Abstract
Microhabitats with distinct biotic and abiotic properties exist within landscapes, and this microhabitat variation can have dramatic impacts on the phenology and physiology of the organisms occupying them. The Antarctic midge Belgica antarctica inhabits diverse microhabitats along the Western Antarctic Peninsula that vary in macrophyte composition, hygric qualities, nutrient input, and thermal patterns. Here, we compare seasonal physiological changes in five populations of B. antarctica living in close proximity but in different microhabitats in the vicinity of Palmer Station, Antarctica. Thermal regimes among our sample locations differed in both mean temperature and thermal stability. Between the warmest and coldest sites, seasonal mean temperatures differed by 2.6˚C and degree day accumulations above freezing differed by a factor of 1.7. Larval metabolic and growth rates varied among the sites, and adult emergence occurred at different times. Distinct microhabitats also corresponded with differences in body composition, as lipid and carbohydrate content of larvae differed across sites. Further, seasonal changes in carbohydrate and protein content were dependent on site, indicating fine-scale variation in the biochemical composition of larvae as they prepare for winter. Together, these results demonstrate that variation in microhabitat properties influences the ontogeny, phenology, physiology, and biochemical makeup of midge populations living in close proximity. These results have implications for predicting responses of Antarctic ecosystems to environmental change.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Landscapes are heterogenous, and even a seemingly uniform landscape can have a vast array of microhabitats that differ in abiotic and biotic characteristics (Risser 1987). Small ectotherms like arthropods can experience considerable variation in environmental conditions across a landscape, either as a consequence of moving among microhabitats or being isolated within a microhabitat. For example, leaves directly exposed to sunlight might be several degrees warmer than leaves in the shade, exposing insects like leaf miners to significantly different thermal conditions even if they are living on the same tree (Pincebourde et al. 2007). Thus, it is important to consider fine-scale variation in microhabitat conditions when assessing phenology, physiology, behavior and potential responses to climate change (see reviews by Chown and Terblanche 2006, and Pincebourde and Woods 2020). During winter and in polar regions, even slight variation in microhabitat conditions can have significant impacts. For example, microclimates can vary considerably depending on whether hibernacula are above or below the snowpack, and these slight differences in microenvironment significantly influence overwintering success and fecundity (Irwin and Lee 2003; Marshall and Sinclair 2012). For the Antarctic springtail Cryptopygus antarcticus, microhabitat has a notable effect on cold acclimatization, as individuals directly exposed to air are more cold hardy than individuals embedded a few centimeters into moss (Hawes et al. 2008). Despite the importance of microhabitat for overwintering arthropods, this fine-scale variation is often overlooked when assessing local adaptation and responses to climate change.
In Antarctica, a small percentage of land is ice-free during the austral summer, and these ice-free areas contain most of the terrestrial biodiversity on the continent (Convey and Stevens 2007; Convey 2013; Convey et al. 2014). These habitats are exceptionally heterogeneous in composition, yielding an array of microhabitats that present unique challenges to their inhabitants (Holdgate 1977). Abiotic factors that contribute to this microhabitat diversity include freshwater availability, topography, proximity to the ocean, and exposure to prevailing winds, among others (Convey, 1997; Convey et al. 2014). Local patterns of biodiversity can also influence microhabitat conditions. For example, localized nitrogen deposition by marine vertebrates (i.e., penguins and seals) creates nutrient-rich areas that support more diverse plant and invertebrate communities (Bokhorst et al. 2019). These differences in plant communities in turn influence soil temperature, as moss-dominated habitats tend to have cooler active layers than habitats dominated by vascular plants (Guglielmin et al. 2008). Together, variation in habitat leads to a diverse thermal profile across terrestrial Antarctic habitats, such that invertebrates experience differences in mean temperature, thermal variability, and timing of freeze–thaw events (Convey et al. 2018). However, while the consequences of microhabitat variation have been addressed for insects at temperate latitudes, particularly in forested ecosystems (Pincebourde and Woods 2020), similar investigations for Antarctic insects are lacking.
The Antarctic midge Belgica antarctica, the only insect endemic to Antarctica, has a 2 + year life cycle spent mainly in the larval stage; adults live only 10–14 days and emerge only during the brief summer period (Lee and Denlinger 2015). Larvae have limited mobility, and although the wingless adults have been found airborne in moderate wind (> 20 km/h) and rafting on the surface of meltwater streams (Edwards and Baust 1981), the midge has an otherwise limited ability to disperse (Peckham 1971; Atchley and Davis 1979). Thus, while many insects can actively select appropriate microclimates and avoid environmental extremes (Woods et al. 2015), larvae of B. antarctica are likely unable to use behavioral strategies to avoid unfavorable conditions and thus can experience considerable environmental variation over small spatial scales. Larvae of B. antarctica are well-equipped to cope with this environmental heterogeneity, as they are freeze tolerant year-round, can withstand a wide pH range, are able to survive extended periods in hypo- and hyperosmotic conditions, and can endure dehydration up to 70% of their original fresh mass (Baust and Lee 1987; reviewed in Lee and Denlinger 2015). Prior to the onset of winter, larvae undergo seasonal metabolic depression that appears to be genetically programmed (Spacht et al. 2020), but whether this metabolic depression is affected by microhabitat conditions has not been assessed.
