Abstract
Decreases in snow cover due to climate change could alter the energetics and physiology of ectothermic animals that overwinter beneath snow, yet how snow cover interacts with physiological thresholds is unknown. We applied numerical simulation of overwintering metabolic rates coupled with field validation to determine the importance of snow cover and freezing to the overwintering lipid consumption of the freeze-tolerant Arctiid caterpillar Pyrrharctia isabella. Caterpillars that overwintered above the snow experienced mean temperatures 1.3°C lower than those below snow and consumed 18.36 mg less lipid of a total 68.97-mg reserve. Simulations showed that linear temperature effects on metabolic rate accounted for only 30% of the difference in lipid consumption. When metabolic suppression by freezing was included, 93% of the difference between animals that overwintered above and below snow was explained. Our results were robust to differences in temperature sensitivity of metabolic rate, changes in freezing point, and the magnitude of metabolic suppression by freezing. The majority of the energy savings was caused by the non-continuous reduction in metabolic rate due to freezing, the first example of the importance of temperature thresholds in the lipid use of overwintering insects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For many abiotic variables, there are critical thresholds beyond which the physiological response of an organism is unpredictable from the linear response preceding it (Gaines and Denny 1993). For example, threshold levels of water flow increase the swimming cost of fish in a non-continuous fashion (Nickerson et al. 1989) and reversible (but potentially injurious) behavioral and physiological changes set upper and lower activity thresholds in ectotherms (Stevens et al. 2010). There is increasing evidence that extreme events are important for many organisms (Jentsch et al. 2007)—for instance, bud-break phenology desynchronized from freezing temperatures can cause widespread frost damage (Augspurger 2009), and incorporating climate extremes in spatial models improves predictions of forest extent in Switzerland (Zimmermann et al. 2009). In coastal bird species, increasing maximum flood levels during extreme flooding events in estuaries in northern Europe are threatening population survival (Van De Pol et al. 2010). Similarly, extreme weather events can result in extreme demographic fluctuations in copepods that influences long-term population growth rates (Drake 2005), and short extreme mid-winter warming events significantly damage Arctic shrubs (Bokhorst et al. 2011) and Collembola communities (Coulson et al. 2000). Thus, in order to predict the importance of increases in extreme events, it is useful to determine the physiological thresholds that underlie fitness responses. Models of climate change predict an increase in extreme weather events (particularly temperature extremes, Easterling et al. 2000), so understanding the nature and effects of physiological thresholds is essential for predicting insect responses to climate change. To date, the majority of studies of biotic responses to climate change assume that the organism has a continuous response to environmental variables (reviewed in Easterling et al. 2000; Benedetti-Cecchi et al. 2006; but see Deutsch et al. 2008).
The temperature associated with the freezing transition in freeze-tolerant insects is a threshold of clear biological importance. Freezing slows or prevents biochemical reactions, disrupts cellular homeostasis, and prevents behavior (reviewed in Storey and Storey 1996; Hochachka and Somero 2002). While insects are frozen, their metabolic and water loss rates drop precipitously (Irwin and Lee 2002; Sinclair et al. 2004), which has been suggested to benefit freeze-tolerant organisms that need to conserve energy and/or water (Voituron et al. 2002). Freezing can also cause disruptions after thaw; for example, the sub-Antarctic caterpillar Pringleophaga marioni has depressed post-thaw metabolism (Sinclair et al. 2004).
In temperate environments, insects with univoltine life cycles spend the majority of their lifespan overwintering, during which feeding does not occur (Hahn and Denlinger 2007). As a result, energy use of insects that overwinter as late-stage juveniles is directly tied to future fitness, since energy stores at the end of winter are the sole fuel source before metamorphosis in the spring, directly determining adult size (Hahn and Denlinger 2007) and thus adult fitness (Oberhauser 1997). Any depression of metabolic rate while frozen and after thawing would then be expected to result in energy savings for freeze-tolerant insects and increased adult fitness (Irwin and Lee 2003). Therefore, changes in winter temperature that affect the likelihood of freezing and the time spent frozen would modify overwinter energy use and impact the abundance and phenology of ectotherm populations in the spring (Bale and Hayward 2010). Overwintering temperatures in leaf litter microhabitats are also generally increased by the presence of snow cover (Zhang 2005), leading to increased overwintering energy consumption. For example, pre-pupae of the freeze-tolerant tephritid Eurosta solidaginis lose more mass overwintering below the snow layer, leading to reduced egg production and therefore lower potential fecundity (Irwin and Lee 2003). A model of overwintering energy use by E. solidaginis indicated that the colder conditions experienced above the snow layer led to energy savings over the winter since the low temperatures decreased metabolic rate (Irwin and Lee 2003).
