Abstract
Food quality determines the growth rate of primary consumers and ecosystem trophic efficiencies, but it is not clear whether variation in primary consumer densities control, or is controlled by, variation in food quality. We quantified variation in the density and condition of an abundant algae-eating cichlid, Tropheus brichardi, with respect to the quality and productivity of algal biofilms within and across rocky coastal sites in Lake Tanganyika, East Africa. Adjacent land use and sediment deposition in the littoral zone varied widely among sites. Tropheus brichardi maximized both caloric and phosphorus intake at the local scale by aggregating in shallow habitats: algivore density decreased with depth, tracking attached algae productivity (rETRMAX) remarkably well (r2 = 0.84, P = 0.00033). In contrast, algivore density was unrelated to among-site variation in algal productivity. Rather, there was significant increase in algal quality (r2 = 0.44, P = 0.011) and decrease in algal biomass (r2 = 0.53, P = 0.0068) with T. brichardi density across sites, consistent with strong top-down control of primary producers. The amount of inorganic sediment on rock surfaces was the strongest predictor of among-site variation in algivore density (r2 = 0.69, P = 0.00096), and algivore gut length increased with sedimentation (r2 = 0.36, P = 0.034). These patterns indicate extrinsic and top-down forcing of algal food quality and quantity across coastal landscapes, combined with adaptive habitat selection by fish at the local scale. Factors that degrade food quality by decreasing algal nutrient content or diluting the resource with indigestible material are likely to depress grazer densities, potentially dampening top-down control in high-light, low-nutrient aquatic ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Eutrophication, climate warming, and terrigenous sediments are transforming coastal marine and freshwater ecosystems (Burkepile and Hay 2006; Crain et al. 2008; Cohen et al. 2016). Nutrients, temperature, and deposited sediments are strong drivers of attached algal biomass and quality (Izagirre et al. 2009; Wagenhoff et al. 2013; Guo et al. 2016; Vadeboncoeur and Power 2017). The emergent effects of these stressors on algal and grazer productivity are difficult to predict because primary consumers simultaneously affect, and are affected by, primary producer biomass and nutrient content (Liess and Hillebrand 2004; Burkepile and Hay 2006; Vadeboncoeur and Power 2017). Ecosystem dynamics may be especially sensitive to the consumer–resource interactions when fish are the dominant grazers because they are nutrient-rich and highly mobile, enhancing their potential to regulate both nutrient availability and algal biomass (Vanni and McIntyre 2016).
Ecosystems in which primary producers have a high-nutrient content exhibit efficient energy transfer from primary producers to herbivores and strong top-down control of primary producer biomass (Cebrian et al. 2009). Attached microalgae support inverted pyramids of trophic level biomass in unimpaired freshwater ecosystems (Vadeboncoeur and Power 2017), in part because microalgae are highly digestible and nutrient-rich compared to other basal resources (Cebrian 1999; Sterner and Elser 2002). High algal nutrient content can boost consumer growth rates (Elser et al. 2000; Sterner and Elser 2002), but it is less clear whether algal quality, rather than quantity, determines the carrying capacity of grazer populations at local or landscape scales.
Algal productivity (rate of carbon fixation) gradients often generate corresponding gradients in grazer densities, demonstrating a direct, bottom-up dependence of grazers on attached algal quantity (Hill et al. 2010). Within and among ecosystems, attached algal productivity is often closely associated with total herbivore biomass (Cebrian et al. 2009) and production (Hill et al. 2010). Indeed, algivorous fish can track algal productivity over large spatial scales (Power 1984a; Vadeboncoeur and Power 2017), rapidly responding to spatial variation in food availability by aggregating in the most productive areas (Power 1984a). Their mobility leads to an ideal free distribution of algivorous fish within contiguous habitat: fish densities correlate positively with algal primary production, but the resulting top-down control yields relatively uniform algal biomass throughout the habitat (Power 1984a). If extrinsic factors impede grazer mobility or reduce grazer densities, algal biomass rapidly accumulates, but it is reduced to background levels even more quickly following restored access by grazers (Power et al. 1988).
