Abstract
The balance between costs and benefits is expected to drive associations between species. While these balances are well understood for strict associations, we have no insights to which extent they determine facultative associations between species. Here, we quantified the costs of living in a facultative association, by studying the effects of red wood ants on the facultatively associated isopod Porcellio scaber. Porcellio scaber frequently occurred in and near hostile red wood ant nests and might outnumber obligate nest associates. The facultative association involved different costs for the isopod. We found that the density of the isopod decreases near the nest with higher ant traffic. Individuals in and near the nest were smaller than individuals further away from the nest. Smaller individuals were also found at sites with higher ant traffic. A higher proportion of wounded individuals was found closer to the nest and with higher ant traffic. We recorded pregnant females and juveniles in the nest suggesting that the life cycle can be completed inside the nests. Lab experiments showed that females died sooner and invested less in reproduction in presence of red wood ants. Porcellio scaber rarely provoked an aggression response, but large numbers were carried as prey to the nest. These preyed isopods were mainly dried out corpses. Our results showed that the ant association incurred several costs for a facultative associate. Consequently, red wood ant nests and their surrounding territory act as an alternative habitat where demographic costs are offset by a stable resource provisioning and protection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Interspecific associations are common and provide benefits as access to resources, shelter, protection and cleaning services (Boucher 1985; Paracer and Ahmadjian 2000). Symbioses are the most intimate associations, shaped by costs-benefits balances in both partners (Doebeli and Knowlton 1998; Mueller et al. 2005; van der Heijden et al. 2015). Loose and facultative associations between organisms are not vital to any of the involved species, but equally widespread (e.g. in Lycaenid butterflies and ants, Pierce et al. 2002). Such loose associations should emerge from similar cost–benefit balances, and organisms that engage in such associations should gain mutual net benefits. Because facultative associations are unstable, costs and benefits are anticipated to be strongly context-dependent (Stadler et al. 2001), and even leading to temporary maladaptive strategies. In some scenarios, costs may even outweigh potential benefits and associating with another organism might become a non-beneficial strategy (White et al. 2007).
A high diversity of facultative associates can be found particularly in and around the structures created by animals such as burrowing mammals, birds, corals and social insects (Patton 1994; Uppstrom 2010; Kurek et al. 2020; Baardsen et al. 2021; Myczko et al. 2021). The engineered environments contain high densities of sometimes critical resources, offer protection against enemies, and dampen the negative effects of physical stressors. Ants are such ecological engineers. Their nests contain high levels of organic matter and refuse (Donisthorpe 1927; O’Keefe 2000; Witte et al. 2008; Rettenmeyer et al. 2011; Parmentier et al. 2014; Parmentier 2020) that provide new niches for other species in and around their nests (Hölldobler and Wilson 1990; Hughes et al. 2008; Parmentier 2020). Besides providing advantages, ants also antagonistically interact with their associates by direct or indirect predation and competition. Interspecific associations will thus only be advantageous as long as these costs are compensated by the benefits. Local maladaptive associations may nevertheless emerge from source-sink dynamics as determined by a net positive flux of one of the partners from more suitable to hostile habitat.
Mound building red wood ants (Formica rufa group) form an iconic group of ecological engineers in Eurasia that affect a multitude of abiotic and biotic components of forest and heath ecosystems (Frouz et al. 2016; Robinson et al. 2016). Red wood ants may structure biological communities in and around their nests by predation, competition and the attraction of associated organisms (Gösswald 1989; Stockan and Robinson 2016; Maák et al. 2021). They are opportunistic predators of a wide array of arthropod species depending on their availability (Skinner 1980; Sörensen and Schmidt 1987; Domisch et al. 2009; Parmentier 2010; Zingg et al. 2018; Trigos-Peral et al. 2021). Apart from predation, red wood ants may impact other species by competition (Haemig 1992; Reznikova and Dorosheva 2004; Jäntti et al. 2007), but also by means of mutualistic interactions (Novgorodova 2005; Depa et al. 2020; Parmentier et al. 2020).
The associated community of red wood ants is dominated by beetles and mites which typically exert neutral to negative effects on their host (Parmentier et al. 2014). Many of these associates are obligate ant nest associates, so-called myrmecophiles that evolved specific adaptations to the hostile ant nest (Parmentier et al. 2017, 2018). Recent work also demonstrated the impact of the red wood ant host on the myrmecophile distribution in and outside the nest (Parmentier et al. 2016a, 2021). Besides these obligate associates, soil organisms such as millipedes, mites, isopods and springtails may facultatively infiltrate red wood ant nests and thereby profit from the provided homeostatic, resource-rich and safe nest environment (Robinson and Robinson 2013; Parmentier et al. 2014; Boer 2021). The facultative symbionts lack strong adaptations to the host, so it remains a puzzling question how they succeed to survive in and around the hostile nest environments. One of the largest facultative associates in red wood ant mounds is the common rough isopod Porcellio scaber Latreille (1804) (Robinson and Robinson 2013; Parmentier et al. 2016b; De Smedt et al. 2020; Boer 2021). Findings of pregnant females as well as juveniles in the nests even suggest that P. scaber can breed in red wood ant nests (Robinson and Robinson 2013). Conversely, the species is brought in large quantities to the nest as prey (Driessen et al. 1984; Loones et al. 2008; Parmentier 2010).
Here, we studied the costs associated with the facultative symbiosis of P. scaber with red wood ants. Porcellio scaber and related species are known to be very responsive to various forms of abiotic (Dallinger and Prosi 1988; Bayley and Baatrup 1996; Fischer et al. 1997; Lardies et al. 2004; Calhôa et al. 2012) and biotic stress (Castillo and Kight 2005; Cazzolla Gatti et al. 2020). We tested the prediction that red wood ants negatively affect the abundance and life history traits of P. scaber in the nest and along an interaction gradient away from the nest. We also tested whether red wood ants decrease reproduction of P. scaber, and whether these putative costs are offset by behavioural interaction with red wood ants.