The North American woolly bear caterpillar Pyrrharctia isabella Smith (Lepidoptera: Arctiidae) overwinters as a final instar larva that ceases feeding in the fall and does not feed in the spring before pupation (Goettel and Philogene 1978), making it a useful model for examining overwintering energetics in a freeze-tolerant insect in the context of potential fitness. P. isabella larvae overwinter underneath the leaf litter layer, and the temperature regime they experience over winter is linked to snow cover: Decreased snow cover results in increased exposure to extreme temperatures (Zhang 2005). P. isabella caterpillars have very little glycogen during their overwintering stage (Marshall and Sinclair 2011), so like many Lepidoptera, lipid is their primary winter fuel.
We used P. isabella to examine the effects of threshold-crossing (freezing) events on whole-winter fitness. Our hypotheses were (1) overwintering exposed to air temperatures is less energetically costly and (2) the non-continuous reduction of metabolic rate during and after freeze–thaw accounts for the majority of this cost savings. We predict that (1) caterpillars overwintering in an exposed microclimate will have more lipids at the end of winter and that (2) energy use calculated with the non-continuous reduction of metabolic rate during freezing events will be significantly lower than if just calculated without that reduction. To test these hypotheses, we developed a numerical simulation model and tested its predictions using real caterpillars overwintering in both exposed and subnivean microhabitats. In our system, the majority of the energy savings result from the non-continuous reduction in metabolism due to freezing, illustrating the importance of temperature thresholds in interpreting ectotherms’ responses to thermal variability.
Materials and methods
Field study
P. isabella caterpillars were collected from fields in Niagara-on-the-Lake, Ontario, Canada (43°04′ N, 79°04′ W) in October 2009. After collection, caterpillars were maintained individually in 15-mL six-well tissue culture plates with moist paper towels on an ad libitum pinto bean diet (Goettel and Philogene 1978). Caterpillars were maintained in the dark and temperatures followed weekly climate maxima and minima (12:12 h) for London, Ontario (43°00′ N, 81°15′ W; www.weatheroffice.ec.gc.ca), until 0°C was first reached, after which they were maintained at 0°C. In early November, caterpillars were weighed and randomized into exposed (n = 20 caterpillars) and subnivean (n = 21 caterpillars) treatment groups.
The overwintering experiment was conducted in a suburban garden in London, ON, Canada, where P. isabella overwinter (B.J. Sinclair, unpublished observations). Each group was placed in a 3.8-L plastic jar filled with soil topped with a 5-cm layer of local unconsolidated leaf litter. Caterpillars were placed between the soil and leaf litter, and the jars closed with a perforated lid. An iButton datalogger (DS1922L, Maxim Integrated Products, Sunnyvale, CA, USA) recorded hourly temperatures alongside the caterpillars in each jar. The subnivean jar was buried in the soil with the soil layer level with the ground (and was snow-covered for much of the winter), and the exposed jar was fixed to a wooden post 1 m above ground level (therefore not snow-covered). In early April, the caterpillars and pupae were sexed and weighed (Genc 2005). Survival was scored based on completing pupation. Sex ratios and survival were compared among treatments using chi-square and Fisher’s exact tests in the program R (R Development Core Team 2010).
Pupae were flash-frozen in liquid nitrogen, while caterpillars were placed in 24 h light in an incubator at 20°C until pupation. All pupae were dried at 70°C for 5 days, and water mass calculated as the difference between dry and wet mass. Pupae were minced with scissors and homogenized in a bullet blender with steel 1.6 mm beads (Next Advance, Averill Park, NY, USA) in 200 μL ice-cold 0.05% Tween 20. Following addition of 800 μL 0.05% Tween 20, the slurry was centrifuged (2 min, 12,000×g) and a 250-fold dilution of the supernatant in 0.05% Tween 20 was frozen until triglyceride and glycerol concentrations were measured (Serum Triglyceride Kit; Sigma-Aldrich, Canada). Lipid mass was compared between treatments using ANCOVA with lipid-free dry mass as a covariate in the program R.