Populations of the midge are scattered along the Western Antarctic Peninsula and adjacent islands, and distribution within islands is often patchy, with densities ranging from few to tens of thousands of individuals per square meter (Peckham 1971; Rico and Quesada 2013; Potts et al., 2020). Larvae are found in a wide array of microhabitats frequently associated with several types of vegetation, including the terrestrial algae Prasiola crispa, several native moss species, and in grassy areas containing the Antarctic hair grass Deschampia antarctica (Tilbrook 1967; Peckham 1971; Gantz et al. 2018). Larvae may also be abundant where vegetation is sparse or absent, such as at the peripheries of seal wallows, penguin rookeries, and within former or active nesting sites of several other bird species (Gressitt 1967; Strong, 1967; Peckham 1971). These habitats reflect significant variation in nutrients (carbon and nitrogen), hygric conditions, and micronutrients, indicating that this species can thrive in a variety of environments (Potts et al. 2020).
While populations of B. antarctica clearly occupy diverse microhabitats, the extent to which this habitat variation influences life history and physiology has not been assessed. For this study, we selected five populations of B. antarctica near Palmer Station, Antarctica that occupy notably different microhabitats. We hypothesized that this variation in microhabitat conditions requires larvae to have flexible seasonal patterns for growth, development, and metabolism. At these sites, we collected continuous temperature data and conducted weekly measurements of larval mass, metabolic rates, and adult emergence patterns to assess development and phenology. Additionally, we conducted body composition analysis across the season to test the hypothesis that variation in diet and temperature would result in variation in energy storage in larvae, which may affect their ability to prepare for the long Antarctic winter.
Materials and methods
Insects
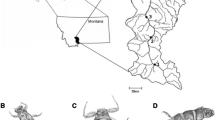
Third and fourth instar larvae of B. antarctica were collected weekly from five locations near Palmer Station, Antarctica: four on Cormorant Island (Cor; specific sites labeled CorA, CorB, CorC, and CorD) and one on Humble Island (Hum) (Figs. 1 and 2; Table 1). The four populations on Cormorant Island were within an area of ~ 1 hectare. Sampling was undertaken during the austral summer, from mid-December 2016 through the end of March 2017. At the start of the season, larvae from CorC were collected from under snow and ice each week until the site thawed in early January. The CorD collection site was inaccessible until the first week of February due to the depth of snow and ice at the site. Larvae were collected from each location within a 2–3 m radius from where the temperature loggers were placed. On each collection day, we also recorded the presence or absence of adults to assess the phenology of adult emergence. Adults were recorded as either present and alive, present and dead, or absent. Relative abundance of adults was recorded, though this information is not reported here because sites were not visited frequently enough to accurately quantify patterns of adult abundance, given the short lifespan of adults (~ 7–10 days) and the relatively short, synchronized period of emergence.
Maps of the Palmer Station Region (a), Cormorant Island (b), and Humble Island (c). Sample locations on Cormorant Island and Humble Island are marked with a red dot and labeled as they are referenced in text. Based on Maps 3, 6, and 16 of the Antarctic Specially Managed Area (ASMA) No. 7 SW Anvers Island and Palmer Basin Management Plan, 2019. Base maps prepared by Environmental Research and Assessment for the United States Antarctic Program
Larvae were transported to the Palmer Station laboratory and extracted from substrate using a modified Berlese setup. Briefly, substrate was placed on metal screens over trays of ice water. Heat lamps placed above the screens were used to drive larvae from the substrate, through the metal screens, and into underlying trays of ice water overnight. The following day, larvae were removed from the ice water for analysis of metabolic rate and body composition.
Field recording of temperature
Temperature at each sampling location was recorded every 30 min using HOBO U23® temperature/relative humidity data loggers (ONSET® Computer Corporation, Bourne, MA, USA). Data loggers were placed just below the substrate surface at each site to record microhabitat temperatures experienced by the larvae. At the beginning of the season, the data logger at CorC was placed under the snow and ice until the site thawed naturally. For a period of three days, the temperature logger at CorC was accidentally dislodged and exposed to ambient air prior to the second week of collection; these dates were excluded from the analyses. A temperature logger was not placed at CorD until the site was accessible during the first week of February. As a proxy for heat accumulation across these sites, we calculated cumulative degree days above 0 °C.
Metabolic rate
Larvae collected weekly from each site were held in the laboratory for fewer than 24 h prior to assessing oxygen consumption as a proxy for metabolic rate. Oxygen consumption was measured using closed system respirometry according to Lee and Baust (1982). Briefly, 10 µl microcapillary tubes were glued into tips of 1 ml plastic syringes affixed with metal washers as weights. For each site, 8 replicates of 10 larvae were placed in a small space near the tips of the syringes with moistened cotton and sealed with plungers. Syringes were then partially submerged in a temperature-controlled circulating bath set to 10˚C. Once in the bath, a 10% solution of KOH was placed into the end of the microcapillary tubes, and the respirometers were allowed to equilibrate for approximately 30 min prior to taking measurements every 30 min over the course of the following hour (for a total of 3 measurements). The KOH solution absorbs CO2 released by larvae, creating negative pressure in the syringes, thus causing the KOH solution to move along the microcapillary tube as the larvae respire. Distances the KOH plugs traveled were measured, and oxygen consumption rate was calculated from these values. Larvae (still in groups of 10) were removed from the respirometer and dried to a constant mass at 65 ˚C, and oxygen consumption was calculated per mg dry mass.
