Abstract
Herbivores assess predation risk in their environment by identifying visual, chemical, and tactile predator cues. Detection of predator cues can induce risk-avoidance behaviors in herbivores that affect feeding, dispersal, and host selection in ways that minimize mortality and reproductive costs. For herbivores that transmit plant pathogens, including many aphids, changes in herbivore behavior in response to predator cues may also affect pathogen spread. However, few studies have assessed how aphid behavioral responses to different types of predator cues affect pathogen transmission. Here, we conducted greenhouse experiments to assess whether responses of pea aphids (Acyrthosiphon pisum) to predation risk and alarm pheromone (E-β-Farnesene), an aphid alarm signal released in response to predation risk, affected transmission of Pea enation mosaic virus (PEMV). We exposed A. pisum individuals to risk cues, and quantified viral titer in aphids and pea (Pisum sativum) host plants across several time periods. We also assessed how A. pisum responses to risk cues affected aphid nutrition, reproduction, and host selection. We show that exposure to predator cues and alarm pheromone significantly reduced PEMV acquisition and inoculation. Although vectors avoided hosts with predator cues, predator cues did not alter vector reproduction or reduce nutrient acquisition. Overall, these results suggest that non-consumptive effects of predators may indirectly decrease the spread of plant pathogens by altering vector behavior in ways that reduce vector competence and pathogen transmission efficiency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Predatory species can affect their prey by killing them (consumptive effects) or by altering their development and behavior (non-consumptive effects) (Preisser et al. 2005; Sih et al. 2010). As insect preys face diverse predator assemblages, they must detect and respond to spatially and temporally variable predation risk. Insects can identify visual, volatile, and contact cues from predators when selecting feeding or oviposition sites, and respond to such cues by altering their behavior, physiology, or morphology in ways that reduce mortality and/or ensure the safety of offspring (Ninkovic et al. 2013; Hermann and Thaler 2014; Beleznai et al. 2015; Hermann and Landis 2017; Culshaw-Maurer et al. 2020). However, changes in insect behavior in response to predators may also affect diet quality, feeding duration, and fecundity (Preisser et al. 2007; Preisser and Bolnick 2008; Jones and Dornhaus 2011). As insect herbivores play key roles in food webs, including acting as vectors of plant pathogens, responses of herbivores to predation risk can lead to cascading indirect effects throughout ecosystems (Peckarsky et al. 2008).
For herbivores that transmit plant pathogens, including many aphids, non-consumptive predator effects may affect vector competence, or the ability of vectors to become infected with, maintain, and transmit an infectious agent. Predation risk can also affect vector fitness, host preferences, feeding behavior, and movement, all of which affect pathogen transmission (Martín et al. 1997; Powell 2005; Fereres and Moreno 2009). For example, in the presence of a spider predator, a leafhopper (Psammotettix alienus) delays initial feeding, and reduces the time spent ingesting phloem, which limits Wheat dwarf virus transmission (Beleznai et al. 2015; Tholt et al. 2018). Additionally, insect predators reduce feeding by Rhopalosiphon padi by stimulating movement, decreasing the prevalence of Cereal yellow dwarf virus (Long and Finke 2015). Increased vector movement caused by predators may also enhance pathogen spread by increasing encounter rates between vectors and hosts (Crowder et al. 2019).
Though studies have assessed herbivore responses to specific categories of predator risk cues (i.e. visual, chemical) and the total effects of predation on pathogen transmission separately, the contribution of predator risk cues to observed rates of transmission has yet to be determined. Despite their relatively short duration, the ubiquity of predation risk cues and their effects on behavioral factors associated with vector competence (Buchanan et al. 2017) suggest their potential in affecting short-term rates of pathogen spread.
Herbivores also respond to predators by releasing alarm signals, such as E-β-Farnesene by aphids, to warn conspecifics of danger (Bowers et al. 1972; Pickett and Griffiths 1980). Alarm signals elicit behavioral and physiological responses, including withdrawal of stylets, dispersal from hosts, and production of winged morphs (Wohlers 1981; Pickett et al. 1992; Kunert et al. 2005; Podjasek et al. 2005). However, while vectors’ anti-predator behaviors in response to alarm pheromones have been observed, whether such changes affect vectors’ ability to transmit pathogens is unknown (Dawson et al. 1982; Lin et al. 2016). This indicates a need for studies that assess pathogen transmission in response to predator cues as well as conspecific risk signals.
Here, we addressed these knowledge gaps by assessing the effects of chemical and visual cues from a lady beetle predator, Hippodamia convergens, as well as alarm pheromone exposure on the ability of pea aphid (Acyrthosiphon pisum) to vector Pea enation mosaic virus (PEMV). We conducted four experiments to evaluate predation risks effects on aphid behavior and life history. Risk treatments were structured to represent scenarios by which aphids might perceive nearby predators: chemical cues left by previous predator visitation, visual and olfactory cues from an adjacent predator, and alarm cues released by a threatened conspecific. First, we measured PEMV titer in aphids that fed on infected peas (acquisition) while exposed to risk cues from both predators and conspecifics, and in pea host plants that were fed upon by infected aphids (inoculation) exposed to the same cues. Each experiment was conducted for several access time periods. We also assessed the effects of risk cues on pea aphid reproduction, host selection, and nutrient ingestion as factors indirectly affecting virus transmission. Overall, our study shows that aphid responses to predator and conspecific risk cues can strongly affect short-term vector competence and pathogen transmission.
Materials and methods
Study system
Acyrthosiphon pisum is a specialist phloem-feeding aphid that feeds on leguminous plants and is the primary vector of PEMV, a persistently transmitted bipartite virus consisting of two single-stranded RNAs, PEMV1 (Enamovirus) and PEMV2 (Umbravirus), which form an obligate symbiosis (Ng and Perry 2004; Rashed et al. 2018). In the Inland Northwest region of the United States, including Washington, Oregon, and Idaho States, outbreaks of A. pisum and PEMV are economically damaging to grain legume crop production (Clement et al. 2010). A diverse community of aphid predators forage in the region, the most abundant being a lady beetle, H. convergens, whose adults and larvae can be regularly observed feeding upon A. pisum in legume fields throughout the growing season (Clark et al. 2019).