Despite the close link between the quantity of algae produced and grazer densities (Power 1984a; Hill et al. 2010), as well as the strong effect of diet quality on grazer growth rate (Sterner and Elser 2002), it is not clear that higher quality forage produces higher densities of grazers (Anderson et al. 2010; Pagès et al. 2014). If there is a substantial metabolic cost to consuming algae with poor nutritional content, then habitats with low-quality algae may support fewer algivores because each individual requires a larger resource base than a comparable individual in a habitat with higher food quality. Conversely, if extrinsic factors such as predation or nutrient loading reduce the effectiveness of top-down control by algivores, then algal quality is likely to decline as algal biomass (C) accumulates (Liess and Hillebrand 2004; Evans-White and Lamberti 2006). Determining the direct effect of algal quality on population densities of grazers is challenging in the face of the top-down effects of grazing on algal quality.
To better understand reciprocal relationships between mobile grazers and primary producers, we quantified grazing fish and algae at both local and landscape scales. We quantified attached algal productivity and nutrient content along with the density of the most abundant algivorous cichlid, Tropheus brichardi at 12 rocky sites in Lake Tanganyika. We surveyed sites that vary in exposure to upwelled nutrients (Corman et al. 2010), anthropogenic nutrients (Kelly et al. 2017), and terrigenous sediments (Alin et al. 1999; McIntyre et al. 2005). Within each site, we also tested for depth gradients in algal productivity and grazer densities. We expected that if algal resources limit grazer populations, then both within- and among-site variation in T. brichardi densities would correlate positively with algal productivity and algal quality, yet algal biomass would be comparatively uniform due to top-down control.
Materials and methods
Study site
Lake Tanganyika is an ancient tectonic lake and is the world’s second largest freshwater lake by depth and volume. Dissolved nutrient concentrations are near detection limits (Corman et al. 2010), and the euphotic zone (> 1% surface light) exceeds 60 m. Its 1830 km shoreline is composed of rocky bedrock outcrops interspersed with expanses of sand, which impose a dispersal barrier to fishes that specialize on eating algae attached to rocks (Sefc et al. 2007). Rocky sites host diverse communities of endemic cichlids, including up to a dozen algivorous species (Takeuchi et al. 2010). Fish in the tribe Tropheini dominate the algivorous assemblages and specialize in consuming algal biofilms attached to the rocks, with typically 1–2 species numerically dominating the assemblage in any region (Konings 2015). Our long-term study sites around Kigoma Tanzania (McIntyre et al. 2005,2006; Corman et al. 2010) host about 30 species of fish, of which 9 specialize on attached algae. The most abundant algivore, Tropheus brichardi, feeds by ripping attached algal filaments and their associated epiphytic diatoms from the rocks. The second most common algivore, Petrochromis spp. feeds by combing epiphytic diatoms from the surface of the algal assemblage (Konings 2015). We quantified the distribution of Tropheus brichardi with respect to habitat complexity and their algal food during the dry seasons (June–August) of 2011, 2012 and 2013 at 12 spatially isolated rocky sites (~ 1 km shoreline between sites) in the Kigoma region of Tanzania. We selected sites across a gradient of land use and wave exposure, two variables that are likely to affect nutrient (Corman et al. 2010; Kelly et al. 2017) and sediment (Cohen et al. 1993; Alin et al. 1999) retention in the shallow littoral habitat.
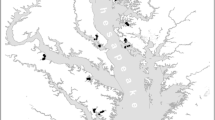
We retained 10 sites (2–7, 9–12) from a previous study (Corman et al. 2010), and extended our study region to the south (Site 13: 4° 55.32S, 29° 36.64E) and north (Site 14: 4° 49.57S, 29° 36.26E) (Fig. 1). We made measurements along depth transects which, unless stated otherwise, included the depths 0.5, 1, 2, 3, 4, 5, 6, 7, and 8 m.