Nutrient analysis
To assess the impact of microhabitat and collection date on biochemical composition of larvae, we measured lipid, carbohydrate, and protein levels. Larvae that were previously dried following respirometry experiments (see above) were stored at − 80 °C until measurement. Lipids were quantified using vanillin phosphoric acid reagent, according to Teets et al. (2011) with some modifications. For each sampling date and location, three groups of two larvae were homogenized in 500 µl 1:1 chloroform:methanol in a bead homogenizer, and tubes were shaken for 15 min at 300 rpm. After centrifugation, the supernatant was transferred to a clean glass test tube and heated at 100 °C for 20 min to evaporate the solvent. 100 µl of sulfuric acid was added to the dried extract and heated for 10 min at 90 °C, after which 1 ml of vanillin phosphoric acid reagent (1.2 mg vanillin /ml in 80% phosphoric acid) was added to each tube for color development. After 20 min at room temperature, three aliquots of each sample were added to a 96-well microtiter plate, and absorbance was measured at 525 nm on a microplate spectrophotometer (CLARIOStar, BMG LABTECH, Cary, NC). Absorbance values were compared to an 8-point standard curve containing 0–300 µg vegetable oil.
For carbohydrate measurements, we used the anthrone assay according to Mercer et al. (2020) with some modifications. For each sampling date and location, three groups of two dried larvae were homogenized in 110 µl phosphate-buffered saline containing 0.05% Tween-20 in a bead homogenizer. Samples were centrifuged, and 100 µl supernatant was combined with 400 µl anthrone reagent (1.4 mg anthrone /ml in 72% sulfuric acid) and heated at 100 °C for 17 min. Samples were then added in triplicate to a 96-well plate and measured at 625 nm on a microplate spectrophotometer. Absorbance values were compared to an 8-point standard curve containing 0–2000 µg/ml glucose in water.
Proteins were measured with the Bicinchonic Acid (BCA) Assay Kit (ThermoFisher Scientific, Waltham, MA) according to the manufacturer’s protocol. For each sampling date and location, three groups of two larvae were homogenized in 200 µl RIPA buffer in a bead homogenizer and centrifuged twice to remove particulates. Samples were pipetted in triplicate onto a 96-well plate, and 200 µl BCA working reagent was added to each well. The plate was incubated 30 min at 37 °C, after which absorbance was measured at 563 nm on a microplate spectrophotometer. Absorbance values were compared to an 8-point standard curve ranging from 0 to 2000 µg/ul BSA.
Statistical analysis
Analyses were carried out using R Version 3.6.3 (R Core Team, 2020), implemented in RStudio Version 1.2.5042 (RStudio Team, 2020) and methods following Thomas (2017). For each variable (dry mass, metabolic rate, carbohydrate, lipid, and protein), a generalized additive model (GAM) in the mgcv package (Wood, 2019) was used. For these models, the response variable was fit as a function of ‘week’, which was smoothed by site to account for non-linear changes over time. ‘Site’ was included in the model to test for differences in each variable across site, and ‘mass’ was included as a covariate (excluding the analysis where ‘mass’ was the independent variable) (Table S1). In each model, the smoothing parameter estimation method was GCV.Cp (General Cross Validation). For nutrient analyses, ‘block’ (i.e., the plate used for analysis of each sample) was included as a random effect to account for plate-to-plate variation in the biochemical assays. Using the dataset collected with HOBO dataloggers, mean temperature during the previous week at each site was also included as a covariate in each model. We initially tested four models to determine the best performance for models including temperature: Model 1) temperature included as a smoothed variable, Model 2) temperature included as a smoothed variable, separated by site, Model 3) temperature as a non-smoothed variable and Model 4) temperature as a non-smoothed variable interacting with Site. Our model-selection criteria (see details below) indicated that model 3 gave the best model performance, and hence Temperature was included as a sole response variable (Table S1). In addition to models with site-specific smoothing terms, we also fit a GAM with a single smoothing term for time. This model was compared with the original model (see details below), and the highest ranking model was consequently selected. If the more complex model (i.e., the model with site-specific smoothing terms) was selected, this result is consistent with changes over time being site dependent. For model selection, including selecting the appropriate error and link functions, we used compare_performance, which ranks models based on five model indices (AIC, BIC, R2, RMSE, and Sigma). To check model diagnostics, we used the functions gam.check and anova.gam (package mgcv) in conjunction with visual confirmation of standardized residuals plotted against fitted values. For body mass and metabolic rate data, N = 8–10 per site for each sampling date. For nutrient composition analyses, N = 3 per site for each sampling date.
Results
Microhabitat temperature
Microhabitat temperatures differed considerably across sites (Fig. 3; Table 1). Mean temperature at the warmest and most thermally stable site, CorA, was 5.6 ± 1.6˚C, while temperature at the coldest and most variable site, CorC, was 3.4 ± 2.8˚C during our sampling period. The temperature at CorD, 3.0 ± 1.5˚C, was slightly lower and more consistent than CorC, but we have incomplete data for this site because it was covered with snow and ice during the early portion of the season. However, when we only include the dates for which CorD was accessible, it remained the coldest of the five sites. Degree days, a measure of accumulated heat units over a period of time, also varied among sites (Table 1). Among sites for which we have a complete record, CorA accumulated the most degree days above 0° (539) and CorC accumulated the fewest (322).