Model assumptions
We extrapolated the relationship between CO2 production rate and temperature for P. isabella from the average value for this relationship at 5°C (Marshall and Sinclair 2011) assuming an exponential relationship and a Q 10 of 1.5 which is within the range of common biological systems and representative of diapausing Lepidoptera (Schmidt-Nielsen 1997; Bennett et al. 1999). To differentiate the metabolic rate at low temperatures from metabolic suppression due to freezing, we estimated freeze–thaw event occurrence assuming a freezing point of −7.6°C (Marshall and Sinclair 2011) and a melting point of −3.4°C estimated from a hemolymph glycerol concentration of 1.82 M for this species (Marshall and Sinclair 2011). Metabolic rate (approximated by carbon dioxide production) was calculated hourly for the winter from the relationship between CO2 production and temperature (as in Irwin and Lee 2003) using hourly temperature data from the appropriate microclimate (from the iButton data loggers in the field experiment). We also ran separate simulations with and without a 15-fold decrease in metabolic rate when caterpillars were predicted to be frozen (freezing depression factor) and with and without a 50% depression in MR for 6 h post-thaw (Sinclair et al. 2004). We were unable to reliably measure these values for P. isabella because it shows discontinuous gas exchange at low temperatures (Marshall and Sinclair 2011), although traces of CO2 during the frozen and thawed state for this species qualitatively showed the same pattern (H. Udaka, unpublished observations). CO2 production was summed for the whole winter and converted to lipid consumed assuming a respiratory exchange ratio of 0.7 (indicating that lipid is the primary overwintering fuel source, cf., Irwin and Lee 2003) and that 2 L of O2 is consumed for each gram of lipid oxidized (Schmidt-Nielsen 1997).
We then used this model to predict P. isabella whole-winter metabolism from microclimate temperature, accounting for the likelihood of the caterpillars being frozen (Fig. 1). We used this model to predict lipid use for four separate states over an entire winter: subnivean without the metabolic depression caused by freezing, subnivean with freezing effects, exposed without freezing effects, and exposed with freezing effects. All numerical simulations were conducted in the program R (R Development Core Team 2010).
Outline of a model predicting total overwintering energy use of P. isabella caterpillars in exposed and subnivean environments, with and without metabolic rate depression induced by freezing. Boxes in gray indicate parameters taken from other studies (references in brackets), while the box with italic lettering indicates empirical data from this study
Model sensitivity
We tested the sensitivity of the predicted lipid use to three parameters: thermal sensitivity of metabolic rate (Q 10), the freezing depression factor, and the freezing point of the caterpillars (which accounted for the possibility of inoculative freezing or changes in SCP in the field, Layne and Blakely 2002). We calculated and compared total overwintering lipid use by varying Q 10 between 1 and 3 with divisions of 0.02, freezing depression factor between 0 and 25 with divisions of 0.25, and freezing point between −20°C and −3.5°C (0.1°C below the melting point) with divisions of 0.165°C, while holding the other values constant. Finally, both freezing point and Q 10 were simultaneously varied between the previously described values to investigate total lipid use for only caterpillars overwintering in the exposed microhabitat including the effect of freezing.
Results
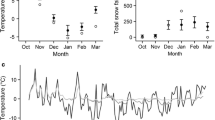
During the field study, the exposed microhabitat was slightly cooler and more variable than the subnivean microhabitat (Table 1; Fig. 2a). Based on empirical freezing points and calculated melting points, P. isabella caterpillars below the snow would not have frozen all winter, while above-snow caterpillars would have experienced repeated freezing (Table 1).