Study sites around Kigoma, Tanzania. In 2009, we added sites 13 and 14 to our original suite of research sites (2–12). Site 13 is on the southwestern tip of a narrow, well-vegetated peninsula. Sites 2–4 are adjacent to the privately owned Jakobsen’s Beach and Guesthouse, which is a large tract of native vegetation. Site 7 is at the base of an escarpment near a hotel compound. Kigoma harbor, a major port on Lake Tanganyika, extends between sites 7 and 9. The land around sites 5, 6, and 9–12, and 14 is a mixture of steep cliffs and hilly grassland with occasional trees
Fish densities often correlate positively with the 3-dimensional structural complexity, or rugosity of a habitat (Gratwicke and Speight 2005), and the size and abundance of cobbles, boulders, and bedrock outcrops varied among sites. We measured 3-dimensional habitat structure at each site in 2011. Three depth transects were deployed perpendicular to the shore between 0 and 8 m. We calculated rugosity as the ratio between the length of a chain laid across the lake bottom to the hypotenuse of a triangle defined by the horizontal distance along the lake surface and the vertical change in water depth (McCormick 1994).
Attached algal resources
We quantified variation in primary productivity along 0–8 m depth transects (N = 2–3 transects per site) using underwater pulse amplitude modulated (PAM, Walz Germany) fluorometry in 2011 and 2012 (Perkins et al. 2011; Devlin et al. 2016). Rapid light curves (RLCs) were collected from horizontally oriented rock surfaces between 10:00 and 14:00. We used the nls function in R (R Core Team 2016) to solve for rETRMAX and α using the hyperbolic tangent function:
where rETR is relative electron transport rate at light intensity E. rETRMAX is the light-saturated relative electron transport rate, and α (photosynthetic efficiency) is the rate of increase in ETR as a function of light intensity in the light-limited region of the photosynthesis irradiance (P-I) curve. We discarded curves if the r2 of the least squared regression of rETR (expected) on rETR (observed) was < 0.90. We coupled PAM fluorometry with direct measurement of primary production in 2011 and 2012 using bulk oxygen exchange methods (Vadeboncoeur et al. 2014; Devlin et al. 2016). In brief, 5 clear (light) and 5 opaque (dark) open-bottomed acrylic chambers were secured to rock surfaces at 2.5 m (2012) and 5 m (2011 and 2012). We measured changes in O2 concentrations using a ProODO oxygen meter (Yellow Springs Instruments, Yellow Springs, Ohio USA) after a 20 min (light) or 2 h (dark) incubation period.
We analyzed biomass and nutrient content of attached algae along depth gradients in 2011 and 2013. In 2011, divers collected epilithic-attached algal scrubs from boulders along a 0–8 m depth transect (N = 3 per site) using a 1 cm diameter modified syringe sampler with a wire brush (Loeb 1981). Two samples from each depth were acidified to remove carbonate C before measuring particulate phosphorus (P) with the acid molybdate method (Stainton et al. 1977). The third sample was frozen and freeze-dried for chlorophyll analysis.
In 2013, divers collected fist-sized cobbles along depth transects (N = 3 per site) at 1, 2.5, 5 and 7 m. We scrubbed a 25.5 cm2 area from the surface of each cobble and freeze-dried (Virtis model 6 K, Gardiner, NY) the samples. The samples were homogenized and subsampled for the following analyses. We combusted subsamples for 4 h at 500 °C for organic matter (AFDM) and inorganic sediment (ash) content of the biofilm. We assessed algal P content with an acid molybdate method (Stainton et al. 1977). Algal C and N content were analyzed at the Cornell Stable Isotope Laboratory with a Vario EL III elemental analyzer (Elemental Americas Corporation, New Jersey, USA). We extracted chlorophyll subsamples in 90% buffered ethanol at 4 °C for 24 h and measured them fluorometrically (Turner Aquafluor: Turner Designs, San Jose, California USA; Vadeboncoeur et al. 2014). We calculated algal C, N, and P content as a fraction of the organic matter (determined by loss on ignition) portion of each sample, such that algal quality and inorganic sediment were not inherently autocorrelated. We extracted phospholipids from ~ 0.3 g subsample using a 2:1 methanol: chloroform mixture, and converted them to fatty acids methyl esters (FAMEs) and separated fatty acids using a HP6890 GC-FID gas chromatograph (DeForest et al. 2016; Agilent Technologies Inc., Santa Clara, California, USA). We identified individual fatty acids as a percent molar fraction of total phospholipid fatty acids using the Sherlock® Microbial Identification System (MIS) (MIDI, Inc., Newark, Delaware, USA).