Acyrthosiphon pisum colonies used in experiments originated from individuals collected in commercial pea fields in Washington State and were maintained on pea plants (Pisum sativum cv. “Banner”) in greenhouses at Washington State University (Pullman, WA, USA) (23 ± 2 °C, light:dark 16:8 h). Our PEMV isolate was obtained from the University of Idaho (Moscow, ID, USA) and maintained by transferring aphids fed on PEMV-infected pea into uninfected colonies, introducing clean plants as needed. Samples from infectious and un-infectious colonies were tested monthly for PEMV presence using reverse transcriptase (RT)-PCR; these samples showed nearly 100% infection levels in the infectious aphid colony and 0% in the un-infectious colony.
Effects of risk cues on PEMV transmission
We conducted two greenhouse experiments to measure effects of predator and alarm pheromone cues on PEMV transmission. The first tested whether risk cues affected PEMV acquisition by un-infectious A. pisum vectors that fed on PEMV-infected pea plants. The second assessed the rate at which un-infectious P. sativum plants were inoculated by PEMV-infectious A. pisum in response to risk cues. Each experiment exposed aphids (un-infectious or infectious) to multiple predator cues, or alarm cues, across several acquisition and inoculation access periods. In all experiments, the treatments of experimental units were randomly assigned within the greenhouse.
For the acquisition experiment, 2-week-old pea plants were inoculated with PEMV by placing four PEMV-infectious 3rd to 4th instar A. pisum nymphs on apical leaves of the plant and allowing them to feed for 24 h. A ‘sham’-inoculated control treatment with un-infectious A. pisum nymphs was also conducted. PEMV-inoculated plants were allowed to develop virus symptoms for 5 days before being used in experiments. This inoculation protocol was used for all PEMV- and ‘sham’- inoculated plants in all experiments in this study. The acquisition experiment was run in two blocks, first with predator-generated risk cues, then with synthetic alarm pheromone cues.
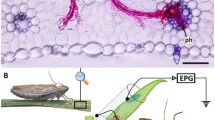
For experiments, four 5-day-old un-infectious A. pisum were confined in fine mesh bags on the three apical, fully formed leaflets of P. sativum plants for 3, 12, or 24 h. Predator risk treatments included chemical contact cues, and sustained visual and volatile chemical cues (“adjacency cues”), applied to PEMV-inoculated pea. Adjacency cues consisted of a single H. convergens adult enclosed within a plastic clip cage attached to a bamboo skewer, suspending the cage adjacent to the plant after A. pisum addition (Fig. 1a). Clip cages were transparent with one side of fine mesh so the predator was visible and any volatile cues could escape. Contact cues were applied by enclosing one adult H. convergens on the three apical fully formed leaflets in a mesh bag for 24 h; individual H. convergens were removed and a clean bag affixed before experiments (Fig. 1b). Hippodamia convergens deposit blends of cuticular hydrocarbons in trails as they walk (Wheeler and Cardé 2014), and several aphid species can detect and respond to these signals (Ninkovic et al. 2013). Individual H. convergens were observed walking upon the surface of enclosed leaves but left no evidence of herbivory or defecation. Conspecific alarm cues were applied by pipetting 5 μl of a solution containing 50 ng or 200 ng pure E-β-Farnesene (Bedoukian, Danbury, CT, USA) dissolved in n-hexane onto a 2 × 2 cm piece of filter paper suspended on a pin below the PEMV-inoculated plant immediately after aphid establishment (Fig. 1c) (Podjasek et al. 2005). These amounts reflect biologically relevant concentrations released by colonies of 5 or 15 individual A. pisum, respectively (Schwartzberg et al. 2008). All groups other that the alarm cue group received a droplet of n-hexane applied similarly as a control. After A. pisum fed for the assigned access period, they were removed from plants, flash-frozen in liquid nitrogen, and tested for PEMV. Non-predator-treated PEMV-inoculated peas, and a control group of sham-inoculated peas, were also used in each block. Each of the 4 treatments groups (contact, adjacency, 50 ng and 200 ng E-β-Farnesene) and 2 control groups (one for each block) contained 4 biological replicates for each of the 3 time periods, resulting in 72 total replicates.
The inoculation experiment was conducted with the same predator cues or alarm pheromone for access periods of 3, 12, and 24 h. Only 50 ng E-β-Farnesene was included, as 50 ng better reflects amounts emitted by colonies of similar size to the number of A. pisum individuals used in the experiment. All experimental plants were ‘sham’-inoculated, and A. pisum used were reared entirely upon PEMV-inoculated P. sativum plants. After the allotted inoculation access period, A. pisum individuals were discarded and the P. sativum host plants were allowed to grow in the greenhouse for 72 h to develop viral titer; 72 h provides adequate time for detectable titers to develop while still being reflective of initial inoculation rates (Wu et al. 2014). After this period, all aboveground plant tissue was collected, wrapped in aluminum foil, frozen in liquid N2, and snap-chilled in dry ice before storing in – 80 °C. The 3 treatment groups (contact, adjacency, and 50 ng E-β-Farnesene) and control group also contained 4 biological replicates for each of the 3 time periods, resulting in 48 total replicates.
Frozen aphid samples were ground with a micro pestle in 1.5 ml microfuge tubes, and pea samples were ground using a mortar and pestle in liquid N2, with 50–100 mg of tissue used for total RNA extraction. Extractions used SV total RNA isolation kits (Promega, Madison, WI) and cDNA from 1 µg of RNA using Bio-Rad iScript cDNA synthesis kits. Gene specific primers for qRT-PCR were designed using the IDT Primer Quest Tool (Table S1). PEMV-specific primers were used in qRT-PCR reactions (10 µl) containing 3 µl of ddH2O, 5 µl of iTaq Univer SYBR Green Supermix, 1 µl of primer mix (forward and reverse), and 1 µl of diluted (1:25) cDNA template. The qRT-PCR program included an initial denaturation for 3 min at 95 °C followed by 40 cycles of denaturation at 95 °C for 15 s, annealing for 30 s at 60 °C, and extension for 30 s at 72 °C. For melting curve analysis, a dissociation step cycle was added (55 °C for 10 s, and then 0.5 °C for 10 s until 95 °C). The relative expression of genes was calculated using the delta-delta Ct method, (\(2^{{ - \Delta \Delta C_{{\text{t}}} }}\)) with Psβ-tubulin and RPL27 as housekeeping genes for peas and aphids, respectively (Livak and Schmittgen 2001; Kozera and Rapacz 2013; Sinha and Smith 2014).