Materials and methods
Spatial demography and fitness correlates of P. scaber in and around red wood ant nests
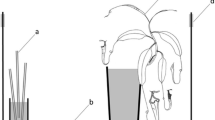
We quantified the distribution of P. scaber along a gradient of red wood ant activity. We therefore selected 18 red wood ant nests (15 Formica polyctena and 3 Formica rufa nests) during summer 2020. All selected nests were located in the province of West-Flanders in the North-West of Belgium, more specifically in Bruges and Poperinge (Fig. 1a). Formica polyctena and F. rufa are closely related and may even hybridize, but typically differ in colony organization with F. polyctena nests having many queens and F. rufa nests a single queen (Seifert 2007). However, the F. rufa colony organization in our study region is similar to F. polyctena as they were also highly polygynous. Nevertheless, the two species can unambiguously be separated based on their pilosity and no hybrids occur in the studied sites (Dekoninck et al 2012). Because of the similar organization and similarly associated fauna (Parmentier et al. 2015a), we expect no effect of host species on P. scaber tolerance. The nests were grouped into seven different clusters, based on their proximity and similarity in the surrounding abiotic conditions (Appendix 1). The three F. rufa nests were located in the same site and were grouped in one cluster. We placed a pitfall in each nest and at three distances outside the nest (1, 5 and 10 m; Fig. 1b). Three pitfalls were installed for each distance outside the nest (Fig. 1b). The pitfalls were positioned parallel to the forest edge to avoid having parallel gradients in other environmental factors associated with edge effects (De Smedt et al. 2018).
a The two study sites of the sampled nests in the province of West-Flanders in the North-West of Belgium, more specifically Bruges (1) and Poperinge (2). b Schematic top view of the setup of the pitfalls around a red wood ant nest to assess the spatial and size distribution. Red wood ant nest (circle), pitfalls (filled rectangles) at 1, 5 and 10 m from the nest and one pitfall in the nest
The pitfalls consisted of plastic, rectangular boxes (25 cm × 7.5 cm × 8 cm) containing an approximate 1 cm bottom layer of plaster. To prevent the desiccation of the isopods the plaster was moistened. Debris of the surrounding also got into the pitfalls due to the wind and passing ants, which created extra humidity and offered hiding places for the isopods. By only collecting living isopods, we avoided the collection of dead isopods that were brought into the pitfalls by foraging ant workers. All pitfalls outside of the nests were dug into the ground with the long side of the pitfall parallel to the nest edge and the top part of the pitfall levelled with the ground surface. An elevated roof was placed on the pitfalls. The elevated roof prevented the pitfall from flooding, but it also left a 1.5 cm slit, large enough for isopods to get caught by the pitfall. The pitfalls inside the nest were dug into the nest and covered again with nest material. A plastic roof, kept in place by rubber bands, was also used to prevent nest material from falling into the pitfalls.
The pitfalls outside the nests were emptied after one week. The ones inside the nests were emptied every one to two days, because they rapidly filled with nest material brought inside by the ants. The collected isopods were stored per pitfall on 70% ethanol. Ants were not collected, but workers in the pitfalls outside the nests were counted. These numbers were used to determine the degree of ant traffic at the location of the pitfall. In 2019 we also collected Porcellio scaber individuals using the same pitfalls in 24 F. rufa nests at the Poperinge site, as part of a study on the dispersal of red wood ant myrmecophiles (Parmentier et al. 2021). These unpublished abundance data, allowed us to compare the intranidal densities of P. scaber with those of strictly associated myrmecophiles, mostly rove beetles (Parmentier et al. 2021).
To document phenotypic effects of ant activity on isopod life history and fitness, we weighted every individual from the 2020 survey. Weight serves a proxy for isopod size. Isopods were placed in an oven set to 60 degrees for 24 h. The dry body mass was measured using a microbalance (Brand: OHAUS; accuracy: 0.1 mg). Sex, state of pregnancy and number of intact antennae for the 7814 collected specimens of P. scaber in the 2020 sampling were determined using a stereomicroscope (Kyowa optical model SD-2P). Sex of P. scaber and woodlice in general can be determined by the presence of external male genitalia used for sperm transfer (Sutton 1980). Because these external male genitalia only develop at the adult stage, we used the dry body mass at which 50% of the animals had external male genitalia as a cut-off value for determining maturity (juveniles < 1.3 mg, adults ≥ 1.3 mg). The presence of a brood pouch (marsupium) holding the eggs (Sutton 1980) indicates female pregnancy. To determine past ant attack, injuries with respect to antenna loss were quantified (Schoener 1979; Ernsting and Fokkema 1983; Ospina et al. 2022).
For the 2019 dataset, we did not have info on fitness correlates such as body size, gender, state of pregnancy and number of remaining antennae of P. scaber.
Statistical analysis
Abundance of adults per pitfall
To analyse the abundance data, we ran a linear mixed model (=LMM) with the fourth-root of the abundances (# isopods / trap) as dependent variable to fulfil normality assumptions. The full model included distance from the nest, ant density (# ants/trap) and the interactions between those two variables as fixed effects. Site and nest were selected as random effects. A likelihood ratio test was used to assess the significance of the predictors.
Abundance of P. scaber vs obligate rove beetles inside a nest
The abundance of P. scaber and obligate rove beetle myrmecophiles in 24 nests of the 2019 dataset were pairwise compared with a non-parametric Wilcoxon paired signed rank test.
Individual dry mass
The dry mass of adult P. scaber individuals around red wood ant nests (excluding individuals caught in the nest) was modelled using a LMM. The full model included distance from the nest (three levels: 1 m, 5 m and 10 m), sex, ant density (fourth root transformed) and all two-way interactions as fixed effects. As random effects, site, nest and pitfall were modelled. We determined the optimal fixed effects structure using backwards model selection with the drop1 function. We removed the least significant predictor at each step until none met the criterion P > 0.10. The natural logarithm of the body mass of the isopods was taken to fulfil normality assumptions and a post-hoc test was conducted.
In addition, we wanted to compare the body mass of individuals found inside the nest with those along the distance gradient outside the nest. As we did not count the ants in the nest pitfalls, we could not control for the factor ‘ant density’ in this analysis. Eventually, we conducted the same model as described above excluding ant density, but with four distance levels (0 m = intranidal, 1 m, 5 m and 10 m).
Proportion of juveniles, females, pregnant females, and individuals with missing antennae
The effects of the predictors distance, ant density and the interaction were assessed on (i) proportion of juveniles, (ii) the proportion of females, (iii) the proportion of pregnant females and (iv) the proportion of individuals with missing antennae. Here we ran four binomial linear mixed models. The full model included distance from the nest, ant density (fourth-root transformed) and the interaction between the variables as fixed effects. As random effects, site and nest were chosen. Overdispersion in these models was checked, but no violations were detected. A post-hoc test was also conducted.
Statistical platform
All models were run in R 4.0.3. For the linear mixed models and binomial mixed models we made use of the lme4 and lmerTest package (Bates et al. 2015; Kuznetsova et al. 2017). For the post-hoc tests we used the package emmeans (Lenth 2021). Overdispersion in the models was checked using the DHARMa package (Hartig 2020).