Fish distributions
We conducted depth-specific visual surveys of algivores in 2011. Each census transect (N = 2 per site) was deployed perpendicular to the shore and was composed of nine contiguous quadrats between 0 and 8 m (see above for depth increments). Quadrats were 5 m wide, and we measured the linear run of each 1 m depth interval. A diver drifted downslope along the transect line, counting T. brichardi as they appeared. We counted each quadrat twice and averaged the values. While censusing T. brichardi, the diver also counted the other relatively common algivore in the region, Petrochromis spp. and the far rarer T. duboisi. The majority of fish in the 2011 survey occurred between 0 and 3 m. Therefore, in 2013, we refined the quantification of T. brichardi using duplicate counts in three 5 m wide quadrats that stretched from the shore to a depth of 3 m.
Although adult T. brichardi are very aggressive and relatively resistant to predation, we quantified the abundance of fish that might prey upon T. brichardi using our annual visual census of the complete fish assemblage (7 × 8 m quadrats, N = 3 per site). Quadrats were oriented perpendicular to shore, with the deep margin set at 5 m and the shallow margin varying between 2 and 4 m depending on slope. A snorkeler drifted slowly across the quadrat at the surface counting all fish in the water column, and then made a series of systematic dives to count species near the substrate throughout the quadrat. This method provided a minimum estimate of total fish densities within the middle part of the depth range covered by our depth transects. There were substantial differences in the censusing methods and the depth strata surveyed between the T. brichardi and fish assemblage analyses. However, this assemblage census, which our group conducted from 2009 to 2015, did provide an estimate of potential predators at each site.
Our research focused primarily on demographic and behavioral responses to variation in resources. However, resource quality can also alter body and gut morphology (Wagner et al. 2009). To test for morphological responses to food quality, 60 T. brichardi were captured with a gill net (1″ × 1″ mesh), measured and weighed from each site in 2013, and from a subset of sites in 2011 and 2012. We sacrificed ten fish from each site each year to measure gut length (stomach to rectum). We calculated the condition factor (k) of each fish by fitting weight and length to a power function (Froese 2006), W = kL2.9, where W is wet mass (g), L is standard length (cm). The scaling exponent was fit as 2.9 based on T. brichardi from Site 13, where body condition was highest, and presumably approached optimal condition for the species (Froese 2006).
Statistical analyses
We used R software (R Core Team 2016) for all analyses. For the 2011 data, we used linear mixed models (lme4, Bates et al. 2015) to test the effects of depth as a fixed factor and site as a random factor to independently predict the following: T. brichardi population density; habitat complexity (rugosity); attached algae phosphorus (%); and chlorophyll a. Additionally, we averaged fish densities, algal P content and rETRMAX across sites for each depth. We used linear models to test the effects of algal P concentration and attached algal productivity on T. brichardi density.
We sampled attached algae at fewer depths in 2013 than in 2011. We used mixed models to assess the effects of depth (fixed effect) and site (random effect) on attached algal quality and quantity in 2013. Data were log10-transformed where necessary to normalize variance. Quantity metrics included organic C (g/m2), N (g/m2), P (g/m2) and chlorophyll a (mg/m2). Metrics of algal quality included % C, % N, % P, N:P (molar), saturated fatty acids (percent mole fraction SAFA), monounsaturated fatty acids (MUFA), polyunsaturated fatty acids (PUFA), and the sum of the omega-three essential fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Diatoms are rich in EPA. We used a t statistic to determine if the slope of the change in attached algal composition with depth was significantly different from zero.