Effects of risk cues on amino acid ingestion
We also measured amino acid content of A. pisum feeding in the presence of contact, adjacency, and E-β-Farnesene alarm cues. Groups of 8 A. pisum individuals were exposed to risk cues or a control in identical experimental set-ups to the PEMV acquisition and inoculation experiments and allowed to feed for 24 h before being collected into liquid N2. For amino acid analysis, A. pisum tissue was lyophilized, ground, and extracted with 20 mM of HCL according to (Patton et al. 2020), with 8 replicates per treatment group, resulting in 64 total replicates. Amino acids were derivatized using AccQ-Fluor kits (Waters, Milford, MA, USA) according to the manufacturer’s instructions, with l-Norleucine as an internal standard. 10 μl from each sample was injected on to a Nova-Pak C18 column with an Agilent 1260 HPLC and fluorescence detector. Peak areas were determined with Agilent Chemstation software and samples were compared to an amino acid standard curve to calculate concentrations (Sigma-Aldrich, St. Louis, MO, USA). The gradient used was 0–0.01 min, 100% A; 0.01–0.5 min, linear gradient to 3% B; 0.5–12 min, linear gradient to 5% B; 12–15 min, linear gradient to 8% B; 15–45 min, 35% B; 45–49 min, linear gradient to 35% B; 50–60 min, 100% B. The flow rate was 1.0 ml min−1.
Effect of risk cues on aphid reproduction
To assess combined effects of predation risk cues and PEMV host or vector infectious status on aphid reproduction, we measured individual adult A. pisum reproduction over 5 days using the same contact, adjacency, and alarm pheromone treatments previously described. Adjacency cues were removed after 24 h to reflect the duration of the other treatments, which could not be reapplied during the experiment. Reproduction was measured for 5 days to capture only the period where risk cues remained perceptible (Ninkovic et al. 2013). Newborn A. pisum were reared for 7 days on sham- or PEMV-inoculated peas, resulting in age standardized un-infectious or PEMV-infectious adult aphids. Individual 7-day-old A. pisum was then placed on the apical leaflets of a 2-week-old plant and could feed and move freely. PEMV-infectious A. pisum were paired with sham-inoculated plants, while un-infectious A. pisum were paired with PEMV-infected plants, reflecting the conditions of transmission experiments. The number of nymphs produced by each adult was recorded daily for 5 days, with 18 adult A. pisum in each of the 3 risk, 1 PEMV-infectious no-cue control, and 1 sham-inoculated control treatments per infectious status pairing, resulting in 180 total replicates.
Effects of predator cues on host selection
To assess A. pisum host preferences in the presence of predation risk cues, we conducted two dual-choice bioassays to compare PEMV-infectious and un-infectious A. pisum settling choices for plants with various risk cues. Choice arenas were constructed using horizontal sections of clear polycarbonate tubing (12 cm long, 3 cm diameter) with a 1.5 cm diameter hole cut in the top of the center of the tube to introduce aphids. In each replicate, a single intact leaflet was placed into each side of the tube and secured with a cotton plug. The first experiment used 5-day-old PEMV-infectious A. pisum and 14-day-old ‘sham’ inoculated P. sativum plants, and the second used 5-day-old un-infectious A. pisum and 14-day-old PEMV-inoculated P. sativum plants. In both experiments, groups of A. pisum individuals were presented untreated leaflets or leaflets with either predator contact cues or adjacency cues. Contact cues were applied by confining a single H. convergens predator onto the leaflet with a fine mesh bag for 24 h prior to the experiment. Adjacency cues consisted of a single dead H. convergens glued to the stem adjacent to the base of the leaflet. Controls for each treatment were either bagged or received a droplet of glue without a predator. E-β-Farnesene was not included, as arenas could not isolate volatile cues on a single host. After treatments were applied, eight A. pisum individuals were then dropped into the tube and allowed to settle freely between leaflets for 6 h before their position was recorded.
Data analysis
To evaluate effects of risk cues on PEMV acquisition and inoculation, we ran generalized linear models (GLMs) for each experiment with viral titer as the response and risk cue, acquisition period, and their interaction as fixed effects. Analyses for viral titer were run on cycle threshold values (Ct), and \(2^{{ - \Delta \Delta C_{{\text{t}}} }}\) (relative expression) was calculated using parameter estimates from the model. Estimated marginal mean of Ct values and standard error of the mean were generated using the “emmeans” package in R (Lenth et al. 2018). Methodology for \(2^{{ - \Delta \Delta C_{{\text{t}}} }}\) followed Rao et al. (2013) and Kozera and Rapacz (2013), using the housekeeping genes RPL-27 for PEMV1 acquisition in A. pisum and β-tubulin for PEMV1 inoculation in P. sativum to normalize expression. “No cue” treatments from the 24 h access periods were used as reference values for expression fold change, as “Sham” groups had no detectable PEMV titers.
To measure effects of risk cues on amino acid uptake, we ran linear mixed models on amino acid concentration with risk cue as a fixed effect, and amino acid type as a random effect. Amino acid data were log-transformed to meet normality assumptions. To analyze the effects of risk cues on aphid reproduction, we ran linear mixed models on total nymphs produced over 5 days with risk cue as a fixed effect and cage as a random effect using the “lme4” package; post hoc tests were calculated using the “emmeans” package. To evaluate predation risk effects on host selection, we analyzed differences in the mean percentage of A. pisum making a settling choice using a paired t test. All analyses were conducted in R ver. 3.5.2 (R Core Team 2020).
Results
Effects of risk cues on PEMV acquisition and inoculation
Compared to no-cue controls, contact predation cues significantly reduced average A. pisum PEMV acquisition by 97% for 12 h (Z = 4.41, n = 12, P < 0.001) and by 84% for 24 h access periods (Z = 2.37, n = 12, P = 0.047) (Fig. 2a). Adjacency predation cues reduced average PEMV acquisition by 87% at 12 h (Z = 2.57, n = 12, P = 0.028) but not at 24 h (Z = 0.67, n = 12, P = 0.78) (Fig. 2a). Release of 200 ng E-β-Farnesene reduced average PEMV acquisition by A. pisum by 70% at 24 h (Z = 2.58, n = 12, P = 0.027), while 50 ng release reduced PEMV acquisition by 65% at 12 h (Z = 2.22, n = 12, P = 0.067) and by 57% at 24 h access periods (Z = 1.81, n = 12, P = 0.17) compared to controls (Fig. 2b).