Porcellio scaber as a prey for red wood ants
To assess whether red wood ants preyed on P. scaber, we monitored the most crowded ant trail of four nests in Bruges in August, 2021. We identified all prey brought to the nests on each of the four trails (during one hour per trail) and checked if the prey items were alive, died recently (preyed upon) or died a while ago (dried out, hence scavenging).
To test whether red wood ants preferred dead or living P. scaber as a prey, we conducted a preference experiment. We installed two pitfalls (dimensions and setup as described above) with ten dead (marked with white enamel dot) and ten living P. scaber individuals (marked with green enamel dot) just outside a red wood ant nest in Bruges. Isopods for this experiment were collected in the Bruges site. Dead isopods were obtained by freezing living individuals, and defrosting them one hour before the start of the experiment. After one hour, the individuals were counted to assess how many individuals were taken out of the pitfalls as prey by the ants. The enamel marking allowed us to see whether living individuals were killed and to differentiate the offered isopods from new individuals that fell or were brought into the traps.
Lab experiment: costs of ants on reproductive investment
We assessed the effect of the presence of red wood ants on reproductive investment (total dry mass of offspring/dry mass of mother) of P. scaber in a controlled lab experiment. First, we collected individuals in early winter from a single population (Location: Ostend), males and females were separated into different containers and put in artificial hibernation in a fridge (4 °C) for two months. Individuals were then put under room temperature, provided with carrot slices and organic material, and after three weeks we checked the pregnancy status of the females. We discarded the females that showed signs of pregnancy as these had been fertilized in the field. Next, we prepared 17 containers (1L, diameter 8.5 cm, height 13 cm) filled with a plaster bottom and 50 mL of organic material and assigned 15 virgin females and 8 males to each of them. In nine of the 17 containers, we added 50 F. polyctena workers (ant + treatment). The other eight containers were used as a control without ants (ant-treatment). Containers were placed in a cabinet with an ambient temperature of 24 °C and a day night cycle of 16D:8N. In each container, we provided two carrot slices and eppendorf tubes filled with water and sugar water (20%), plugged with cotton as nourishment for the isopods and ants. Organic material and sugar water were replaced every week, carrot slices every 4–5 days. Dead ants were replaced every two days, and dead isopods were removed. We moistened the surface of the containers every 4–5 days. Preliminary experiments indicated that the minimal gestation time was 23 days under the used conditions. Therefore, we isolated the surviving females individually after 22 days, otherwise we would not have been able to assess the gestation time and reproductive investment of each individual female. Females were individually housed in snap lid vials (height 2 cm, diameter = 4.5 cm) with a 0.5 cm bottom layer of plaster. A small slice of carrot was provided, and we added 1 red wood ant worker to the vials with females that were subjected to the ant + treatment. This prolonged the effect of ant stress on these females. The females were monitored every day to record the gestation time. We ended the trial when the mother gave birth. We assessed the dry mass of the mother and of her offspring.
Statistical analysis
Female investment (dry mass offspring/dry mass mother) was modelled using a mixed-effects beta regression (package glmmTMB (Brooks et al. 2017)). Treatment (ant + vs ant-), gestation time and their interaction were included as fixed factors in this model, container as a random factor. Residuals were diagnosed with the DHARMa package, but no violations were detected. We conducted backward variable selection using ‘drop1’ function and removed the least significant predictor at each step until none met the criterion P > 0.10.
Behavioural interaction between red wood ants and P. scaber
We conducted behavioural assays in the lab to assess how red wood ants and P. scaber individuals behave when interacting. For this experiment, red wood ants (Formica polyctena) and 20 isopod individuals were collected in Bruges.
The behavioural experiments were conducted in cylindrical plastic containers (diameter = 6.5 cm, height = 7 cm). The container had a moistened plaster bottom of ca. 1 cm and the inner side was coated with fluon preventing ants and isopods from escaping. Seven medium sized ants were put into the arenas to acclimatize for 1 h. The choice for medium sized ants was based on previous research that demonstrated that smaller red wood ant workers react more aggressively (Parmentier et al. 2015b). After the acclimatization period of the ants, an isopod individual was introduced into the arena. Ten seconds following the introduction of the isopod, the interactions were filmed for ten minutes. The videos were later analyzed, frame by frame, with the program “VirtualDub”. Interactions were divided in three groups, i.e. initial behaviour of the isopod, behaviour of the ant when contacting the isopod and the response of the isopod on the ant’s behaviour. We differentiated the following initial behavioural acts in the isopod: not moving, walking or running. We scored whether the behaviour of the ant was aggressive (opening mandibles, chasing, biting, acid spraying) or not (ignoring, antennation, inspecting). The isopod reacted on the behaviour of the ant by not moving, doing a short stop, walking or running. The first 20 interactions were assessed for each individual. We repeated the behavioural trial with 20 unique individuals and ants.
Statistical analysis
Effect initial behaviour isopod on ant aggression
A binomial GLMM was modelled including in the full model the initial behaviour of the isopod as a fixed effect and the ID of the isopod as a random effect. No overdispersion was detected, and a post-hoc test was conducted.
Effect behaviour of the ants on the response of the isopod
A multinomial model was run to test whether P. scaber responded differently (proportion of 4 behavioural categories: moving, running, walking, making short stops) to aggressive versus non-aggressive ants.
The binomial GLMM was modelled using the packages lme4 and lmerTest, as well as the emmeans package for the post-hoc test. The multinomial model was coded in the nnet package (Venables and Ripley 2002), and significance was tested with the lsmeans (Lenth 2016) package.
Results
Spatial demography and fitness correlates of P. scaber
Porcellio scaber frequently occurred around red wood ant nests, we collected 7814 specimens around red wood ant nests (at 1, 5 and 10 m distance) in 2020: 493 around three F. rufa nests, 7241 around 15 F. polyctena nests. A total of 80 P. scaber individuals were found in the nests: 40 individuals in the three F. rufa nests and 40 individuals distributed over 8 of the 15 sampled F. polyctena nests.
Abundance of adults per pitfall
The interaction effect between ant density and sampling distance significantly affected the adult abundance of P. scaber per pitfall (F = 4.5, df = 2, P = 0.012) (Fig. 2). The abundance decreased with an increasing ant density at 1 m of the nest, while at 5 and 10 m no such changes were present (Fig. 2). The abundance of adults per pitfall and the total Porcellio biomass per pitfall (Pearson’s r = 0.98, P < 0.001) was strongly correlated.