a Overwintering temperature conditions during the winter of 2009–2010 measured hourly in either exposed (solid line) or subnivean (fine dotted line) microclimates. Horizontal dashed line indicates the freezing point of P. isabella (Marshall and Sinclair 2011). b Predicted carbon dioxide production rate (milliliters per hour) in P. isabella caterpillars overwintering in either exposed (solid line) or subnivean (fine dotted line) microclimates during the winter of 2009–2010, including the metabolic depression of freezing. Also plotted is the cumulative difference in lipid saved (milligrams) by caterpillars overwintering in the exposed microclimate relative to those in the subnivean microclimate while either including the effect of freezing (thick solid line) or not (dashed line)
The number or sex of pupae recovered and the wet mass before winter did not differ significantly between overwintering locations (Table 2). Sex had no effect on estimated lipid mass, so the sexes were pooled. The pupal mass of above-snow caterpillars was significantly greater than below-snow caterpillars, and this difference was reflected in an average 18.36 ± 7.24 mg difference in lipid mass between the two groups (Table 2).
Based on our model of overwinter energy use, we estimated a total consumption of 161.23 mg lipid by caterpillars overwintering in the subnivean microclimate and 144.13 mg lipid by exposed caterpillars—a 17.10-mg total difference. Removing metabolic depression associated with freezing from the model reduced this difference to 5.57 mg extra lipid used in the exposed microclimate (Fig. 2b).
The model was insensitive to variation in Q 10 between 1.5 and 2.0. Total predicted lipid use by caterpillars that overwintered in the exposed microclimate varied by only 25 mg through the entire range of Q 10’s examined (Fig. 3a). The difference between the exposed and subnivean groups’ lipid use was maintained throughout the entire range of Q 10 values tested when freezing was accounted for and up to a Q 10 value of 2.9 when freezing was not accounted for. Similarly, the factor by which freezing depressed metabolic rate did not significantly change overwintering energy use at values above 1 (Fig. 3b). Varying the freezing point in the simulation showed that total winter energy use was the lowest when the metabolic depression caused by freezing was included at almost all temperatures investigated and that inoculative freezing would actually exacerbate this effect (Fig. 3c). Varying freezing point and Q 10 simultaneously demonstrated that these two variables both contribute significantly to overwintering energy use in our system, with the effect of freezing point predominating at high freezing points (demonstrated by more horizontal divisions above −10°C), while Q 10 effects predominate at lower freezing points (demonstrated by more vertical divisions below −10°C, Fig. 4).
a Sensitivity of model predicting overwintering energy use (in milligram lipid) by P. isabella to changes in temperature sensitivity (in Q 10 values) in the subnivean microclimate (solid line), exposed but not accounting metabolic depression during freezing (dashed line), or exposed and accounting for metabolic depression during freezing (dotted line). Vertical line indicates the Q 10 value used in the final simulation (Fig. 2). b Sensitivity of model predicting overwintering energy use (in mg lipid) by P. isabella in the exposed microclimate to changes in the amount of metabolic depression by freezing. Vertical line indicates a freezing depression factor of 1 (i.e., no change with freezing). c Sensitivity of model predicting overwintering energy use (in milligram lipid) by P. isabella both above (solid lines) and below (dashed lines) the snow layer either including (black lines) or not including (gray lines) metabolic depression of freezing to changes in freezing point. The freezing point of P. isabella as measured in the laboratory (Marshall and Sinclair 2011) is represented by a solid vertical line
Interactive effects of changing freezing point and Q 10 simultaneously on overwintering lipid use of caterpillars exposed to air temperature in London, Ontario, when the metabolic rate depression of freezing is included. Freezing point was varied between −20°C and −3.5°C in increments of 0.165°C, while Q 10 was varied between values of 1 and 3 in increments of 0.02. Grayscale indicates total lipid use
Discussion
Our simulation indicated that the threshold-crossing impact of freezing on metabolic rate is a significant determinant of overwintering energy use in P. isabella. The field validation confirmed that caterpillars that overwintered in the exposed microclimate consumed less lipid than caterpillars overwintering in the subnivean microclimate, and our simulation indicates that the majority (93%) of this reduction was due to the increased frequency of freezing temperatures above the snow layer, rather than simply a decrease in mean temperature. Thus, physiological thresholds such as the freezing transition can greatly affect the fitness of an organism in its environment, and the microclimate determines the frequency with which the threshold is encountered.