Temperature of each site over the course of the field season (a–e), and a violin plot summarizing distribution and frequency of temperatures at each site (f). Closed circles in the violin plot represent mean temperature for the season, and error bars represent standard deviation. The gap in the CorC panel (c) is due to the HOBO logger being inadvertently dislodged and exposed to ambient air for three days before being replaced in the proper location
Development: dry mass
Larval dry mass, metabolic rate, and adult presence/absence at collecting sites were used as proxies for developmental progression. At all sites, mass tended to increase over time (Fig. 4a–b), with weight increasing 106% on average over the season. However, changes over time were site-specific, as determined by AIC comparison of GAM models with and without site-specific smoothing terms (DAIC = 334.3). We observed a significant negative correlation between temperature in the preceding week and mass (t-value = − 2.75, p < 0.001). Absolute weights also varied by site (p-value for effect of site < 2E-16), with CorC and CorD having the largest larvae and CorA and CorB having the smallest larvae. Complete results for the GAM analysis are presented in Table S2, mass for each site is separately plotted in Figure S1, and prediction plots for dry mass are plotted in Figure S2a.
Weekly changes in dry mass and metabolic rate for each of the five microhabitats. Weekly profiles are shown in (a) and (c), while early, mid, and late-season levels are plotted in (b) and (d) to illustrate seasonal trends. In (a) and (c), the shaded area around the lines represents standard error, while in (b) and (d), symbols represent mean ± sem. In (a) and (c), text in the bottom-left summarizes the results of GAM analyses test the effects of site and time on each variable. For “site”, asterisks indicate a significant effect of site, with * for p < 0.01, ** for p < 0.01, and *** for p < 0.0001. For “time,” we fit site-specific smoothing terms, and the asterisks indicates that at least one of the sites reached the indicated significance level. The “site-specific changes over time?” indicates the results of model-selection analysis where a GAM with site-specific smoothing terms was compared to a GAM with a universal smoothing term. “Yes” indicates that the model with site-specific smoothing terms had stronger support, while “No” indicates better support for a model with a single smoothing term
Development: metabolic rate
At all sites, metabolic rate decreased over time, with an average decrease of 66% from the beginning to end of the sampling period (Fig. 4c–d). Like body size, changes over time were site specific, as determined by AIC comparison of GAM models (DAIC = 134.5). We observed no correlation between mean temperature in the preceding week and metabolic rate (t-value = 1.381, p = 0.168). Metabolic rates also varied strongly by site (p-value for effect of site = 6.11E-10), with Humble having a greater metabolic rate than CorA (t-value = 3.752, p = < 0.001). No significant differences were found between CorA and CorB (t-value = 0.553, p = 0.58), and between CorA and CorC (t-value = − 0.083, p = 0.193). Mass-specific metabolic rate was also highly dependent on size, with larger larvae having lower mass-specific metabolic rates (t-value = − 9.058, p < 2.2E-18). The complete results for the GAM analysis are presented in Table S3, metabolic rates for each site are separately plotted in Figure S3, and prediction plots of metabolic rates for each site are separately plotted in Figure S2b.
Development: adult presence/absence
The timing of adult emergence varied among sites (Table 2). Earliest adult emergences were recorded at Hum, CorA, and CorB, and adults were present during each week we sampled in December. Given that these sites had already thawed prior to the start of our sampling, it is likely that we missed the earliest adult emergence events at each of these sites. Adults were observed at CorC for all but one week in January, and there was little or no overlap with the other sites. At CorD, adults were first observed in the final week of January and were present every week thereafter until the first week of March. No adults were observed at any location during the final three weeks of sampling.
Carbohydrate content
Carbohydrate content varied over time, with most sites decreasing early in the season and increasing at the end of the season (Fig. 5a–b). We observed no significant correlation between mean temperature in the preceding week and carbohydrate content (t-value = 0.917, p = 0.36). Changes over time were site specific, as determined by comparisons of GAM models (DAIC = 39.14). Carbohydrate content also varied by site (F = 2.895, p-value for effect of site = 0.024,), with CorC and Humble tending to have the highest carbohydrate levels, especially in the middle of the season. Unlike other variables, body size was not a strong predictor of mass-specific carbohydrate content (t-value = − 1. 164, p = 0.246). The complete results for GAM analysis are presented in Table S4, carbohydrate contents for each site are separately plotted in Figure S4, and prediction plots of carbohydrate content for each site are separately plotted in Figure S2c.
Lipid content
Unlike other variables, lipid content was relatively constant over time (Fig. 5c–d), with levels fluctuating early in the season but converging by the end of the sampling period. Also, changes over time were not site specific, as the GAM model with a universal smoothing term was slightly better supported than a complex model with site-specific smoothing terms DAIC = 2.618). The universal smoothing term also indicated that any changes in lipid over time were not significant (edf = 1.212, ref.df = 1.395, F = 3.084, p = 0.124). However, lipid did vary by site (F = 3.36, p value for effect of site = 0. 011). Lipid content was significantly lower at CorB than CorA (t-value = − 2.317; p < 0.0217). Both CorC and Humble were not statistically different from CorA (t-value = 1.514, p = 0.132 and t-value = 0.056, p = 0.955, respectively). We observed no significant correlation between mean temperature in the preceding week and lipid content (F = 1.634, p = 0.203). Body size was also a strong predictor of mass-specific lipid content, with larger larvae having lower mass-specific lipid content (t-value = − 5.410, p = 2.11E-07). The complete results for the GAM analysis are presented in Table S5, lipid content for each site is separately plotted in Figure S5, and prediction plots of lipid content for each site and of lipid content per mg fresh mass are separately plotted in Figures S2d and S2e, respectively.