Fold Change in Expression value of PEMV across host access periods as measured by qRT-PCR in A. pisum groups feeding upon PEMV-infected P. sativum plants a exposed to contact and adjacency predation cues, b exposed to E-β-Farnesene, and c in whole pea plants herbivorized by PEMV-infected A. pisum groups exposed to predation and E-β-Farnesene. Error bars represent 95% confidence intervals. Letters indicate a significant difference between treatments within time points (Tukey’s HSD, p < 0.05)
In P. sativum plants fed upon by PEMV-infectious A. pisum, release of 200 ng E-β-Farnesene reduced average PEMV titer by 93% at 3 h (Z = 6.74, n = 16, P < 0.001) and 62% at 24 h access periods (Z = 2.42, n = 16, P = 0.015). Contact predation cues reduced PEMV titer in P. sativum plants by 86% at 3 h (Z = 5.00, n = 16, P < 0.001), 75% at 12 (Z = 2.65, n = 16, P = 0.008), and 80% at 24 h access periods (Z = 4.06, n = 16, P < 0.001). In contrast, adjacency predation cues did not reduce PEMV titer in P. sativum for any access period (Fig. 2c). ‘Sham’ controls in both experiments had no detectable PEMV titer.
Effects of predator cues on amino acid uptake by A. pisum
In un-infectious A. pisum feeding upon PEMV-infected P. sativum plants, we identified a significant effect of predator risk cues on average concentrations of amino acids when considered individually (χ2 = 111.1, df = 3, P < 0.001) (Fig. S1a). Compared to other groups, contact predation cues significantly increased the total amino acid concentration in A. pisum (Fig. 3a, Tukey HSD). In infectious A. pisum feeding upon uninfected P. sativum plants, all three predator risk cues significantly increased individual amino acid concentrations compared to controls (χ2 = 18.37, df = 3, P < 0.001) (Fig. S1b), though the total amino acid concentrations did not differ (Fig. 3b, Tukey HSD).
Mean concentrations (nmol/mg dry weight) among 16 amino acids in groups of a uninfectious A. pisum on PEMV-infected pea plants and b PEMV-infected A. pisum on ‘sham’ inoculated pea plants exposed to risk cues. Error bars represent 95% confidence intervals. Asterisk indicates significant difference from other groups (p < 0.05, Tukey HSD)
Effect of risk cues on aphid reproduction
In the acquisition group, ‘sham’ group A. pisum (uninfectious A. pisum on uninfected P. sativum plants) produced more nymphs over 5d than “no-cue” and “adjacency cue” groups, which fed on infected P. sativum plants (t85 = 3.13, P = 0.01; t85 = 3.34, P = 0.01, respectively) (Fig. S2a). In the inoculation group, risk cues did not significantly affect A. pisum nymph production across treatments groups over 5 days (χ2 = 5.74, df = 4, P = 0.22) (Fig. S2b).
Effects of predator cues on host selection
Un-infectious A. pisum individuals settling on PEMV-infected P. sativum plants had significant preferences for plants without adjacency and contact predator cues (t19 = 2.83, P = 0.01; and t19 = 2.89, P = 0.009, respectively) (Fig. 4a). PEMV-infectious A. pisum settling on ‘sham’-inoculated P. sativum plants also significantly avoided adjacency cues (t19 = 2.59, P = 0.018), but did not avoid contact cues (t19 = − 0.22, P = 0.83) (Fig. 4b).
Mean percentage of uninfected A. pisum settling choice between PEMV-infected pea plants with no cues vs. a adjacency or b contact predator cues, and PEMV-infected A. pisum settling choice between ‘sham’ inoculated pea plants with no cues vs. c adjacency or d contact predator cues. Error bars represent 95% confidence intervals. Asterisks denote significance in the mean percentage of settled aphids’ choice (t-tests: p < 0.05)
Discussion
Our study shows that perception of predation risk by A. pisum vectors significantly decreased the ability of un-infectious vectors to quickly acquire PEMV, while also decreasing the ability of infectious A. pisum to inoculate healthy plants with PEMV (Fig. 2). While previous studies have suggested that anti-predator responses by vector species could influence transmission of specific plant viruses (Finke 2012; Tholt et al. 2018), we provide the first direct evidence of risk affecting measurable viral titers in both vector and host organisms. As vectors’ initial encounters with hosts determine whether the vector or host becomes infected, initial settling and feeding events are crucial to virus transmission success (Fereres and Moreno 2009). Lady beetles and other aphid predators often arrive in pea fields prior to the establishment of aphids (Ninkovic et al. 2001), thus aphid vectors may be perceiving and responding to predation cues throughout their first colonization events, affecting behaviors important to initial pathogen spread.
Early season predation events are considered important to initial establishment and spread of plant viruses in agriculture by affecting vector abundance (Landis and Van der Werf 1997). Here, we provide further evidence that non-consumptive predator effects could also contribute to observed reductions in virus spread. Initial delays in transmission may be particularly impactful to the spread of persistently transmitted pathogens, which require lengthy feeding bouts for acquisition or inoculation to occur (Hogenhout et al. 2008). Similarly, transmission of Cereal yellow dwarf virus by Rhopalosiphon padi vectors was reduced when predators were present (compared to absent) independent of vector abundance, which was attributed to reduced feeding time, and greater interplant movement, reducing transmission efficiency (Long and Finke 2015). Our results demonstrate that predation risk cues, despite their transience, may reduce pathogen transmission even without direct interaction with predators, indicating broader spatial impact of predation risk on transmission efficiency. Recent theoretical models indicate that while vector behaviors like feeding duration and movement can have variable effects on infection dependent on pathogen characteristics and interactions, transmission efficiency is a stable determinant of rates of virus spread (Shaw et al. 2017, 2019; Crowder et al. 2019). If predation cues consistently lower transmission efficiency independent of other vector traits, reductions in pathogen spread may be consistent. While this would require regular reapplication of predation cues to new hosts throughout the season, which may become less frequent as foraging predators preferentially select dense colonies of vectors (Ives et al. 1993), even temporary fluctuations in acquisition and inoculation efficiency have the potential to affect patho-systems dramatically (Keissar et al. 2020). However, our study did not address the effects of risk cues on the generation of alate or ‘winged’ aphid morphs, which can be induced by contact with predators or alarm pheromone release (Weisser et al. 1999; Kunert et al. 2005). As alate aphids can increase the range of pathogen dispersal (Blua and Perring 1992; Fereres and Moreno 2009), induction of alate production by predators may affect pathogen prevalence over long distances.
