unpublished data).
To date, two complementary N resorption indices have been proposed, i.e., N resorption efficiency and proficiency. Previous studies found that N resorption proficiency was more responsive to plant N status than N resorption efficiency, and N resorption proficiency was proposed to be a valuable metric for testing hypotheses regarding the evolutionary significance of N resorption (see Introduction). Nitrogen resorption efficiency should be closely related to protein degradability, but we found that this metric did not reflect a small but crucial physiological difference between legumes and nonlegumes, i.e., the lower structural protein degradability in legumes (Fig. 2b). On the other hand, both metabolic and structural protein contents in fully senesced leaves were higher in legumes than in nonlegumes, consistent with between-group difference in senesced leaf N content.
Conclusions
Contrary to our expectations, the metabolic proteins were highly degraded in legumes and in nonlegumes. Nonetheless, legumes retained more metabolic proteins in their senesced leaves than nonlegumes, because symbiotic N2 fixation improved the initial metabolic protein content in green leaves. The structural proteins were moderately degraded in nonlegumes and almost not in legumes, and more structural proteins were left in the senesced leaves in legumes than in nonlegumes. The higher protein contents in the senesced leaves of legumes properly explained the less proficient N resorption.
Availability of data and materials
All data generated or analyzed during this study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Abbreviations
- [P MET,GR]:
-
Metabolic protein content in green leaf
- [P MET,SE]:
-
Metabolic protein content in fully senesced leaf
- [P STR,GR]:
-
Structural protein content in green leaf
- [P STR,SE]:
-
Structural protein content in fully senesced leaf
- P D,MET :
-
Proportion of the metabolic proteins degraded during leaf senescence
- P D,STR :
-
Proportion of the structural proteins degraded during leaf senescence
- m :
-
Mass loss correcting factor (i.e., senesced leaf mass/green leaf mass)
References
Adams MA, Turnbull TL, Sprent JI, Buchmann N (2016) Legume are different: leaf nitrogen, photosynthesis, and water use efficiency. Proc Natl Acad Sci USA 113:4098–4103. https://doi.org/10.1073/pnas.1523936113
Aerts R (1996) Nutrient resorption from senescing leaves of perennials: are there general patterns? J Ecol 84:597–608. https://doi.org/10.2307/2261481
Chapin FS III (1980) The mineral nutrition of wild plants. Ann Rev Ecol Syst 11:233–260. https://doi.org/10.1146/annurev.es.11.110180.001313
Chapin FS III, Shaver GR, Kedrowski RA (1986) Environmental controls over carbon, nitrogen and phosphorus fractions in Eriophorum vaginatum in Alaskan tussock tundra. J Ecol 74:167–195. https://doi.org/10.2307/2260357
Charles-Edwards DA, Stutzel H, Ferraris R, Beech DF (1987) An analysis of spatial variation in the nitrogen content of leaves from different horizons within a canopy. Ann Bot 60:421–426. https://doi.org/10.1093/oxfordjournals.aob.a087463
Drenovsky RE, Koehler CE, Skelly K, Richards JH (2013) Potential and realized nutrient resorption in serpentine and non-serpentine chaparral shrubs and trees. Oecologia 171:39–50. https://doi.org/10.1007/s00442-012-2396-7
Drenovsky RE, Pietrasiak N, Short T (2019) Global temporal patterns in plant nutrient resorption plasticity. Glob Ecol Biogeogr 28:728–743. https://doi.org/10.1111/geb.12885
Evans JR, Seemann JR (1989) The allocation of protein nitrogen in the photosynthetic apparatus: costs, consequences, and control. In: Brigs WR (ed) Photosynthesis. Liss, New York, pp 183–205
Field C, Mooney HA (1986) The photosynthesis–nitrogen relationship in wild plants. In: Givnish TJ (ed) On the economy of plant form and function. Cambridge University Press, Cambridge, pp 25–55