Abundance of P. scaber vs obligate rove beetles inside a nest
We recorded P. scaber in 16 of 24 F. rufa nests in the Poperinge site (mean number per nest: 12.8, median = 2.5, 0.5 quantile: 0.0–12.8) in 2019. The mean number of obligate rove beetle myrmecophiles in these nests was 10.9 (median = 6.5, 0.5 quantile: 3.0–11.3). No significant differences were found between the number of P. scaber and myrmecophilous rove beetles in these nests (Wilcoxon signed rank test with continuity correction, V = 198.5, P = 0.17). Porcellio was more abundant than myrmecophilous rove beetle individuals in 7 of the 24 nests (Appendix 2, Fig. S1).
Individual dry mass
The dry mass of individual P. scaber was on average lower with increasing ant densities (F = 7.5, df = 1, P = 0.008) (Fig. 3). Moreover, individual P. scaber dry mass was lower close to the nest than further away from the nest (F = 9.5, df = 2, P = 0.001). Both females and males at 1 m (estimated marginal mean of log mass female at mean ant density ± SE: EMM1m = −5.46 ± 0.07, males: EMM1m = −5.46 ± 0.07) weighed less than those at 5 and 10 m (females: EMM5m: −5.25 ± 0.06, EMM10m: −5.23 ± 0.06, P < 0.001; males: EMM5m: −5.34 ± 0.07, EMM10m: −5.28 ± 0.06: P < 0.053). Dry mass at 5 and 10 m was not affected by ant density in both female (P = 0.909) and male (P = 0.353) P. scaber.
Male mass in the nests (mean log mass = −5.61 ± 0.12) was on average lower than male mass at 5 and 10 m (mean5m = −5.35 ± 0.08, mean10m = −5.27 ± 0.08, P = 0.029, P = 0.002, respectively), but was similar to male mass at 1 m (mean1m = −5.50 ± 0.08, P = 0.660) (Appendix 3: Fig. S2). We found a comparable, but non-significant, trend in female body mass, (Appendix 3: Fig. S2).
Proportion of juvenile individuals
The proportion of juveniles changed according to the interaction between sampling distance and ant density (F = 6.0, df = 2, P = 0.048). The proportion of juveniles increased with ant density at 1 m distance only.
Proportion of females
The proportion of females significantly increased further away from the nests (F = 7.9, df = 2, P = 0.019) but only the difference between 1 and 10 m showed statistically significance: 60.4% (95% CI 57.4–63.4%) of all caught adults were female at 1 m and this proportion increased to 64.3% (95% CI 61.2–66.8%) at a distance of 10 m (P = 0.023).
Proportion of pregnant females
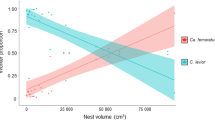
The proportion of pregnant females significantly increased with distance away from the nest (F = 9.7, df = 2, P = 0.007) (Appendix 4, Fig. S3). The proportion of pregnant females was larger at 10 compared to 1 m distance (estimated marginal mean proportion [95% CI] at average ant density: EMM1m = 0.12 [0.07–0.18], EMM10m = 0.17 [0.11–0.25], P = 0.005), whereas the proportion of pregnant females at 5 m (EMM5m = 0.14 [0.09–0.22]) did not differ from the other sampling distances. The proportion of pregnant females also increased significantly with increasing ant density irrespective of the sampling distance (F = 6.2, df = 1, P = 0.012) (Appendix 4, Figure S3). We found 12 pregnant females in the nests, but they were not included in the model.
Proportion of individuals with missing antennae
There was a significant increase in the number of isopods missing one or two antennae with increasing ant densities (F = 3.9, df = 1, P = 0.047) (Fig. 4). The proportion of individuals with missing antennae was significantly higher closer to the nest (estimated marginal mean proportions [95% CI] at average ant density: EMM1m = 0.11 [0.07–0.16], EMM5m = 0.07 [0.05–0.10], EMM10m = 0.04 [0.03–0.06], F = 43.7, df = 2, P < 0.001, post hoc tests: P1/5 = 0.003; P1/10 < 0.001; P5/10 = 0.001) (Fig. 4).
P. scaber as a prey for red wood ants
Porcellio scaber was the most carried prey in four red wood ant nests in Bruges: 65 out of 173 collected prey items were P. scaber (37.6%). Hymenoptera (solitary bees, other ants…) composed another important part of the diet (33.5%), a minor fraction of the diet composed of Coleoptera (6.4%), Diptera (6.4%), Lepidoptera (5.8%), and diverse groups of other arthropods and invertebrates (10.4%). All of the Porcellio individuals were dead: 47 were dry carcasses, 18 individuals were not dried out.
We also found that red wood ants did not take any of the 20 living individuals out of the two pitfalls. By contrast, 18 of the 20 dead isopods were taken by the ants out of the pitfalls.
Lab experiment: costs of ants on reproductive investment
Treatment (ant+ or ant−) was the only fixed predictor retained in the final model, gestation time and its interaction with treatment were not included in this final model. Female isopods invested less in offspring (calculated as the dry mass of the released offspring divided by the dry mass of the mother) in presence (17.8%, 95% CI 15.3–19.0%) than in absence of ants (20.8%, 95% CI 19.0–22.7%) (F = 6.8, df = 1, P = 0.009, Fig. 5).
Behavioural experiments
Effect initial behaviour isopod on ant aggression
When P. scaber was running, the probability of ant aggression was 61%. Ant aggression decreased to respectively 17 and 11% when isopods walked or did not move (F = 63.7, df = 2, P < 0.001).
Effect behaviour of the ants on the response of the isopod
When ants non-aggressively approached P. scaber, the isopods mostly did not move (43%), followed by walking away (31%), running (14%) or making a short stop (12%). The isopods response was considerably different when approached aggressively (F = 58.1, df = 3, P < 0.001). The probability of running away then increased towards 49%, whereas the probability of walking (16%), not moving (33%) and short stopping (3%) decreased.
Discussion
The facultative ant associate Porcellio scaber was frequently recorded in and around hostile red wood ant nests. They constituted a significant proportion of the arthropod fauna in the sampled red wood ant nests and may even outnumber specialized myrmecophilous beetles within these nest fortresses. We found that the loose and facultative association involved multiple costs to the isopod as the ants strongly affect the distribution and some key life history traits of the isopod.