Both chemical cues from predators and E-β-Farnesene reduced viral titers in A. pisum vectors and P. sativum hosts. Alarm pheromone release can elicit cascading behavioral responses over short distances (Pickett et al. 1992), meaning a single predator disturbance event could affect transmission efficiencies throughout colonies and between host plants. However, the nature and intensity of responses to alarm pheromone can vary broadly across aphid species and pheromone blends or concentrations (Vandermoten et al. 2012; Basu et al. 2021). Certain alarm chemicals, including E-β-Farnesene, also have the potential to mediate trophic interactions by influencing predator recruitment and behavior directly (Vosteen et al. 2016; Li et al. 2019). Thus, whether interactions between perception of predator risk cues and alarm pheromones contribute to changes in vector behavior require further investigation. Moreover, while quantities of alarm pheromone released by aphids are documented (Schwartzberg et al. 2008), it is unclear whether perception of chemical or visual cues alone can induce alarm pheromone release and vector responses. Interestingly, PEMV titer detected in P. sativum plants decreased as inoculation access period increased, though the effects of contact and conspecific alarm cues were consistent over time (Fig. 2c). To prevent false negatives, host plants were given 72 h after inoculation to develop detectable titers before sampling, which may have allowed for host immune responses to affect titer (Mandadi and Scholthof 2013).
We show that un-infectious A. pisum generally avoided settling on riskier host plants that were infected with PEMV (Fig. 4), supporting previous studies linking risk cues and settling behavior (Ninkovic et al. 2013; Tamai and Choh 2019). This may mitigate some effects of predation risk on feeding behaviors if only enemy-free hosts are selected. If vectors choose to avoid risky hosts after settling and feeding, contact with additional uninfected hosts may accelerate the spread of pathogens, as has been observed with both physical disturbance by predators and alarm pheromone release (Smyrnioudis et al. 2001; Hodge et al. 2011; Lin et al. 2016). However, increased movement between hosts exposes vectors to additional environmental hazards, including desiccation and predator species (Losey and Denno 1998). Chemical cues deposited by predators did not deter settling of PEMV-infectious A. pisum on uninfected hosts (Fig. 4b); however, infectious aphids did respond to adjacency cues. Recently, Bera et al. (2020) demonstrated un-infectious A. pisum prefer to settle pea plants with oxylipin signaling inhibited, however, PEMV-infectious A. pisum had no preference. These results suggest PEMV infection may have a direct impact on vectors’ perception of some chemical cues. Virus infection can directly manipulate host choice in aphid vectors to promote settling of infectious vectors on un-infectious hosts, and vice versa (Ingwell et al. 2012), which models suggest can greatly accelerate transmission (Shoemaker et al. 2019). Thus, preventing an infectious vector’s anti-predator response to guarantee feeding on an un-infectious host could provide short-term benefits to pathogen transmission greater than costs to vector survival (Finke 2012).
Predation risk cues alone did not play a substantial role in determining rates of A. pisum reproduction (Fig. S2). Though effective in reducing aphid population abundance in the field, aphid predators encounter and disturb far more prey than they consume. In the case of H. convergens, as few as 1 in 30 encounters with A. pisum result in consumption (Nelson and Rosenheim 2006). Physical disturbance of prey by predators and alarm pheromone release have been shown to reduce aphid reproductive capacity (Nelson et al. 2004; Su et al. 2006), yet we demonstrate that chemical and visual cues from predators, and conspecific alarm cues, may not affect short-term vector reproduction, despite their effects on initial rates of pathogen transmission. Monitoring vector populations alone may thus fail to capture some indirect effects of predation risk on short-term vector-borne pathogen dynamics, though these effects are likely less influential that physical disturbance or consumption by predators. We did however find increased nymph production by un-infectious A. pisum feeding upon ‘sham’ uninfected P. sativum plants compared to those feeding on PEMV-infected P. sativum plants. This is likely attributable to PEMV infection reducing leaf area and increasing density constraints in our experiment, rather than a direct effect of infection, as previous studies have indicated that PEMV-infectious status can increase A. pisum fecundity over 5 days (Hodge and Powell 2010). This explanation would be consistent with findings from similar persistently transmitted plant virus systems (i.e. Barley yellow dwarf virus, Bean leafroll virus), where infection regularly improves host quality and enhances vector reproduction (Mauck et al. 2012; Patton et al. 2020).
We predicted that amino acid content in A. pisum would be reduced by predation risk cues, reflecting fewer feeding events or decreased feeding duration than arenas without cues (Dixon and Agarwala 1999; Hermann and Thaler 2014; Fan et al. 2017), coinciding with observed reductions in PEMV transmission when cues were present (Fig. 2). However, cues left by prior predator visitation slightly increased total amino acid concentrations in groups of un-infectious A. pisum feeding upon PEMV-infected P. sativum plants (Fig. 3a), despite this treatment consistently reducing PEMV titers in both aphids and plants (Fig. 2a, c). Feeding by Homopteran insects occurs in a series of probing, insertion, salivation, and ingestion phases, during which pathogens can be acquired or transmitted at specific phases according to their transmission mode (Fereres and Moreno 2009). PEMV is primarily transmitted by A. pisum during sub-phase II-2 of intracellular salivation, which precedes the phloem ingestion phases where amino acid acquisition occurs (Powell 2005). Our measurement of amino acid concentration was intended to provide a metric of both aphid feeding duration and nutritional status, but was unable to differentiate changes in feeding-phase duration important to virus inoculation However, our findings suggest that reductions in the number of feeding events or overall feeding duration are likely not responsible for observed reductions in virus transmission. Further work using electrical penetration graph (EPG) techniques, which use electrical currents to measure the duration of specific insect feeding phases, in combination with predation risk cues would be useful in evaluating risk effects on specific patterns of vector feeding phases important to pathogen transmission.