Protein content
Protein levels varied over time at most sites, with levels increasing early in the season and decreasing at the end of the season (Fig. 5e–f). Changes over time were site-specific, as determined by comparison of GAM models (DAIC = 53.2). Protein levels also varied strongly by site (F = 7.638, p-value for effect of site = 1.19E-5), with larvae at CorB having the highest protein levels over time. Larvae at CorC and Hum had lower protein levels than larvae at CorA, whilst CorB presented higher protein levels (Table S6). Mass was also a strong predictor of protein content, with larger larvae having lower mass-specific protein content (t-value = − 7.672, p = 1.64E-12). We observed no significant correlation between mean temperature in the preceding week and protein content (t-value = -0.696, p = 0.487). The complete results for the GAM analysis are presented in Table S6, protein content for each site is separately plotted in Figure S6, and prediction plots of protein content for each site are separately plotted in Figure S2f.
Discussion
When modeling distributions and climate change responses in small animals like insects, it can be challenging to work at an appropriately small scale that is relevant for the species of interest (Pincebourde and Woods, 2021). In fact, it has long been known that microclimatic and microhabitat variation influence arthropod physiology, behavior, and development (Uvarov 1931; Cloudsley-Thompson 1962; Huey 1991). Insects often select microhabitats for characteristics such as temperature and water availability (Edney et al. 1974; Harris et al. 2015), thereby maintaining body temperature, limiting desiccation, and maintaining energy reserves when overwintering (Sinclair 2015). For polar environments like Antarctica, growing seasons are incredibly short, so slight microclimatic variation in temperature can have major impacts on soil-dwelling organisms, especially when temperatures often fluctuate around the lower limits for locomotion – B. antarctica larvae become inactive at temperatures between 0 and − 2 ˚C, and adults don’t resume activity until ambient temperature reaches ~ 5 ˚C (Peckham 1971; Convey and Block 1996). Here, we observed considerable variation in thermal conditions among closely associated microhabitats (Fig. 3a-f; Table 1), with a 2.6 ˚C difference in average temperature between the warmest and coldest sites. Thermal buffering was also different among sites, with some sites such as CorC and Hum showing considerable thermal variation (SD = 2.8 ˚C), while CorA was much more stable (SD = 1.6 ˚C). Because the body temperature of small insects tends to closely track surface temperatures (Pincebourde et al. 2021), this variation in microclimate would lead to differences in body temperature, which likely explains some of the observed physiological differences between these populations.
While these results indicate considerable differences in microhabitat conditions across closely situated sites, our experiments do invoke the question of how fine-scale is fine enough? Larvae were sampled within a ~ 2–3 m radius of the logger, but factors such as solar radiation on rocks or evaporative cooling could cause variation within each sampling site. For some other small arthropods, temperature differences upwards of 10 °C can be observed over a few cm, for example between sun-exposed rocks and surrounding soil, or between peaks and valleys on the surface of bark (Nicolai 1986; Pike et al. 2012). Thus, we likely failed to completely capture the thermal heterogeneity present in our sampling locations. Indeed, a recent review by Pincebourde and Woods (2020) indicates that the relevant microscale for an insect the size of B. antarctica (~ 5 mm in length) is ~ 20 linear cm, but a limited number of dataloggers and the destructive nature of our sampling prevented us from sampling at this resolution. Despite these limitations, we observed significant differences in several parameters across sites, indicating that local environmental conditions are likely having a direct impact on the physiology of larvae.
In general, colder sites had larger larvae with lower metabolic rates, while warmer sites had smaller larvae with higher metabolic rates. When microhabitat temperature the week prior to sampling was included as a covariate in our models, it was negatively correlated with body size, and this is likely a result of temperature and body size showing opposite trends across the season (i.e., temperature tended to decrease across the season while larvae continued to grow). In contrast, temperature the week prior to measurement did not influence metabolic rate, which is consistent with our previous work in the laboratory (Spacht et al. 2020). However, in general the warmer sites (i.e., CorA and CorB) tended to have higher metabolic rates than the cooler sites (CorC), suggesting that overall temperature conditions across the season may be influencing metabolic rate as expected. It is worth noting that all metabolic rate measurements were conducted in the laboratory at a constant temperature (10 °C), indicating that larvae from different environments retained different metabolic rates even when measured at a constant laboratory condition. While the hypothesis of metabolic cold adaptation would predict elevated metabolic rate in the populations from cold environments (Addo-Bediako et al. 2002), B. antarctica does not appear to have metabolic cold adaptation or the capacity for compensatory acclimation (Lee and Baust 1982). Rather, differences in metabolic rate across sites may be primarily driven by differences in size. Much like other animals, smaller body sizes typically correspond with higher metabolic rates in insects (Brown et al., 2004), and our results are consistent with this trend, as body size was negatively correlated with metabolic rate. The extreme variation in body size also indicates asynchronous development rates across sites, and therefore ontogenetic changes in metabolism may be influencing the variation in metabolic rates observed here. Nonetheless, our results indicate that fine-scale variation in microhabitat conditions can influence seasonal growth and metabolism.