We used principal components analysis (PCA; vegan function) to identify minimum sets of attached algae composition predictors from 2013 that might explain among-site differences in fish density and condition. We reduced the data set by averaging algal composition data from the 1 and 2.5 m depths from each site, which corresponded to the depth range where T. brichardi were most abundant. Metrics related to algal standing crop included C (g/m2), P (g/m2), N (g/m2), and chlorophyll (mg/m2). Quality metrics were % N and % P. We wanted to include essential fatty acids in our PCA, but we lacked fatty acid data for site 6. Therefore, we ran a second PCA on 11 sites using all of the above given descriptors of algal quantity and quality plus the sum of EPA and DHA. We used regression analysis to assess whether among-site variation in T. brichardi densities or condition were functions of the principal components axes.
We used generalized linear models to test for relationships between inorganic sediments and T. brichardi densities, relative gut length, and condition factor. To address predation and habitat availability as potential additional influences on algivore density, we tested for associations between piscivore densities or rugosity and T. brichardi densities using generalized linear models.
Results
Attached algal quantity and quality
Attached algal productivity measured as PAM fluorescence declined with depth (Fig. 2a) at all sites except site 7, which was the site most impacted by sediment (Fig. 1). In 2011, site averages of attached algal gross primary productivity measured with oxygen exchange methods ranged from 23 to 80 mg C m−2 h−1 at 5 m. In 2012, average algal productivity ranged from 43 to 80 mg C m−2 h−1 at 2.5 m, and 30–78 mg C m−2 h−1 at 5 m. Productivity was significantly higher at 2.5 m than at 5 m at all sites except Site 7.
Variation in resource availability and algivore densities with depth in 2011. Each point is average value for 12 sites. a Attached algal productivity was measured in situ with PAM fluorometry. Relative electron transport rate (rETRMAX) is an index of maximum light-saturated photosynthesis. b Attached algal %P on boulders; c Tropheus brichardi densities measured by snorkel surveys. Error bars are ± 1 standard error for N = 12 sites
The quantity of chlorophyll (g/m2) on rocks did not change significantly with depth in 2011 or 2013 (Table 1). The quantity of P (mg/m2) in algae decreased significantly with depth on both boulders (2011: coefficient of log P with depth = − 0.063, t72 = − 3.172, P = 0.0024) and cobbles (2013, Table 1). We measured the quantity of algal C, N, and AFDM per square meter only in 2013 and there was no trend with depth (Table 1).
There was a significant decline in attached algal quality with depth in both years. On boulders (2011), attached algal %P declined with depth (Fig. 2b: coefficient of log %P = − 0.035, t70 = − 2.890, P = 0.0051). On cobbles (2013), algal %P and %N declined significantly with depth, N:P molar increased with depth, and there was no change in algal %C (Table 1). The most abundant essential fatty acids (EFAs) were EPA and linoleic acid (LA), which constituted 1.5 and 3.2%, respectively, of the total fatty acids. DHA constituted less than 1% of total fatty acids. Percent mole fraction of polyunsaturated fatty acids (PUFAs) and the sum of EPA and DHA decreased significantly with depth, whereas saturated fatty acids (SAFAs) increased with depth (Table 1).
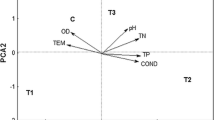
The first two axes of the principal component analysis (PCA) accounted for 96% of the variance in attached algal composition among sites (Fig. 3a). PC1 was highly correlated with the quantity of organic carbon (Pearson’s correlation, r = 0.99), the quantity of N (r = 0.95), and the quantity of chlorophyll per square meter of rock (r = 0.89). PC2 was highly negatively correlated with attached algal % P (Pearson’s correlation, r = − 0.82) and %N (r = − 0.79). There was little among-site variability in gross primary productivity (mg C/m2/h), and it did not load on PC1 or PC2. Eleven of the sites were distributed along a gradient ranging from high quality, low quantity to low quality, high quantity. However, the southernmost site (13) had both a high quality and a high quantity of algae (Fig. 3a). In analyses relating algal quantity to fish densities, we omitted site 13 (see below), but site 13 was not omitted from regressions of fish densities on algal quality. Omitting site 6 from the PCA to include EFA’s in the analysis did not change the above given results. Interestingly, EFAs loaded on PC1 with metrics of attached algal quantity, not with attached algal quality metrics on PC2.