Overall, our results indicate that perception of predation risk cues, and conspecific alarm cues, have the potential to stall the initial spread of persistent plant viruses, and that this effect may not be discernable through other changes in vector populations or behavior. Continuous exposure to predation risk cues may affect long term vector population dynamics and pathogen prevalence throughout the season, as vectors make settling and feeding decisions based on dynamic levels of risk, host characteristics, and population densities. As individual risk cues can contribute differently to vector behaviors, their characteristics will have to be considered when predicting the magnitude and directionality of predator effects on pathogen transmission.
Variation in individual vectors’ traits has been increasingly recognized as important to pathogen transmission rates (Crowder et al. 2019; Cator et al. 2020). However, empirical studies have been understandably limited in the number and scale of factors affecting vector traits and are thus not yet representative of real-world ecological systems. In our study, for example, a greater understanding of the persistence of risk cues in natural settings and how perception of risk affects vector populations of varying sizes would be required to make predictions about in-field transmission rates. Efforts to examine disease dynamics through a community ecology framework are considered an important priority to inform disease management (Johnson et al. 2015), and fine-scale studies have been able identify potential mechanisms through which interactions between species might affect transmission outcomes. However, future research examining known interactions between vectors and communities at greater spatial and temporal scales are needed to evaluate the contributions of such interactions to pathogen transmission in natural settings.
References
Basu S, Clark RE, Fu Z, Lee BW, Crowder DW (2021) Insect alarm pheromones in response to predators: ecological trade-offs and molecular mechanisms. Insect Biochem Mol Biol. https://doi.org/10.1016/j.ibmb.2020.103514
Beleznai O, Tholt G, Tóth Z, Horvath V, Marczali Z, Samu F (2015) Cool headed individuals are better survivors: non-consumptive and consumptive effects of a generalist predator on a sap feeding insect. PLoS ONE. https://doi.org/10.1371/journal.pone.0135954
Bera S, Blundell R, Liang D, Crowder DW, Casteel CL (2020) The oxylipin signaling pathway is required for increased aphid attraction and retention on virus-infected plants. J Chem Ecol 46:771–781. https://doi.org/10.1007/s10886-020-01157-7
Blua MJ, Perring TM (1992) Alatae production and population increase of aphid vectors on virus-infected host plants. Oecologia 92:65–70
Bowers WS, Nault LR, Webb RE, Dutky SR (1972) Aphid alarm pheromone: isolation, identification, synthesis. Science 80(177):1121–1122. https://doi.org/10.1126/science.177.4054.1121
Buchanan AL, Hermann SL, Lund M, Szendrei Z (2017) A meta-analysis of non-consumptive predator effects in arthropods: the influence of organismal and environmental characteristics. Oikos 126:1233–1240. https://doi.org/10.1111/oik.04384
Cator LJ, Johnson LR, Mordecai EA, El Moustaid F, Smallwood TRC, LaDeau SL, Johansson MA, Hudson PJ, Boots M, Thomas MB, Power AG, Pawar S (2020) The role of vector trait variation in vector-borne disease dynamics. Front Ecol Evol 8:189. https://doi.org/10.3389/fevo.2020.00189
Clark RE, Basu S, Lee BW, Crowder DW (2019) Tri-trophic interactions mediate the spread of a vector-borne plant pathogen. Ecology. https://doi.org/10.1002/ecy.2879
Clement SL, Clement SL, Husebye DS, Eigenbrode SD (2010) ecological factors influencing pea aphid outbreaks in the US pacific Northwest. In: Kindlmann P, Dixon AFG, Michaud JP (eds) Aphid biodiversity under environmental change patterns and processes. Springer Netherlands, Dordrecht, pp 107–128. https://doi.org/10.1007/978-90-481-8601-3
Crowder DW, Li J, Borer ET, Finke DL, Sharon R, Pattemore DE, Medlock J (2019) Species interactions affect the spread of vector-borne plant pathogens independent of transmission mode. Ecology 100:1–10. https://doi.org/10.1002/ecy.2782
Culshaw-Maurer M, Sih A, Rosenheim J (2020) Bugs scaring bugs: enemy-risk effects in biological control systems. Ecol Lett. https://doi.org/10.1111/ele.13601
Dawson GW, Gibson RW, Griffiths DC, Pickett JA, Rice AD, Woodcock CM (1982) Aphid alarm pheromone derivatives affecting settling and transmission of plant viruses. J Chem Ecol 8:1377–1388. https://doi.org/10.1007/BF01403101
Dixon AFG, Agarwala BK (1999) Ladybird-induced life-history changes in aphids. Proc R Soc Lond Ser B Biol Sci 266:1549–1553. https://doi.org/10.1098/rspb.1999.0814
Fan L, Ouyang F, Su J, Ge F (2017) Adaptation of defensive strategies by the pea aphid mediates predation risk from the predatory lady beetle. J Chem Ecol. https://doi.org/10.1007/s10886-017-0908-y
Fereres A, Moreno A (2009) Behavioural aspects influencing plant virus transmission by homopteran insects. Virus Res 141:158–168. https://doi.org/10.1016/j.virusres.2008.10.020
Finke DL (2012) Contrasting the consumptive and non-consumptive cascading effects of natural enemies on vector-borne pathogens. Entomol Exp Appl 144:45–55. https://doi.org/10.1111/j.1570-7458.2012.01258.x
Hermann SL, Landis DA (2017) Scaling up our understanding of non-consumptive effects in insect systems. Curr Opin Insect Sci 20:54–60. https://doi.org/10.1016/j.cois.2017.03.010
Hermann SL, Thaler JS (2014) Prey perception of predation risk: volatile chemical cues mediate non-consumptive effects of a predator on a herbivorous insect. Oecologia 176:669–676. https://doi.org/10.1007/s00442-014-3069-5