González-Zurdo P, Escudero A, Mediavilla S (2015) N resorption efficiency and proficiency in response to winter cold in three evergreen species. Plant Soil 394:87–98. https://doi.org/10.1007/s11104-015-2509-2
Harrison MT, Edwards EJ, Farquhar GD, Nicotra AB, Evans JR (2009) Nitrogen in cell walls of sclerophyllous leaves accounts for little of the variation in photosynthetic nitrogen-use efficiency. Plant Cell Environ 32:259–270. https://doi.org/10.1111/j.1365-3040.2008.01918.x
Kikuzawa K (1983) Leaf survival of woody plants in deciduous broad-leaved forest. 1. Tall Trees Can J Bot 61:2133–2139. https://doi.org/10.1139/b84-346
Killingbeck KT (1986) The terminological jungle revisited: making a case for use of the term resorption. Oikos 46:263–264. https://doi.org/10.2307/3565477
Killingbeck KT (1996) Nutrients in senesced leaves: keys to the search for potential resorption and resorption proficiency. Ecology 77:1716–1727. https://doi.org/10.2307/2265777
Kobe RK, Lepczyk CA, Iyer M (2005) Resorption efficiency decreases with increasing green leaf nutrients in a global data set. Ecology 86:2780–2792. https://doi.org/10.1890/04-1830
Komsta L (2011) outliers: tests for outliers. https://CRAN.R-project.org/package=outliers
Lamport DTA (1966) The protein component of primary cell walls. Adv Bot Res 2:151–218. https://doi.org/10.1016/S0065-2296(08)60251-7
Legendre P (2014) lmodel2: model II regression. R package version 1.7-2. http://CRAN-R-project.org/package=lmodel2
Lü X-T, Freschet GT, Flynn DFB, Han X-G (2011) Plasticity in leaf and stem nutrient resorption proficiency potentially reinforces plant-soil feedback and microscale heterogeneity in a semi-arid grassland. J Ecol 100:144–150. https://doi.org/10.1111/j.1365-2745.2011.01881.x
Maekawa T, Kokubun M (2005) Correlation of leaf nitrogen, chlorophyll and Rubisco contents with photosynthesis in a supernodulating soybean genotype Sakukei 4. Plant Prod Sci 8:419–426. https://doi.org/10.1626/pps.8.419
McGrath R (1972) Protein measurement by ninhydrin determination of amino acids released by alkaline hydrolysis. Anal Biochem 49:95–102. https://doi.org/10.1016/0003-2697(72)90245-X
Mediavilla S, García-Iglesias G-Z, Escudero A (2014) Nitrogen resorption efficiency in mature trees and seedlings of four tree species co-occurring in a Mediterranean environments. Plant Soil 385:205–215. https://doi.org/10.1007/s11104-014-2230-6
Niinemets Ü, Tamm Ü (2005) Species differences in timing of leaf fall and foliage chemistry modify nutrient resorption efficiency in deciduous temperate forest stands. Tree Physiol 25:1001–1014. https://doi.org/10.1093/treephys/25.8.1001
Noodén LD (1988) The phenomena of senescence and aging. In: Noodén LD, Leopold AC (eds) Senescence and aging in plants. Academic Press, New York, pp 281–328
Oikawa S, Matsui Y, Oguro M, Okanishi M, Tanabe R, Tanaka T, Togashi A, Itagaki T (2020) Legumes are not proficient at resorbing nitrogen from senescing leaves. J Plant Res 133:639–648. https://doi.org/10.1007/s10265-020-01211-1
Onoda Y, Hikosaka K, Hirose T (2004) Allocation of nitrogen to cell walls decreases photosynthetic nitrogen-use efficiency. Func Ecol 18:419–425. https://www.jstor.org/stable/3599203
Onoda Y, Wright IJ, Evans JR, Hikosaka K, Kitajima K, Niinemets Ü, Poorter H, Tosens T, Westoby M (2017) Physiological and structural tradeoffs underlying the leaf economic spectrum. New Phytol 214:1447–1463. https://doi.org/10.1111/nph.14496
R development Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. See http://www.R-project.org
Ratnam J, Sankaran M, Hanan NP, Grant RC, Zambatis N (2008) Nutrient resorption patterns of plant functional groups in a tropical savanna: variation and functional significance. Oecologia 157:141–151. https://doi.org/10.1007/s00442-008-1047-5