Principal components analysis (PCA) of attached algal quality and quantity at 12 sites in Lake Tanganyika in 2013. a PCA axis 1 (PCA1) was strongly correlated with metrics of algal quantity including chlorophyll (mg/m2) and organic carbon (g/m2). PCA axis 2 (PC2) was negatively correlated with algal quality, including %N and %P. b Variation in T. brichardi densities (each point is a mean of 3 quadrat counts) among sites was unrelated to PCA1, but PCA2 (algal quality) explained 44% the variation in T. brichardi densities among sites; Tropheus brichardi densities increased with increasing algal %N and %P
Fish distributions
We counted 814 Tropheus brichardi in 2011 and 616 T. brichardi in 2013. Tropheus brichardi densities within sites were positively correlated between 2011 and 2013 (Pearson’s correlation = 0.65, P = 0.02). Tropheus brichardi densities declined with depth at all sites (Fig. 2c), as did densities of Petrochromis. Depth-specific average densities of the two algivores were strongly correlated (Pearson’s correlation = 0.95, P = 0.00012). The third algivore, T. duboisi, was too uncommon to assess trends with depth. Variation in algivore densities with depth was a positive function of attached algal photosynthesis rate (rETRMAX) (T. brichardi: F1,7 = 42.27, r2 = 0.84, P = 0.00033. Petrochromis: F1,7 = 19.48, r2 = 0.79, P = 0.0031). Algal %P also declined with depth, but the Akaike information criterion (AIC) value was lower when rETRMAX alone was included in the model. The 3-dimensional habitat structure (rugosity) was a unimodal function of depth, peaking at about 3 m, and did not explain variation in algivore density with depth.
Among sites, T. brichardi densities varied positively with attached algal quality, negatively with algal standing crop, and were not correlated with algal productivity. Their densities were unrelated to PC1 (F1,10 = 0.27, P = 0.61), but the regression of fish densities on PC2 (%P, %N) was significant (Fig. 3b; F1,10 = 9.81, r2= 0.44, P = 0.011). Sediment accumulation had a significant negative effect on T. brichardi densities (F1,9 = 23.16, r2 = 0.69, P = 0.00096; Fig. 4a). In addition, relative gut length of T. brichardi increased across the sediment gradient (Fig. 4b). There was no relationship between T. brichardi density and substrate rugosity (r2= 0.001, P = 0.8, 2011; and r2= 0.01, P = 0.7 for 2013) or piscivore densities (r2 = 0.15; P > 0.05). Based on our annual censuses of the entire fish community at each site, the total density of algivores was nearly three times higher than that of piscivorous species.
a Tropheus brichardi densities at 12 sites (N = 3 quadrats per site) were negatively related to sediment accumulation on rocks. b Across sites, average relative gut length (gut length/total length; N = 10 fish per site) increased as sediment accumulation on rocks increased. Values for the outlier, Site 13 (triangle), were not included in the regressions
With the exception of site 13, fish appeared to have a strong top-down effect on the quantity of algae attached to rocks. The total amount of attached algal C (Fig. 5a; F1,9 = 18.62, r2 = 0.64, P = 0.0019) and chlorophyll (Fig. 5b; F1,9 = 12.17, r2 = 0.53, P =0.0068) per square meter of rock surface declined significantly as a function of T. brichardi density. There was a significant negative relationship between T. brichardi density and molar C:P (r2 = 0.4, P = 0.034). The body condition of T. brichardi also varied significantly among sites. Fish condition factor and fish density were not correlated across sites in 2013 (Pearson’s r = 0.3, P = 0.5), and none of the metrics of attached algal quality or quantity explained among-site variation in condition factor. Interestingly, average body condition declined over the study period at the five sites for which we had 3 years of data. The mean condition factor for T. brichardi was 2.24 in 2011, 2.15 in 2012, and 2.04 in 2013.