Porcellio scaber adults were considerably smaller closer to the nests of red wood ants. Individuals caught inside the nests also tended to be smaller than those away from the nest. The body mass of adults at a given distance outside the nest was negatively linked to the number of ants passing by. A general pattern found in obligate ant associates is that they are typically small compared to their host ant (Hughes et al. 2008; Witte et al. 2008; Parmentier et al. 2020). Moreover, there is growing evidence that a smaller body size in ant associates decreases their detection by the host (Parmentier et al. 2016a; von Beeren et al. 2021). Many small ant-associated arthropods such as mites, bark lice, springtails and millipedes usually stay undetected in ant nests without resorting to advanced deception strategies (Donisthorpe 1927; Kistner 1982; Eickwort 1990; Witte et al. 2008; Uppstrom 2010; Parmentier 2020; Rocha et al. 2020). As a facultative generalist, P. scaber does not possess specialist traits such as chemical mimicry or morphological adaptations. The observed effect on body mass is likely the result from larger individuals avoiding high ant densities. In our behavioral trials, we also observed that the isopods avoided the ants. This is a simple and wide-spread strategy to avoid attacks of enemies (Parmentier et al. 2018; Ospina et al. 2022). It was also documented that Porcellio species make use of avoidance behaviour when confronted with other negative conditions, such as the presence of toxins (Zidar et al. 2019) or predator cues (Hegarty and Kight 2014). In addition, they likely benefit from a hard, protective exoskeleton (cf von Beeren et al. 2021) and low concentrations of chemical recognition cues on the cuticle (Parmentier et al. 2017). Juveniles and small adults will likely be less noticed and will better tolerate high ant densities. The relationship between small size and reduced enemy avoidance is not limited to ant interactions (Blanckenhorn 2000) but has been demonstrated in diverse agonistic interactions such as in bats and their prey (Barclay and Brigham 1991). Because of the positive association between body size and detection, it is likely that larger P. scaber individuals experience more stress and are more inclined to avoid the nest and sites with high ant densities around the nest. An alternative explanation for the lower body mass in or close by the nest might be that the stress imposed by the hostile ants can lead to lower growth rates in the isopods (Lavy et al. 2001; Dixie et al. 2015). An additional hypothesis for the negative correlation between body mass and ant stress is that larger individuals were detected and predated more by the ants than smaller isopods. The selective predation of larger individuals cannot be ruled out, but is likely of minor importance as we did not observe red wood ants hunting for living isopods in our experiments.
Previous research demonstrated that ants may strongly affect the spatial distribution of arthropods (Halaj et al. 1997; Hawes et al. 2002; Reznikova and Dorosheva 2004; Cembrowski et al. 2014). Here, we found evidence that ants also drive the distribution of P. scaber. The abundance of P. scaber adults and the total biomass of all P. scaber individuals in a pitfall stayed fairly constant at 5 and 10 m from the nest with changing red wood ant activity, while at 1 m, P. scaber abundance strongly declined with increasing ant activity. There are fewer hiding places near the nest, as the concentration of ant trails is higher near the nest. This may result in a stronger avoidance response of the isopod when exposed to high levels of ant interactions near the nest. The observed size distributions might also be explained by ant predation rather than avoidance by the isopod. The concentration of ants near the nest could equally lead to a higher predation rate of the isopod, but we did not find support for active hunting of the isopod in this study. The higher proportion of juveniles around the nest when ant densities were high demonstrates the size-dependence in spatial distribution. The corresponding patterns in adult abundance and total biomass in a pitfall were expected as the total pitfall mass is mainly driven by the adult individuals and not by the small juveniles. Crucially, the isopod distribution pattern was opposite to the ones detected in obligate red wood ant associates. The densities of these species increased towards the nest (Parmentier et al. 2021). Moreover, higher densities of obligate red wood ant myrmecophiles were recorded at areas, such as ant trails, with high ant densities (Parmentier et al. 2021).
The proportion of pregnant females increased away from the nests. Contrary to our expectations that pregnant females would avoid ants because of their reduced mobility and agility, we found that the proportion of pregnant females increased with ant density. A possible explanation is that further away from the nests, pregnant isopods reside along ant trails to avoid predators. In line with Robinson and Robinson (2013), we found large numbers of pregnant females and juveniles inside the red wood ant nests. These observations strongly suggest that the isopod could complete its entire life cycle in the hostile nest environment. However, our controlled lab experiment demonstrated that females in association with red wood ants invest less in reproduction than free-living individuals. Interestingly, an experiment with non-myrmecophilous carabid beetles showed a more drastic decrease in fecundity in presence of red wood ants (Hawes et al. 2013). A previous study also found that the presence of ants may shorten the breeding period of isopods, but this effect was not detected in this study (Castillo and Kight 2005).
Another indication that the presence of red wood ants can be stressful and costly, is the fact that isopods were more likely to miss one or two antennae closer to the nest and with increasing ant density. Porcellio scaber is not able to hide its antennae under its hard exoskeleton so they are exposed to ant attacks. The higher the ant density, the more likely an isopod encounters an ant attack and loses one or two of its antennae.
Porcellio scaber did receive low levels aggression by red wood ants. It was shown that the isopod provoked less aggression than most obligate red wood ant myrmecophiles (Parmentier et al. 2016b). Interestingly, Porcellio scaber is also able to infiltrate nests of other social insect species, such as honeybees (Kärcher and Ratnieks 2010), social wasps (pers. observations TP) and Lasius ants (pers. observations TP), where it likely provokes low aggression as well.
It is puzzling that Porcellio scaber frequently occurs in and near red wood ants, even though they may form an important component of the allocated prey, as demonstrated in previous studies (Driessen et al. 1984; Loones et al. 2008; Parmentier 2010) and here. However, we observed that all the P. scaber individuals brought to the nest were dead, and mostly dried out. So, this observation suggests that red wood ants generally do not hunt for living individuals, but rather scavenge on already dead individuals. Our preference experiment with dead and living P. scaber individuals also indicated that only dead individuals were collected. The relatively low aggression towards P. scaber, its morphological protection and avoidance behaviour support the idea that ants unlikely directly kill P. scaber individuals.
We found that P. scaber isopods associated with red wood ants were smaller, invested less in reproduction, were less numerous in localities with high ant densities near the nest and suffered from more injuries. Previously, it has also been shown that living for a long time in red wood ant lab nests resulted in increased mortality in P. scaber (Parmentier et al. 2016a). Overall, all these results indicate that living with the ants involves costs for the studied facultative associate. So why do they live in the nests? They could be forced into the territory and nests of red wood ants because of saturation of suitable habitat nearby. Alternatively, red wood ant nests could act as ecological traps for the isopods if they choose red wood ant nests over more suitable habitat (Robertson and Hutto 2006). In both scenarios, isopods might only persist in association with red wood ants if there is a constant flux of individuals from more suitable habitat. On the other hand, we can expect that the isopod may profit from thermoregulated, moist and protected nests, rich in resources (Hughes et al. 2008; Kronauer and Pierce 2011). Nevertheless, it is much harder to assess the positive effects of an association with red wood ants. At this point, it is unknown whether, and in what context, the negative effects outlined in this study are offset by potential positive effects. Future studies are needed to further improve our understanding of the dynamics of facultative associations.