Stewart JR, Kennedy GJ, Landes RD, Dawson JO (2008) Foliar-nitrogen and phosphorus resorption patterns differ among nitrogen-fixing and nonfixing temperate-deciduous trees and shrubs. Int J Plant Sci 169:495–502. https://doi.org/10.1086/528749
Stoddart JL, Thomas H (1982) Leaf senescence. In: Boulter D, Parthier B (eds) Nucleic acids and proteins in plants, I, vol 14. Encyclopedia of plant physiology. Springer, Berlin, pp 592–636
Takashima T, Hikosaka K, Hirose T (2004) Photosynthesis or persistence: nitrogen allocation in leaves of evergreen and deciduous Quercus species. Plant Cell Environ 27:1047–1054. https://doi.org/10.1111/j.1365-3040.2004.01209.x
Tang J, Sun B, Cheng R, Shi Z, Luo D, Liu S, Centritto M (2019) Seedling leaves allocated lower fractions of nitrogen to photosynthetic apparatus in nitrogen fixing trees than in non-nitrogen fixing trees in subtropical China. PLoS ONE 14:e0208971. https://doi.org/10.1371/journal.pone.0208971
van Heerwaarden LM, Toet S, Aerts R (2003) Current measures of nutrient resorption efficiency lead to a substantial underestimation of real resorption efficiency: facts and solutions. Oikos 101:664–669. https://doi.org/10.1034/j.1600-0706.2003.12351.x
Vergutz L, Manzoni S, Porporato A, Novais RF, Jackson PB (2012) Global resorption efficiencies and concentrations of carbon and nutrients in leaves of terrestrial plants. Ecol Monogr 82:205–220. https://doi.org/10.1890/11-0416.1
Vitousek PM, Cassman K, Cleveland C, Crews T, Field CB, Grimm NB, Howarth RW, Marino R, Martinelli L, Rastetter EB, Sprent JI (2002) Towards an ecological understanding of biological nitrogen fixation. Biogeochemistry 57(58):1–45. https://doi.org/10.1023/A:1015798428743
Wolf AA, Funk JL, Menge DNL (2017) The symbionts made me do it: legumes are not hardwired for high nitrogen concentrations but incorporate more nitrogen when inoculated. New Phytol 213:690–699. https://doi.org/10.1111/nph.14303
Yasumura Y, Ishida A (2011) Temporal variation in leaf nitrogen partitioning of a broad-leaved evergreen tree, Quercus myrsinaefolia. J Plant Res 124:115–123. https://doi.org/10.1007/s10265-010-0358-x
Yasumura Y, Hikosaka K, Hirose T (2006) Seasonal changes in photosynthesis, nitrogen content and nitrogen partitioning in Lindera umbellata leaves grown in high or low irradiance. Tree Physiol 26:1315–1323. https://doi.org/10.1093/treephys/26.10.1315
Yasumura Y, Hikosaka K, Hirose T (2007) Nitrogen resorption and protein degradation during leaf senescence in Chenopodium album grown in different light and nitrogen conditions. Func Plant Biol 34:409–417. https://doi.org/10.1071/FP06307
Yonekura K, Kajita T (2003) BG plant index Y-List. http://ylist.info
Acknowledgements
We thank Noriyuki Osada and Tomoki Tanaka for experimental protocols and advice; we also thank Syunki Harigai for assisting with the measurements reported here; and Ülo Niinemets and two anonymous reviewers for exceptionally helpful comments on the manuscript.
Funding
Support for this work was provided by JSPS KAKENHI Grant number 17K07554.
Author information
Authors and Affiliations
Contributions
RT and SO designed the research. RT and SM performed the experiments and acquired the experimental data. All authors contributed to the interpretation of the data, reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Communicated by Ülo Niinemets .
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tanabe, R., Miyazawa, SI., Kitade, O. et al. Effect of symbiotic N2 fixation on leaf protein contents, protein degradation and nitrogen resorption during leaf senescence in temperate deciduous woody species. Oecologia 200, 79–87 (2022). https://doi.org/10.1007/s00442-022-05264-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-022-05264-y