There has been a steady decline in winter snow cover in the Northern Hemisphere over the past several decades (Déry and Brown 2007), and this decline is predicted to continue (Alexander et al. 2010). Given that overwintering above snow leads to lower overwintering energy use due to both lower temperatures and increased chance of freezing, we suggest that declining snow cover will have a net positive effect on overwintering freeze-tolerant ectotherms. However, most freeze-tolerant organisms, including P. isabella, freeze at relatively high sub-zero temperatures (usually between −4°C and −12°C, Lee 2010) which are close to the mean overwintering temperature in Southern Ontario. Thus, if winter mean temperatures increase in step with decreased snow cover, the potential benefits of reduced snow cover could be balanced by the increased energetic costs of fewer freezing events overall. Thus, understanding the way that snow cover will modulate the effects of climate change is a key part of understanding changes in fitness in organisms whose winter microhabitats are affected by snow (see also Bale and Hayward 2010).
Our final model slightly overestimated total lipid use and slightly underestimated the difference in energy expenditure between above- and below-snow caterpillars. It is possible that the Q 10 of 1.5 that we chose to extrapolate metabolic rates is an overestimate of temperature sensitivity in this species, while the 15-fold reduction in metabolic rate with freezing may be an underestimate, since both of these numbers are derived from studies another species (the sub-Antarctic tineid P. marioni, Sinclair et al. 2004). However, even if different Q 10 and reductions in metabolic rates are used, the relative importance of the energy savings of freezing remains (Fig. 3a, b) since metabolic rate is so low at sub-zero temperatures that the magnitude of reduction of metabolic rate is unimportant as long as the threshold is present. Similarly, although previous work has shown that P. isabella held at −5°C are susceptible to inoculative freezing (Layne and Blakely 2002), our conclusions hold at a broad range of potential freezing points (Fig. 3c). It is also likely that pupation was a metabolic cost that we did not include in our model, and we assume that this cost is constant for caterpillars in both environments.
We recognize that the field component of our study compared caterpillars in two different locations only, without replication within each environment. However, it is well established that subnivean temperatures are, over the course of a winter, warmer than temperatures above the snow layer (Zhang 2005), and this has already been empirically demonstrated to increase energy use in the freeze-tolerant fly E. solidaginis (Irwin and Lee 2003). The purpose of this study was not simply to reiterate this result but to investigate the relative importance of extreme physiological events (the freezing transition) on whole-winter energy use. As such, we feel the strength of our study is that for the first time we were able to dissect the energy savings due to freezing events from the energy savings due to lower mean temperatures in our modeling approach.
The relationship between freezing point and Q 10 (Fig. 4) demonstrates the importance of the freezing threshold in this system. Freeze-tolerant insects generally have relatively high supercooling points, and this is thought to facilitate controlled ice formation (Sinclair et al. 2009); however, we show that high SCPs are also energetically advantageous for freeze-tolerant insects. However, there are fitness costs to repeated freeze–thaw cycles in both P. isabella and P. marioni (Sinclair and Chown 2005; Marshall and Sinclair 2011) which likely constrains the optimal freezing point. Shifting Q 10 has a relatively small effect at high freezing points, while at lower freezing points, Q 10 effects predominate. This is likely driven by the temperature regime used in our model. London, Ontario experiences a relatively moderate continental climate with high snow volume due to the proximity of the Great Lakes. However, it is apparent that this broad relationship between the relationship of Q 10 and freezing point on overwintering energy use will hold regardless of the temperature regime encountered.
Thus, temperature thresholds are an important component of the fitness of overwintering insects. However, the thresholds themselves may be plastic; for example, repeated freezing and thawing appears to depress the freezing point of hoverfly larvae (Syrphus ribesii; Brown et al. 2004) and sub-Antarctic beetles (Hydromedion sparsutum; Bale et al. 2001). While the freezing point of P. isabella does not vary with repeated freezing, it can shift by 4°C between years (Marshall and Sinclair 2011). Modifying freezing point does not change the relative amount of energy use between microclimate locations; however, it can affect absolute energy use within a location by altering the frequency with which freezing occurs (Fig. 3c). We suggest that identifying the physiological mechanisms and limits of the plasticity of biological thresholds can help inform models of organismal response to extreme events (Chevin et al. 2010), along with understanding the impacts of the frequency and timing of extreme values of abiotic variables such as temperature, wave forces, salinity, and water availability (Gaines and Denny 1993). As climate change leads to changes in thermal variability concurrent with shifts in mean temperatures, an important contribution of comparative physiology to global change biology will be the identification of key thresholds and their impacts.