Availability of data and material
Datasets will be deposited at https://github.com/tjparmen/Dissecting-the-costs-of-a-facultative-symbiosis upon acceptance.
Code availability
The code to run the analyses is available from the corresponding author on reasonable request.
References
Baardsen LF, De Bruyn L, Adriaensen F et al (2021) No overall effect of urbanization on nest-dwelling arthropods of great tits (Parus major). Urban Ecosyst 24:959–972. https://doi.org/10.1007/s11252-020-01082-3
Barclay RMR, Brigham RM (1991) Prey detection, dietary niche breadth, and body size in bats: why are aerial insectivorous bats so small? Am Nat 137:693. https://doi.org/10.1086/285188
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Bayley M, Baatrup E (1996) Pesticide uptake and locomotor behaviour in the woodlouse: an experimental study employing video tracking and 14C-labelling. Ecotoxicology 5:35–45. https://doi.org/10.1007/BF00116322
Blanckenhorn WU (2000) The evolution of body size: what keeps organisms small? Q Rev Biol 75:385–407
Boer P (2021) De Nederlandse mieren. https://www.nlmieren.nl/websitepages/WOODANTMOUNDS.html. Accessed 26 Oct 2021
Boucher D (1985) The biology of mutualism: ecology and evolution. Oxford University Press, New York
Brooks M, Kristensen K, van Benthem K et al (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J 9:378–4000
Calhôa CF, Soares AMVM, Loureiro S (2012) Effects on survival and reproduction of Porcellio dilatatus exposed to different Cd species. Ecotoxicology 21:48–55. https://doi.org/10.1007/s10646-011-0762-6
Castillo M, Kight S (2005) Response of terrestrial isopods, Armadillidium vulgare and Porcellio laevis (Isopoda: Oniscidea) to the ant Tetramorium caespitum: morphology, behavior and reproductive success. Invertebr Reprod Dev 3:183–190
Cazzolla Gatti R, Messina G, Tiralongo F et al (2020) Learning from the environment: how predation changes the behavior of terrestrial Isopoda (Crustacea Oniscidea). Ethol Ecol Evol 32:29–45. https://doi.org/10.1080/03949370.2019.1640799
Cembrowski AR, Tan MG, Thomson JD, Frederickson ME (2014) Ants and ant scent reduce bumblebee pollination of artificial flowers. Am Nat 183:133–139. https://doi.org/10.1086/674101
Dallinger R, Prosi F (1988) Heavy metals in the terrestrial isopod Porcellio scaber Latreille. II. Subcellular fractionation of metal-accumulating lysosomes from hepatopancreas. Cell Biol Toxicol 4:97–109. https://doi.org/10.1007/BF00141289
De Smedt P, Baeten L, Berg MP et al (2018) Desiccation resistance determines distribution of woodlice along forest edge-to-interior gradients. Eur J Soil Biol 85:1–3. https://doi.org/10.1016/j.ejsobi.2017.12.002
De Smedt P, Boeraeve P, Arijs G, Segers S (2020) De landpissebedden van België. Spinicornis, Bonheiden
Dekoninck W, Ignace D, Vankerkhoven F, Wegnez P (2012) Verspreidingsatlas van de mieren van België. Bull La Société R Belge D’entomologie/bulletin Van K Belgische Ver Voor Entomol 148:95–186
Depa Ł, Kaszyca-Taszakowska N, Taszakowski A, Kanturski M (2020) Ant-induced evolutionary patterns in aphids. Biol Rev 95:1574–1589. https://doi.org/10.1111/brv.12629
Dixie B, White H, Hassall M (2015) Effects of microclimate on behavioural and life history traits of terrestrial isopods: Implications for responses to climate change. Zookeys 2015:145–157. https://doi.org/10.3897/zookeys.515.9399
Doebeli M, Knowlton N (1998) The evolution of interspecific mutualisms. Proc Natl Acad Sci USA 95:8676–8680. https://doi.org/10.1073/pnas.95.15.8676
Domisch T, Finér L, Neuvonen S et al (2009) Foraging activity and dietary spectrum of wood ants (Formica rufa group) and their role in nutrient fluxes in boreal forests. Ecol Entomol 34:369–377. https://doi.org/10.1111/j.1365-2311.2009.01086.x
Donisthorpe HSJK (1927) The guests of British ants, their habits and life-histories. George Routledge and Sons, London
Driessen GJJ, Van Raalte AT, De Bruyn GJ (1984) Cannibalism in the red wood ant, Formica polyctena (Hymenoptera: Formicidae). Oecologia 63:13–22
Eickwort G (1990) Associations of mites with social insects. Annu Rev Entomol 35:469–488. https://doi.org/10.1146/annurev.ento.35.1.469
Ernsting G, Fokkema D (1983) Antennal damage and regeneration in springtails (Collembola) in relation to predation. Netherlands J Zool 33:476–484
Fischer E, Farkas S, Hornung E, Past T (1997) Sublethal effects of an organophosphorous insecticide, dimethoate, on the isopod Porcellio scaber Latr. Comp Biochem Physiol - C Pharmacol Toxicol Endocrinol 116:161–166. https://doi.org/10.1016/S0742-8413(96)00164-8
Frouz J, Jílková V, Sorvari J (2016) Contribution of wood ants to nutrient cycling and ecosystem function. In: Stockan J, Robinson E (eds) Wood ant ecology and conservation. Cambridge University Press, Cambridge, pp 207–220
Gösswald K (1989) Die Waldameise Band 2 Die Waldameise im Ökosystem Wald, ihr Nutzen und ihre Hege. Aula-Verlag, Wiesbaden
Haemig PD (1992) Competition between ants and birds in a Swedish Forest. Oikos 65:479–483. https://doi.org/10.2307/3545565
Halaj J, Ross DW, Moldenke AR (1997) Negative effects of ant foraging on spiders in Douglas-fir canopies. Oecologia 109:313–322. https://doi.org/10.1007/s004420050089
Hartig F (2020) DHARMa: Residual diagnostics for hierarchical regression models. R package version 0.4.4.