While there is limited information on thermal thresholds necessary for development of B. antarctica, developmental rate is strongly influenced by ambient temperature (Harada et al., 2014). Our sites had considerable differences in degree day accumulation above freezing (Table 1), and these differences generally corresponded with the timing of adult emergence (Table 2), rates of mass gain (Fig. 4a), and metabolic rate (Fig. 4b) throughout the season. Larvae from the two sites with the highest degree days gained the most mass throughout the season (CorA = 66.1% increase, CorB = 51.6% increase). Curiously, the lowest mass gain was at Hum (26.8% increase), even though it had the third highest degree days value, and the coldest sites had surprisingly high mass gain throughout the season (CorC = 46.6% increase, CorD = 30.1% increase). These results suggest that larvae may have the capacity for compensatory feeding, a behavior in which animals adjust food intake to meet specific nutritional needs or to maintain growth rates (Clissold et al., 2013). Sites did not thaw synchronously, with CorA, CorB, and Hum thawing prior to our arrival, and two sites, CorC and CorD, thawing after our arrival, weeks apart. Thus, the available time to feed and develop is not uniform across locations, and in a previous field season one of our sample sites, CorD, failed to thaw during the summer months. While extended frozen periods could result in larval mortality if energy stores are depleted, it is also possible that some populations of B. antarctica have life cycles extending beyond 2 years, an idea proposed by Harada et al. (2014). Temporal isolation due to microclimate variation could serve as a reproductive barrier among conspecific populations, as seen in the stonefly Leuctra hippopus (Boumans et al., 2017). While we did not evaluate the genetic relatedness of these populations, it is reasonable to hypothesize that physiological divergence, and perhaps speciation, could be the long-term effects of such temporal and spatial isolation (Rundle and Nosil 2005).
Microhabitat differences also influenced the biochemical composition of larvae, as reflected by differences in carbohydrate, lipid, and protein content among sites (Fig. 5a–c). Temperature is an important modulator of nutrient uptake and assimilation in insects (reviewed in Clissold and Simpson 2015) and a number of other ectotherms, including crustaceans (Croll and Watts 2007), reptiles (Du et al. 2007; Pafalis et al. 2007), and fish (Handeland et al. 2008; Fatma and Ahmed, 2020). However, temperature of the preceding week did not predict macronutrient content of larvae, indicating that week-to-week variation in biochemical composition across sites is not directly related to recent temperature conditions. Instead, differences in biochemical composition may be related to variation in habitat composition, as sites varied in the quantity and types of food available for larvae (Table 1). While some insects can select optimal microhabitats to meet nutritional demands (Clissold et al. 2013), the limited dispersal ability of B. antarctica likely prevents active foraging, so larvae are restricted to food sources in the vicinity of where females oviposit. B. antarctica is a generalist and can feed on a variety of detritus, microorganisms, and plant matter, but there is evidence they preferentially feed on the terrestrial algae Prasiola crispa when available (Peckham 1971). P. crispa was readily available at only one of our sites, Hum, and either absent or present in limited quantities at the other locations. CorB had no visible vegetation and was also one of the warmest sites, and larvae from this site were the smallest and had the lowest lipid content (after accounting for the effect of body size, which had a strong negative effect on mass-specific lipid content). Thus, the combination of consistently high temperatures and absence of vegetation may have hindered growth and lipid accumulation at this site and could explain why larvae at CorB were smaller than a similarly warm site, CorA, which had lots of decaying moss and grass present. Together, our results suggest that a combination of abiotic (i.e., temperature) and biotic (i.e., vegetation composition) are contributing to the observed physiological differences, although manipulative studies are needed to tease apart these factors.
Weekly carbohydrate, lipid, and protein content for each of the five microhabitats. Weekly profiles are shown in (a), (c), and (e), while early, mid, and late-season levels are plotted in (b), (d), and (f) to illustrate seasonal trends. In (a), (c), and (e), lines indicate the mean and shaded areas around lines represents standard error, while in (b), (d), and (f) symbols represent mean ± sem. In (a), (c), and (e), text in the bottom left summarizes the results of GAM analyses test the effects of site and time on each variable. For “site”, asterisks indicate a significant effect of site, with * for p < 0.01, ** for p < 0.01, and *** for p < 0.0001. For “time,” we fit site-specific smoothing terms, and the asterisks indicates that at least one of the sites reached the indicated significance level. The “site-specific changes over time?” indicates the results of model-selection analysis where a GAM with site-specific smoothing terms was compared to a GAM with a universal smoothing term. “Yes” indicates that the model with site-specific smoothing terms had stronger support, while “No” indicates better support for a model with a single smoothing term
Investigations of physiological ecology frequently do not account for the influence of microhabitat on basic aspects of an organism’s physiology and life history. Indeed, local adaptation can happen on geographic scales much finer (e.g., tens of kilometers or tens of meters) than typically considered (Richardson et al. 2014), and so it is important to consider fine-scale variation in microenvironmental conditions when assessing ecological factors that influence an organism’s physiology. Given the heterogeneity of terrestrial Antarctic ecosystems and the relative immobility of B. antarctica, we expected to find diversity in the physiology and phenology among populations. Our results demonstrate that there are considerable temperature differences among the microhabitats occupied by B. antarctica, and these microhabitat differences likely contribute to the observed physiological differences among the populations we sampled. Ongoing analyses investigating genomic variation in these populations will begin to tease apart the relative contributions of genotype and environment in explaining these observed differences across populations. The work presented here supports a growing body of literature that considering fine-scale heterogeneity in habitats is important for predicting how this species and other terrestrial organisms in Antarctica will respond to future environmental perturbation.