References
Alexander MA, Tomas R, Deser C, Lawrence DM (2010) The atmospheric response to projected terrestrial snow changes in the late twenty-first century. J Climate 23:6430–6437
Augspurger CK (2009) Spring 2007 warmth and frost: phenology, damage and refoliation in a temperate deciduous forest. Func Ecol 23:1031–1039
Bale JS, Hayward SA (2010) Insect overwintering in a changing climate. J Exp Biol 213:980–994
Bale JS, Worland MR, Block W (2001) Effects of summer frost exposures on the cold tolerance strategy of a sub-Antarctic beetle. J Insect Physiol 47:1161–1167
Benedetti-Cecchi L, Bertocci I, Vaselli S, Maggi E (2006) Temporal variance reverses the impact of high mean intensity of stress in climate change experiments. Ecology 87:2489–2499
Bennett VA, Kukal O, Lee RE (1999) Metabolic opportunists: feeding and temperature influence the rate and pattern of respiration in the High Arctic woollybear caterpillar Gynaephora groenlandica (Lymantriidae). J Exp Biol 202:47–53
Bokhorst S, Bjerke JW, Street LE, Callaghan TV, Phoenix GK (2011) Impacts of multiple extreme winter warming events on sub-Arctic heathland: phenology, reproduction, growth, and CO2 flux responses. Global Change Biol 17:2817–2830
Brown CL, Bale JS, Walters KF (2004) Freezing induces a loss of freeze tolerance in an overwintering insect. Proc R Soc London, Ser B 271:1507–1511
Chevin LM, Lande R, Mace GM (2010) Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PLoS Biology 8:e1000357
Coulson SJ, Leinaas HP, Ims RA, Søvik G (2000) Experimental manipulation of the winter surface ice layer: the effects on a High Arctic soil microarthropod community. Ecography 23:299–306
Déry SJ, Brown RD (2007) Recent Northern Hemisphere snow cover extent trends and implications for the snow-albedo feedback. Geophys Res Lett 34:L22504
Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR (2008) Impacts of climate warming on terrestrial ectotherms across latitude. Proc Natl Acad Sci USA 105:6668–6672
R Development Core Team (2010) R: a language and environment for statistical computing. R Foundation for Statistical Computing. http://www.R-project.org
Drake JM (2005) Population effects of increased climate variation. Proc R Soc London, Ser B 272:1823–1827
Easterling DR, Meehl GA, Parmesan C, Changnon SA, Karl TR, Mearns LO (2000) Climate extremes: observations, modeling, and impacts. Science 289:2068–2074
Gaines SD, Denny MW (1993) The largest, smallest, highest, lowest, longest and shortest: extremes in ecology. Ecology 74:1677–1692
Genc H (2005) Determination of sex in pupae of Phyciodes phaon (Lepidoptera: Nymphalidae). Fla Entomol 88:536–537
Goettel MS, Philogene BJR (1978) Laboratory rearing of the banded woollybear Pyrrharctia (Isia) isabella (Lepidoptera: Arctiidae), on different diets with notes on the biology of the species. Can Entomol 110:1077–1086
Hahn DA, Denlinger DL (2007) Meeting the energetic demands of insect diapause: nutrient storage and utilization. J Insect Physiol 53:760–773
Hochachka PW, Somero GN (2002) Temperature. In: Hochachka PW, Somero GN (eds) Biochemical adaptation: mechanism and process in physiological evolution. Oxford University Press, New York, pp 290–360
Irwin JT, Lee RE (2002) Energy and water conservation in frozen vs. supercooled larvae of the goldenrod gall fly, Eurosta solidaginis (Fitch) (Diptera: Tephritidae). J Exp Zool Part A 292:345–350
Irwin JT, Lee RE (2003) Cold winter microenvironments conserve energy and improve overwintering survival and potential fecundity of the goldenrod gall fly, Eurosta solidaginis. Oikos 100:71–78