Hawes C, Stewart A, Evans H (2002) The impact of wood ants (Formica rufa) on the distribution and abundance of ground beetles (Coleoptera: Carabidae) in a Scots pine plantation. Oecologia 131:612–619. https://doi.org/10.1007/s00442-002-0916-6
Hawes C, Evans HF, Stewart AJA (2013) Interference competition, not predation, explains the negative association between wood ants (Formica rufa) and abundance of ground beetles (Coleoptera: Carabidae). Ecol Entomol 38:315–322. https://doi.org/10.1111/een.12021
Hegarty KG, Kight SL (2014) Do predator cues influence turn alternation behavior in terrestrial isopods Porcellio laevis Latreille and Armadillidium vulgare Latreille? Behav Processes 106:168–171. https://doi.org/10.1016/j.beproc.2014.06.005
Hölldobler B, Wilson EO (1990) The ants. Harvard University Press, Cambridge
Hughes DP, Pierce NE, Boomsma JJ (2008) Social insect symbionts: evolution in homeostatic fortresses. Trends Ecol Evol 23:672–677. https://doi.org/10.1016/j.tree.2008.07.011
Jäntti A, Suorsa P, Hakkarainen H et al (2007) Within territory abundance of red wood ants Formica rufa is associated with the body condition of nestlings in the Eurasian treecreeper Certhia familiaris. J Avian Biol 38:619–624. https://doi.org/10.1111/j.2007.0908-8857.03926.x
Kärcher MH, Ratnieks FLW (2010) Honey bee guards recognise allospecific intruders via “different odours” not “harmful-intruder odours.” J Apic Res 49:270–277. https://doi.org/10.3896/IBRA.1.49.3.07
Kistner DH (1982) The social insects’ bestiary. In: Hermann HR (ed) Social insects, vol 3. Academic Press, London, pp 1–244
Kronauer DJC, Pierce NE (2011) Myrmecophiles. Curr Biol 21:208–209. https://doi.org/10.1016/j.cub.2011.01.050
Kurek P, Nowakowski K, Rutkowski T et al (2020) Underground diversity: Uropodina mites (Acari: Mesostigmata) from European badger (Meles meles) nests. Exp Appl Acarol 82:503–513. https://doi.org/10.1007/s10493-020-00563-6
Kuznetsova A, Brockhoff P, Christensen R (2017) lmerTest Package: Tests in linear mixed effects models. J Stat Softw 82:1–26
Lardies MA, Carter MJ, Bozinovic F (2004) Dietary effects on life history traits in a terrestrial isopod: The importance of evaluating maternal effects and trade-offs. Oecologia 138:387–395. https://doi.org/10.1007/s00442-003-1447-5
Lavy D, Van Rijn MJ, Zoomer HR, Verhoef HA (2001) Dietary effects on growth, reproduction, body composition and stress resistance in the terrestrial isopods Oniscus asellus and Porcellio scaber. Physiol Entomol 26:18–25. https://doi.org/10.1046/j.1365-3032.2001.00211.x
Lenth RV (2016) Least-squares means: the R Package lsmeans. J Stat Softw 69:1–33. https://doi.org/10.18637/jss.v069.i01
Lenth R V (2021) emmeans: Estimated marginal means, aka least-squares means. R package version 1.7.0. https://CRAN.R-project.org/package=emmeans
Loones J, Maelfait J, Van Rhijn J et al (2008) De rode bosmier in Vlaanderen: voorkomen, bedreigingen en herstelmaatregelen aan de hand van een detailstudie in de Sixtusbossen (Poperinge-Vleteren). Rapporten van het Instituut voor Natuur- en Bosonderzoek 2008 (INBO.R.2008.01). Instituut voor Natuur en Bosonderzoek, Brussel
Maák IE, Sondej I, Juhász O et al (2021) Unexpected distribution of subordinates around nests of the wood ants. Acta Oecologica 110:103709. https://doi.org/10.1016/j.actao.2021
Mueller UG, Gerardo NM, Aanen DK et al (2005) The evolution of agriculture in insects. Annu Rev Ecol Evol Syst 36:563–595. https://doi.org/10.1146/annurev.ecolsys.36.102003.152626
Myczko Ł, Kurek P, Tryjanowski P et al (2021) Where to overwinter: burrows of medium-sized carnivores as winter places for invertebrates in temperate environment. Ecol Entomol 46:1177–1184. https://doi.org/10.1111/een.13062
Novgorodova TA (2005) Red wood ants (Formicidae) impact on multi-species complexes of aphids (Aphididae) in the forest-park zone of Novosibirsk. Euroasion Entomol J 4:117–120
O’Keefe ST (2000) Ant-like stone beetles, ants, and their associations (Coleoptera: Scydmaenidae; Hymenoptera: Formicidae; Isoptera). J New York Entomol Soc 108:273–303
Ospina B, Jonathan J, Lerma JM (2022) Intruders in the nest : Interaction of Attaphila paucisetosa (Blattodea : Blaberoidea) with Atta cephalotes Workers (Hymenoptera: Formicidae). J Insect Behav. https://doi.org/10.1007/s10905-022-09794-4
Paracer S, Ahmadjian V (2000) Symbiosis: an introduction to biological associations, 2nd edn. Oxford University Press, New York
Parmentier T (2020) Guests of social insects. In: Starr C (ed) Encyclopaedia of social insects. Springer, Cham
Parmentier T, Dekoninck W, Wenseleers T (2014) A highly diverse microcosm in a hostile world: a review on the associates of red wood ants (Formica rufa group). Insectes Soc 61:229–237. https://doi.org/10.1007/s00040-014-0357-3
Parmentier T, Dekoninck W, Wenseleers T (2015a) Metapopulation processes affecting diversity and distribution of myrmecophiles associated with red wood ants. Basic Appl Ecol 16:553–562. https://doi.org/10.1016/j.baae.2015.04.008
Parmentier T, Dekoninck W, Wenseleers T (2015b) Context-dependent specialization in colony defence in the red wood ant Formica rufa. Anim Behav 103:161–167. https://doi.org/10.1016/j.anbehav.2015.02.023
Parmentier T, Dekoninck W, Wenseleers T (2016a) Survival of persecuted myrmecophiles in laboratory nests of different ant species can explain patterns of host use in the field (Hymenoptera : Formicidae). Myrmecol News 23:71–79
Parmentier T, Dekoninck W, Wenseleers T (2016b) Do well-integrated species of an inquiline community have a lower brood predation tendency? A test using red wood ant myrmecophiles. BMC Evol Biol 16:12. https://doi.org/10.1186/s12862-016-0583-6
Parmentier T, Dekoninck W, Wenseleers T (2017) Arthropods associate with their red wood ant host without matching nestmate recognition cues. J Chem Ecol 43:644–661. https://doi.org/10.1007/s10886-017-0868-2