Hodge S, Powell G (2010) Conditional facilitation of an aphid vector, Acyrthosiphon pisum, by the plant pathogen, pea enation mosaic virus. J Insect Sci 10:1–14. https://doi.org/10.1673/031.010.14115
Hodge S, Hardie J, Powell G (2011) Parasitoids aid dispersal of a nonpersistently transmitted plant virus by disturbing the aphid vector. Agric for Entomol 13:83–88. https://doi.org/10.1111/j.1461-9563.2010.00493.x
Hogenhout SA, Ammar E-D, Whitfield AE, Redinbaugh MG (2008) Insect vector interactions with persistently transmitted viruses. Annu Rev Phytopathol 46:327–359. https://doi.org/10.1146/annurev.phyto.022508.092135
Ingwell LL, Eigenbrode SD, Bosque-Pérez NA (2012) Plant viruses alter insect behavior to enhance their spread. Sci Rep 2:578. https://doi.org/10.1038/srep00578
Ives AT, Kareiva P, Perry R (1993) Response of a predator to variation in prey density at three hierarchical scales: lady beetles feeding on aphids. Ecology 74:1929–1938. https://doi.org/10.2307/1940836
Johnson PTJ, de Roode JC, Fenton A (2015) Why infectious disease research needs community ecology. Science 349:1–9. https://doi.org/10.1126/science.1259504
Jones EI, Dornhaus A (2011) Predation risk makes bees reject rewarding flowers and reduce foraging activity. Behav Ecol Sociobiol 65:1505–1511. https://doi.org/10.1007/s00265-011-1160-z
Keissar O, Scharf I, Sadeh A (2020) Predator modulation of plant pathogen spread through induced changes in vector development rates. Ecol Entomol 45:213–222. https://doi.org/10.1111/een.12790
Kozera B, Rapacz M (2013) Reference genes in real-time PCR. J Appl Genet 54:391–406. https://doi.org/10.1007/s13353-013-0173-x
Kunert G, Otto S, Röse USR, Gershenzon J, Weisser WW (2005) Alarm pheromone mediates production of winged dispersal morphs in aphids. Ecol Lett 8:596–603. https://doi.org/10.1111/j.1461-0248.2005.00754.x
Landis DA, Van Der Werf W (1997) Early-season predation impacts the establishment of aphids and spread of beet yellows virus in sugar beet. Entomophaga 42:499–516. https://doi.org/10.1007/BF02769810
Lenth RV (2018) Least-squares means: the R package lsmeans. J Stat Softw. https://doi.org/10.18637/jss.v069.i01
Li J, Hu H, Mao J et al (2019) Defense of pyrethrum flowers: repelling herbivores and recruiting carnivores by producing aphid alarm pheromone. New Phytol 223:1607–1620. https://doi.org/10.1111/nph.15869
Lin FJ, Bosquée E, Liu YJ, Chen J, Liu Y, Francis F (2016) Impact of aphid alarm pheromone release on virus transmission efficiency: When pest control strategy could induce higher virus dispersion. J Virol Methods 235:34–40. https://doi.org/10.1016/j.jviromet.2016.05.009
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Long EY, Finke DL (2015) Predators indirectly reduce the prevalence of an insect-vectored plant pathogen independent of predator diversity. Oecologia 177:1067–1074. https://doi.org/10.1007/s00442-014-3194-1
Losey JE, Denno RF (1998) The escape response of pea aphids to foliar-foraging predators: factors affecting dropping behaviour. Ecol Entomol 23:53–61. https://doi.org/10.1046/j.1365-2311.1998.00102.x
Mandadi K, Scholthof KG (2013) Plant immune responses against viruses: how does a virus cause disease? Plant Cell 25:1489–1505. https://doi.org/10.1105/tpc.113.111658
Martin B, Collar JL, Tjallingii WF, Fereres A (1997) Intracellular ingestion and salivation by aphids may cause the acquisition and inoculation of non-persistently transmitted plant viruses. J Gen Virol 78:2701–2705. https://doi.org/10.1099/0022-1317-78-10-2701
Mauck K, Bosque-Pérez NA, Eigenbrode SD, De Moraes CM, Mescher MC (2012) Transmission mechanisms shape pathogen effects on host-vector interactions: evidence from plant viruses. Funct Ecol 26:1162–1175. https://doi.org/10.1111/j.1365-2435.2012.02026.x
Nelson EH, Rosenheim JA (2006) Encounters between aphids and their predators: the relative frequencies of disturbance and consumption. Entomol Exp Appl 118:211–219. https://doi.org/10.1111/j.1570-7458.2006.00378.x
Nelson EH, Matthews CE, Rosenheim J (2004) Predators reduce prey population growth by inducing changes in prey behavior. Ecology 85:1853–1858. https://doi.org/10.1890/03-3109
Ng JCK, Perry KL (2004) Transmission of plant viruses by aphid vectors. Mol Plant Pathol 5:505–511. https://doi.org/10.1111/J.1364-3703.2004.00240.X
Ninkovic V, Al AS, Pettersson J (2001) The influence of aphid-induced plant volatiles on ladybird beetle searching behavior. Biol Control 195:191–195. https://doi.org/10.1006/bcon.2001.0935
Ninkovic V, Feng Y, Olsson U, Pettersson J (2013) Ladybird footprints induce aphid avoidance behavior. Biol Control 65:63–71. https://doi.org/10.1016/j.biocontrol.2012.07.003
Patton MF, Bak A, Sayre JM, Heck ML, Casteel CL (2020) A polerovirus, Potato leafroll virus, alters plant–vector interactions using three viral proteins. Plant Cell Environ 43:387–399. https://doi.org/10.1111/pce.13684
Peckarsky BL, Abrams PA, Bolnick DI, Dill LM, Grabowski JH, Luttbeg B, Orrock JL, Peacor SD, Preisser EL, Schmitz OJ, Trussel GC (2008) Revisiting the classics: considering nonconsumptive effects in textbook examples of predator—prey interactions. Ecology 89:2416–2425
Pickett JA, Griffiths DC (1980) Composition of aphid alarm pheromones. J Chem Ecol 6:349–360. https://doi.org/10.1007/BF01402913
Pickett J, Wadhams L, Woodcock C (1992) The chemical ecology of Aphids. Annu Rev Entomol 36:895–913. https://doi.org/10.1093/plankt/fbu025
Podjasek JO, Bosnjak LM, Brooker DJ, Mondor EB (2005) Alarm pheromone induces a transgenerational wing polyphenism in the pea aphid, Acyrthosiphon pisum. Can J Zool 83:1138–1141. https://doi.org/10.1139/z05-108