Jentsch A, Kreyling J, Beierkuhnlein C (2007) A new generation of climate-change experiments: events, not trends. Front Ecol Environ 5:365–374
Layne JR, Blakely DL (2002) Effect of freeze temperature on ice formation and long-term survival of the woolly bear caterpillar (Pyrrharctia isabella). J Insect Physiol 48:1133–1137
Lee RE (2010) A primer on insect cold-tolerance. In: Denlinger DL, Lee RE (eds) Insect low temperature biology. Cambridge University Press, New York, pp 3–34
Marshall KE, Sinclair BJ (2011) The sub-lethal effects of repeated freezing in the woolly bear caterpillar Pyrrharctia isabella. J Exp Biol 214:1205–1212
Nickerson DM, Facey DE, Grossman GD (1989) Estimating physiological thresholds with continuous two-phase regression. Physiol Zool 62:866–887
Oberhauser KS (1997) Fecundity, lifespan and egg mass in butterflies: effects of male-derived nutrients and female size. Func Ecol 11:166–175
Schmidt-Nielsen K (1997) Energy metabolism. In: Schmidt-Nielsen K (ed) Animal physiology: adaptation and environment, 5th edn. Cambridge University Press, New York, pp 169–216
Sinclair BJ, Chown SL (2005) Deleterious effects of repeated cold exposure in a freeze-tolerant sub-Antarctic caterpillar. J Exp Biol 208:869–879
Sinclair BJ, Klok CJ, Chown SL (2004) Metabolism of the sub-Antarctic caterpillar Pringleophaga marioni during cooling, freezing and thawing. J Exp Biol 207:1287–1294
Sinclair BJ, Gibbs AG, Lee WK, Rajamohan A, Roberts SP, Socha JJ (2009) Synchrotron X-ray visualisation of ice formation in insects during lethal and non-lethal freezing. PLoS One 4:e8259
Stevens MM, Jackson S, Bester SA, Terblanche JS, Chown SL (2010) Oxygen limitation and thermal tolerance in two terrestrial arthropod species. J Exp Biol 213:2209–2218
Storey KB, Storey JM (1996) Natural freezing survival in animals. Annu Rev Ecol Syst 27:365–386
Van De Pol M, Ens BJ, Heg D, Brouwer L, Krol J, Maier M, Exo KM, Oosterbeer K, Lok T, Eising CM (2010) Do changes in the frequency, magnitude and timing of extreme climatic events threaten the population viability of coastal birds. J Appl Ecol 47:720–730
Voituron Y, Mouquet N, de Mazancourt C, Clobert J (2002) To freeze or not to freeze? An evolutionary perspective on the cold-hardiness strategies of overwintering ectotherms. Am Nat 160:255–270
Zhang T (2005) Influence of the seasonal snow cover on the ground thermal regime: an overview. Rev Geophys 43:RG4002
Zimmermann NE, Yoccoz NG, Edwards TC, Meier ES, Thuiller W, Guisan A, Schmatz DR, Pearman PB (2009) Climatic extremes improve predictions of spatial patterns of tree species. Proc Natl Acad Sci USA 106:19723–19728
Acknowledgments
We thank Moriah Sokolowski for technical assistance, Bonnie Tarnowski for assisting with caterpillar collection, Mhairi McFarlane for property access, Hiroko Udaka and Heath MacMillan for comments, and Hugh Henry, Yolanda Morbey, and several anonymous referees for constructive comments on earlier versions of the manuscript. This research was supported by the Canadian Foundation for Innovation, an Ontario Early Researcher Award and an NSERC Discovery grant to BJS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Sven Thatje
An erratum to this article can be found at http://dx.doi.org/10.1007/s00114-011-0879-8
Rights and permissions
About this article
Cite this article
Marshall, K.E., Sinclair, B.J. Threshold temperatures mediate the impact of reduced snow cover on overwintering freeze-tolerant caterpillars. Naturwissenschaften 99, 33–41 (2012). https://doi.org/10.1007/s00114-011-0866-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-011-0866-0