Parmentier T, De Laender F, Wenseleers T, Bonte D (2018) Prudent behavior rather than chemical deception enables a parasite to exploit its ant host. Behav Ecol 29:1225–1233. https://doi.org/10.1093/beheco/ary134
Parmentier T, De Laender F, Bonte D (2020) The topology and drivers of ant – symbiont networks across Europe. Biol Rev 95:1664–1688. https://doi.org/10.1111/brv.12634
Parmentier T, Claus R, De Laender F, Bonte D (2021) Moving apart together: co-movement of a symbiont community and their ant host, and its importance for community assembly. Mov Ecol 9:25. https://doi.org/10.1186/s40462-021-00259-5
Parmentier T (2010) Taakverdeling en voedselecologie bij de werksters van Formica rufa en Formica polyctena. Master thesis, Department of Biology, Ghent University, Ghent, Belgium
Patton WK (1994) Distribution and ecology of animals associated with branching corals (Acropora spp.) from the Great Barrier Reef, Australia. Bull Mar Sci 55:193–211
Pierce N, Braby M, Heath A et al (2002) The ecology and evolution of ant association in the Lycaenidae (Lepidoptera). Annu Rev Entomol 47:733–771
Rettenmeyer CW, Rettenmeyer ME, Joseph J, Berghoff SM (2011) The largest animal association centered on one species: the army ant Eciton burchellii and its more than 300 associates. Insectes Soc 58:281–292. https://doi.org/10.1007/s00040-010-0128-8
Reznikova Z, Dorosheva H (2004) Impacts of red wood ants Formica polyctena on the spatial distribution and behavioural patterns of ground beetles (Carabidae). Pedobiologia 48:15–21. https://doi.org/10.1016/j.pedobi.2003.06.002
Robertson BA, Hutto RL (2006) A framework for understanding ecological traps and an evaluation of existing evidence. Ecology 87:1075–1085
Robinson NA, Robinson EJH (2013) Myrmecophiles and other invertebrate nest associates of the red wood ant Formica rufa (Hymenoptera Formicidae) in Northwest England. Br J Entomol Nat Hist 26:67–88
Robinson EJH, Stockan JA, Iason GR (2016) Wood ants and their interactions with other organisms. In: Stockan J, Robinson E (eds) Wood ant ecology and conservation. Cambridge University Press, Cambridge, pp 177–207
Rocha F, Lachaud J, Pérez-lachaud G (2020) Myrmecophilous organisms associated with colonies of the ponerine ant Neoponera villosa (Hymenoptera: Formicidae) nesting in Aechmea bracteata bromeliads: a biodiversity hotspot. Myrmecol News 30:73–92
Schoener TW (1979) Inferring the properties of predation and other injury-producing agents from injury frequencies. Ecology 60:1110–1115
Seifert B (2007) Die Ameisen Mittel- und Nordeuropas. lutra Verlags- und Vertriebsgesellschaft, Görlitz
Skinner GJ (1980) The feeding habits of the wood-ant, Formica rufa (Hymenoptera: Formicidae), in limestone woodland in North-West England. J Anim Ecol 49:417–433. https://doi.org/10.2307/4255
Sörensen U, Schmidt GH (1987) Vergleichende Untersuchungen zum Beuteeintrag der Waldameisen (Genus: Formica, Hymenoptera) in der Bredstedter Geest (Schleswig-Holstein). J Appl Entomol 103:153–177. https://doi.org/10.1111/j.1439-0418.1987.tb00974.x
Stadler B, Fiedler K, Kawecki TJ, Weisser WW (2001) Costs and benefits for phytophagous myrmecophiles: When ants are not always available. Oikos 92:467–478. https://doi.org/10.1034/j.1600-0706.2001.920308.x
Stockan JA, Robinson EJH (2016) Wood ant ecology and conservation. Cambridge University Press, Cambridge
Sutton SL (1980) Woodlice. Pergamon Press Ltd, England
Trigos-Peral G, Juhász O, Kiss PJ et al (2021) Wood ants as biological control of the forest pest beetles Ips spp. Sci Rep 11:1–10. https://doi.org/10.1038/s41598-021-96990-5
Uppstrom KA (2010) Mites (Acari) associated with the ants (Formicidae) of Ohio and the harvester ant, Messor pergandei, of Arizona. Master thesis, Ohio State University, USA
van der Heijden MGA, Martin FM, Selosse MA, Sanders IR (2015) Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol 205:1406–1423. https://doi.org/10.1111/nph.13288
Venables WN, Ripley BD (2002) Modern applied statistics with S, 4th edn. Springer, New York
Von Beeren C, Brückner A, Hoenle PO et al (2021) Multiple phenotypic traits as triggers of host attacks towards ant symbionts: body size, morphological gestalt, and chemical mimicry accuracy. Front Zool. https://doi.org/10.1186/s12983-021-00427-8
White JW, Grigsby CJ, Warner RR (2007) Cleaning behavior is riskier and less profitable than an alternative strategy for a facultative cleaner fish. Coral Reefs 26:87–94. https://doi.org/10.1007/s00338-006-0161-2
Witte V, Leingärtner A, Sabaß L et al (2008) Symbiont microcosm in an ant society and the diversity of interspecific interactions. Anim Behav 76:1477–1486. https://doi.org/10.1016/j.anbehav.2008.05.010
Zidar P, Kos M, Ilič E et al (2019) Avoidance behaviour of isopods (Porcellio scaber) exposed to food or soil contaminated with Ag- and CeO2- nanoparticles. Appl Soil Ecol 141:69–78. https://doi.org/10.1016/j.apsoil.2019.05.011
Zingg S, Dolle P, Voordouw MJ, Kern M (2018) The negative effect of wood ant presence on tick abundance. Parasit Vectors 11:1–9. https://doi.org/10.1186/s13071-018-2712-0
Acknowledgements
We thank the Flemish Agency for Nature and Forest (ANB) and the provincie West-Vlaanderen for granting permission to sample at their sites
Funding
This work was supported by FWO and FNRS (1203020N/30257865 to TP).
Author information
Authors and Affiliations
Contributions
JZ, FDW, LDB and TP conceived and designed the experiments. JZ, FDW and TP performed the experiments. LDB and DB participated in the coordination of the study. All authors analyzed the data. JZ and TP wrote the manuscript, FDW, LDB and DB critically revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Not applicable.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Communicated by Stefan Scheu.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zarka, J., De Wint, F.C., De Bruyn, L. et al. Dissecting the costs of a facultative symbiosis in an isopod living with ants. Oecologia 199, 355–366 (2022). https://doi.org/10.1007/s00442-022-05186-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-022-05186-9