Powell G (2005) Intracellular salivation is the aphid activity associated with inoculation or non-persistently transmitted viruses. J Gen Virol 86:469–472. https://doi.org/10.1099/vir.0.80632-0
Preisser EL, Bolnick DI (2008) The many faces of fear: comparing the pathways and impacts of nonconsumptive predator effects on prey populations. PLoS ONE 3:5–8. https://doi.org/10.1371/journal.pone.0002465
Preisser EL, Bolnick DI, Benard ME (2005) Scared to death? The effects of intimidation and consumption in predator-prey interactions . Ecology 86:501–509
Preisser L, Orrock L, Schmitz J (2007) Predator hunting mode and habitat domain alter nonconsumptive effects in predator-prey interactions. Ecology 88:2744–2751
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Rao X, Huang X, Zhou Z, Lin X (2013) An improvement of the 2ˆ(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat Bioinforma Biomath 3:71–85
Rashed A, Feng X, Prager SM, Porter LD, Knodel JJ, Karasev A, Eigenbrode SE (2018) Vector-borne viruses of pulse crops, with a particular emphasis on North American cropping system. Ann Entomol Soc Am 111:205–227. https://doi.org/10.1093/aesa/say014
Schwartzberg EG, Kunert G, Stephan C, David A, Rose USR, Gershenzon J, Boland E, Weisser WW (2008) Real-time analysis of alarm pheromone emission by the pea aphid (Acyrthosiphon pisum) under predation. J Chem Ecol 34:76–81. https://doi.org/10.1007/s10886-007-9397-8
Shaw AK, Peace A, Power AG, Bosque-Pérez NA (2017) Vector population growth and condition-dependent movement drive the spread of plant pathogens. Ecology 98:2145–2157. https://doi.org/10.1002/ecy.1907
Shaw AK, Igoe M, Power AG, Bosque-Pérez NA, Peace A (2019) Modeling approach influences dynamics of a vector-borne pathogen system. Bull Math Biol 81:2011–2028. https://doi.org/10.1007/s11538-019-00595-z
Shoemaker LG, Hayhurst E, Weiss-Lehman CP, Porath-Krause A, Borer ET, Seabloom EW, Shaw AK (2019) Pathogens manipulate the preference of vectors, slowing disease spread in a multi-host system. Ecol Lett 22:1115–1125. https://doi.org/10.1111/ele.13268
Sih A, Bolnick DI, Luttbeg B, Orrick JL, Peacor SD, Pintor LM, Preisser E, Rehage JS, Vonesh JR (2010) Predator-prey naïveté, antipredator behavior, and the ecology of predator invasions. Oikos 119:610–621. https://doi.org/10.1111/j.1600-0706.2009.18039.x
Sinha DK, Smith CM (2014) Selection of reference genes for expression analysis in diuraphis noxia (Hemiptera: Aphididae) fed on resistant and susceptible wheat plants. Sci Rep 4:1–6. https://doi.org/10.1038/srep05059
Smyrnioudis IN, Harrington R, Clark SJ, Katis N (2001) The effect of natural enemies on the spread of barley yellow dwarf virus (BYDV) by Rhopalosiphum padi (Hemiptera: Aphididae). Bull Entomol Res 91:301–306. https://doi.org/10.1079/BER2001110
Su J, Zhu S, Zhang Z, Ge F (2006) Effect of synthetic aphid alarm pheromone (E)-β-farnesene on development and reproduction of Aphis gossypii (Homoptera: Aphididae). J Econ Entomol 99:1636–1640. https://doi.org/10.1093/jee/99.5.1636
Tamai K, Choh Y (2019) Previous exposures to cues from conspecifics and ladybird beetles prime antipredator responses in pea aphids Acyrthosiphon pisum (Hemiptera: Aphididae). Appl Entomol Zool 54:277–283. https://doi.org/10.1007/s13355-019-00623-3
Tholt G, Kis A, Medzihradszky A, Szita É, Tóth Z, Havelda Z, Samu F (2018) Could vectors’ fear of predators reduce the spread of plant diseases? Sci Rep 8:8705. https://doi.org/10.1038/s41598-018-27103-y
Vandermoten S, Mescher MC, Francis F, Haubruge E, Verheggen FJ (2012) Aphid alarm pheromone: an overview of current knowledge on biosynthesis and functions. Insect Biochem Mol Biol 42:155–163. https://doi.org/10.1016/j.ibmb.2011.11.008
Vosteen I, Weisser WW, Kunert G (2016) Is there any evidence that aphid alarm pheromones work as prey and host finding kairomones for natural enemies? Ecol Entomol 41:1–12. https://doi.org/10.1111/een.12271
Weisser WW, Braendle C, Minoretti N (1999) Predator-induced morphological shift in the pea aphid. Proc R Soc B 266:1175–1181. https://doi.org/10.1098/rspb.1999.0760
Wheeler CA, Cardé RT (2014) Following in their footprints: cuticular hydrocarbons as overwintering aggregation site markers in Hippodamia convergens. J Chem Ecol 40:418–428. https://doi.org/10.1007/s10886-014-0409-1
Wohlers P (1981) Effects of the alarm pheromone (E)-Β-farnesene on dispersal behaviour of the pea aphid Acyrthosiphon Pisum. Entomol Exp Appl 29:117–124. https://doi.org/10.1111/j.1570-7458.1981.tb03049.x
Wu Y, Davis TS, Eigenbrode SD (2014) Aphid behavioral responses to virus-infected plants are similar despite divergent fitness effects. Entomol Exp Appl 153:246–255. https://doi.org/10.1111/eea.12246
Acknowledgements
This research was supported by USDA-NIFA Grant 2019-67011-29602 to BWL and USDA-NIFA Grant 2017-67013-26537 to DWC and CLC. We thank Crowder lab undergraduate assistants, Megan Asche for graphics creation, and D. Shorts and T. C. Koji for inspiration.
Author information
Authors and Affiliations
Contributions
BWL, SB, and DWC conceived the experiments. BWL, SB, SB, and CLC performed the experiments. BWL analyzed the data. BWL and DWC wrote the manuscript. All authors provided editorial assistance.
Corresponding author
Additional information
Communicated by Sylvain Pincebourde.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, B.W., Basu, S., Bera, S. et al. Responses to predation risk cues and alarm pheromones affect plant virus transmission by an aphid vector. Oecologia 196, 1005–1015 (2021). https://doi.org/10.1007/s00442-021-04989-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-021-04989-6