References
Addo-Bediako A, Chown SL, Gaston KJ (2002) Metabolic cold adaptation in insects: a large-scale perspective. Funct Ecol 16:332–338
Atchley WR, Davis BL (1979) Chromosomal variability in the Antarctic insect, Belgica antarctica (Diptera: Chironomidae). Ann Entomol Soc Am 72:246–252
Baust JG, Lee RE (1987) Multiple stress tolerance in an Antarctic terrestrial arthropod: Belgica antarctica. Cryobiology 24:140–147
Bokhorst S, Convey P, Aerts R (2019) Nitrogen inputs by marine vertebrates drive abundance and richness in Antarctic terrestrial ecosystems. Curr Biol 29:1721–1727
Boumans L, Hogner S, Brittain J, Johnsen A (2017) Ecological speciation by temporal isolation in a population of the stonefly Leuctra hippopus (Plecoptera, Leuctridae). Ecol Evol 7:1635–1649
Brown JH, Gillooly JF, Allen AP, Savage VM, West GB (2004) Toward a metabolic theory of ecology. Ecology 85:1771–1789
Chown SL, Terblanche JS (2006) Physiological diversity in insects: Ecological and evolutionary contexts. Adv Insect Physiol 33:50–152
Clissold FJ, Simpson SJ (2015) Temperature, food quality and life history traits of herbivorous insects. Curr Opin Insect Sci 11:63–70
Clissold FJ, Coggan N, Simpson SJ (2013) Insect herbivores can choose microclimates to achieve nutritional homeostasis. J Exp Biol 216:2089–2096
Cloudsley-Thompson JL (1962) Microclimates and the distribution of terrestrial arthropods. Annu Rev Entomol 7:199–222
Convey P (1997) How are the life history strategies of Antarctic terrestrial invertebrates influenced by extreme environmental conditions? J Therm Biol 22:429–440
Convey P (2013) Antarctic ecosystems. In: Levin SA (ed) Encyclopedia of Biodiversity. Academic Press, USA, pp 179–188
Convey P, Block W (1996) Antarctic Diptera: Ecology, physiology, and distribution. Eur J Entomol 93:1–13
Convey P, Stevens MI (2007) Antarctic biodiversity. Science 317:1877–1878
Convey P, Chown SL, Clarke A, Barnes DKA, Bokhorst S, Cummings V, Ducklow HW, Frati F, Green TGA, Gordon S, Griffiths HJ, Howard-Williams C, Huiskes AHL, Laybourn-Parry J, Lyons WB, McMinn A, Morley SA, Peck LS, Quesada A, Robinson SA, Schiaparelli S, Wall DH (2014) The spatial structure of Antarctic biodiversity. Ecol Monogr 84:203–244
Convey P, Coulson SJ, Worland MR, Sjoblom A (2018) The importance of understanding annual and shorter-term temperature patterns and variation in the surface levels of polar soils for terrestrial biota. Polar Biol 41:1587–1605
Croll SL, Watts SA (2007) The Effect of Temperature on Feed Consumption and Nutrient Absorption in Procambarus clarkii and Procambarus zonangulus. Journal of the World Aquaculture Society 35:478–488.
Du W, Lu Y, Shu L, Bao Y (2007) Thermal dependence of food assimilation and locomotor performance in juvenile blue-tailed skinks, Eumeces elegans. Anim Biol 57:29–38
Edney EB, Haynes S, Gibo D (1974) Distribution and activity of the desert cockroach Arenivaga investigata (Polyphagidae) in relation to microclimate. Ecology 55:420–427
Edwards JS, Baust J (1981) Sex ratio and adult behavior of the Antarctic midge Belgica antarctica (Diptera, Chironomidae). Ecological Entomology 6:239–243
Fatma S, Ahmed I (2020) Effect of water temperature on protein requirement of Heteropneustes fossilis (Bloch) fry as determined by nutrient deposition, hemato-biochemical parameters and stress resistance response. Fisheries and Aquatic Sciences 23:1
Gantz JD, Spacht DE, Lee RE (2018) A preliminary survey of the terrestrial arthropods of the Rosenthal Islands. Antarctica Polar Research 37:1500266
Gressitt JL (1967) Notes on arthropod populations in the Antarctic Peninsula – South Shetland Islands – South Orkney Islands area. In: Gressitt JL (ed) Entomology of Antarctica. American Geophysical Union, USA, pp 373–391
Guglielmin M, Evans CJE, Cannone N (2008) Active layer thermal regime under different vegetation conditions in permafrost areas. A case study at Signy Island (Maritime Antarctica). Geoderma 144:73–85
Handeland SO, Imsland AK, Stefansson SO (2008) The effect of temperature and fish size on growth, feed intake, food conversion efficiency and stomach evacuation rate of Atlantic salmon post-smolts. Aquaculture 283:36–42
Harada E, Lee RE, Denlinger DL, Goto SG (2014) Life history traits of adults and embryos of the Antarctic midge Belgica antarctica. Polar Biol 37:1213–1217
Harris RMB, McQuillan P, Hughes L (2015) The effectiveness of common thermo-regulatory behaviours in a cool temperate grasshopper. J Therm Biol 54:12–19
Hawes TC, Bale JS, Worland MR, Convey P (2008) Trade-offs between microhabitat selection and physiological plasticity in the Antarctic springtail, Cryptopygus antarcticus (Willem). Polar Biol 31:681–689
Holdgate MW (1977) Terrestrial ecosystems in the Antarctic. Philos Trans R Soc B 279:5–25
Huey RB (1991) Physiological consequences of habitat selection. Am Nat 137:S91–S115
Irwin JT, Lee RE (2003) Cold winter microenvironments conserve energy and improve overwintering survival and potential fecundity of the goldenrod gall fly, Eurosta solidaginis. Oikos 100:71–78
Lee RE, Baust JG (1982) Absence of metabolic cold adaptation and compensatory acclimation in the Antarctic fly, Belgica antarctica. J Insect Physiol 28:725–729
Lee RE, Denlinger DL (2015) Stress tolerance in a polyextremophile: the southernmost insect. Can J Zool 93:679–686
Marshall KE, Sinclair BJ (2012) Threshold temperatures mediate the impact of reduced snow cover on overwintering freeze-tolerant caterpillars. Naturwissenschaften 99:33–41
Mercer NH, Teets NM, Bessin RT, Obrycki JJ (2020) Supplemental foods affect energetic reserves, survival, and spring reproduction in overwintering adult Hippodamia convergens (Coleoptera: Coccinellidae). Environ Entomol 49:1–9
Nicolai V (1986) The bark of trees: thermal properties, microclimates and fauna. Oecologica 69:148–160
Pafalis P, Foufopoulos J, Poulakakis N, Lymberakis P, Valakos E (2007) Digestive performance in five Mediterranean lizard species: effects of temperature and insularity. J Comp Physiol B 77:49–60
Peckham V (1971) Notes on the chironomid midge Belgica antarctica Jacobs at Anvers Island in the maritime Antarcitc. Pacific Insects Monograph 25:145–166
Pike DA, Webb JK, Shine R (2012) Hot mothers, cool eggs: nest-site selection by egg-guarding spiders accommodates conflicting thermal optima. Funct Ecol 26:469–475
Pincebourde S, Woods HA (2020) There is plenty of room at the bottom: microclimates drive insect vulnerability to climate change. Current Opinion in Insect Science 41:63–70
Pincebourde S, Sinoquet H, Combes D, Casas J (2007) Regional climate modulates the canopy mosaic of favourable and risky microclimates for insects. J Anim Ecol 76:424–438
Pincebourde S, Dillon ME, Woods HA (2021) Body size determines the thermal coupling between insects and plant surfaces. Funct Ecol 00:1–13
Potts LJ, Gantz JD, Kawarasaki Y, Philip BN, Gonthier DJ, Law A, Moe L, Unrine JM, McCulley RL, Lee RE, Denlinger DL, Teets NM (2020) Environmental factors influencing fine-scale distribution of Antarctica’s only endemic insect. Oecologia. (in press).
Richardson JL, Urban MC, Bolnick DI, Skelly DK (2014) Microgeographic adaptation and the spatial scale of evolution. Trends Ecol Evol 29:165–176
Rico E, Quesada A (2013) Distribution and ecology of chironomids (Diptera, Chironomidae) on Byers Peninsula, Maritime Antarctica. Antarct Sci 25:288–291
Risser, PG (1987) Landscape Ecology: State of the Art. In: Turner M.G. (eds) Landscape Heterogeneity and Disturbance. Ecological Studies, vol 64. Springer, New York, NY, USA.
Rundle HD, Nosil P (2005) Ecological speciation. Ecol Lett 8:336–352
Sinclair BJ (2015) Linking energetics and overwintering in temperate insects. J Therm Biol 54:5–11
Spacht DE, Gantz JD, Lee RE, Denlinger DL (2020) Onset of seasonal metabolic depression in the Antarctic midge Belgica antarctica appears to be independent of environmental cues. Physiol Entomol 45:16–21
Strong J (1967) Ecology of terrestrial arthropods at Palmer Station, Antarctic Peninsula. In: Gressitt JL (ed) Entomology of Antarctica. American Geophysical Union, USA, pp 357–371
Teets NM, Kawarasaki Y, Lee RE, Denlinger DL (2011) Survival and energetic costs of repeated cold exposure in the Antarctic midge, Belgica antarctica: a comparison between frozen and supercooled larvae. J Exp Biol 214:806–814
Thomas RJ (2017) Data analysis with R statistical software: A guidebook for scientists. Eco-explore, Caerphilly
Tilbrook PJ (1967) Arthropod ecology in the maritime Antarctic. In: Gressitt JL (ed) Entomology of Antarctica. American Geophysical Union, USA, pp 331–356
Uvarov BP (1931) Insects and climate. Transactions of the Entomological Society of London 79:1–247
Woods HA, Dillon ME, Pincebourde S (2015) The roles of microclimatic diversity and of behavior in mediating the responses of ectotherms to climate change. J Therm Biol 54:86–97
Acknowledgements
We thank the staff and crew of Palmer Station and the R/V Laurence M Gould for their tireless support of our research efforts in Antarctica. We thank Colin Harris and Katharina Lorenz of Environmental Research and Assessment for contributing the maps in Fig. 1. We also thank the reviewers for their careful reading of our manuscript, and for providing constructive feedback which contributed to improving the overall quality of the manuscript.
Funding
This project was supported by National Science Foundation Grants PLR-1341385 to REL, PLR-1231393 to DLD, and OPP-1850988 to NMT, and Hatch Project 1010996 from the USDA National Institute of Food and Agriculture to NMT.
Author information
Authors and Affiliations
Contributions
DES, JDG, DLD, and REL: originally conceived the idea. DES and JDG: formulated methodology and conducted fieldwork. DES, JDG, EAM, and NMT: performed laboratory work. JJD and NMT: performed statistical modeling. DES, NMT, and JJD: assembled figures and wrote the manuscript. All authors contributed to editing the manuscript.
Corresponding author
Additional information
Communicated by Sylvain Pincebourde.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Spacht, D.E., Gantz, J.D., Devlin, J.J. et al. Fine-scale variation in microhabitat conditions influences physiology and metabolism in an Antarctic insect. Oecologia 197, 373–385 (2021). https://doi.org/10.1007/s00442-021-05035-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-021-05035-1