Algivorous fish appeared to exert strong top-down control on algal biomass. There was little among-site variation in algal productivity but both (a) organic carbon and (b) chlorophyll a accumulation on rocks decreased with increase in Tropheus brichardi densities. Each point represents the average of six cobbles collected from the site. Values for the outlier, Site 13 (triangle), were not included in the regressions
Discussion
Relationships between Tropheus brichardi densities and food resources differed at local and landscape scales. Within a site, these grazing fish closely tracked the productivity of their algal resource, but among-site variation in T. brichardi densities was unrelated to algal productivity. Rather, variation in T. brichardi densities along the coastline appears to drive differences in algal biomass (organic carbon and chlorophyll) across sites as a top-down effect. Furthermore, densities of these fish correlate with the quality (nutrient and EFA content) of algal resources both within and among sites. The positive correlation between algivores and food quality is consistent with a metabolic cost to consuming low-quality food, but it could also indicate a top-down effect of grazing on algal nutrient content.
Consumer–resource relationships at a local scale
Within sites, T. brichardi aggregated in shallow areas where attached algal biomass and productivity were the highest (Fig. 2). Light limitation causes decline in algal productivity with depth (Vadeboncoeur et al. 2014), and it is remarkable how closely T. brichardi densities tracked attached algal photosynthetic rate (rETRMAX) with depth (F1,7 = 42.27, r2 = 0.84, P = 0.00033). We observed no change in chlorophyll with depth despite the substantial variation in primary productivity, suggesting that these grazers achieve an ideal free distribution with respect to the quantity of algae available. Such behavioral tracking of resources has been reported previously in grazing tropical fish (Power 1984a), and increases in the territory size of algivorous cichlids in Lake Tanganyika with depth have been attributed to gradients in algal productivity (Karino 1998). However, our concurrent analyses of fish densities, algal productivity, and algal biomass are the first to demonstrate that algivores track light-limited primary productivity patterns at a local scale, thereby creating uniformly low algal standing stocks across all depths.
The depth distribution of grazing fish also was correlated with the elevated phosphorus content of algae in the upper 3 m (Fig. 2). Based on the light-nutrient hypothesis developed for phytoplankton (Sterner et al. 1997), we had expected decrease in algal C:P ratios with depth due to declining rates of carbon fixation (Fig. 2). However, we observed the opposite pattern: algal C:P and C:N increased with depth. This result indicates that nutrient availability declined with depth, and did so at a rate that was relatively higher than the decline in light. Dissolved inorganic nutrients were near detection limits at all sites (< 2 µg L−1 for soluble reactive P, NH4-N, and NO3-N; McIntyre et al. 2006; Corman et al. 2010). However, depth gradients in nutrient availability, rather than concentration, could be generated by gradients in production of excreted wastes by fish (McIntyre et al. 2008) and by depth-dependent N-fixation by attached algae (Higgins et al. 2001). The stoichiometry of attached algae in Lake Tanganyika (mean N:P = 37; mean C:P = 869) suggests strong P-limitation (Hillebrand and Sommer 1999). Fish excretion can provide as much as 50% of nutrient demands for algal growth (Andre et al. 2003, McIntyre et al. 2007), and the depth distribution of algivores likely accelerates recycling of nutrients in shallow waters. Furthermore, the abundance of grazers in shallow areas could depress algal C:P and N:P by removing senescing algae and maintaining biofilms in a high-growth state (Liess and Hillebrand 2004).
Essential fatty acids of algal biofilms also decreased with depth (Table 1), adding a second major dimension of depth-dependent variation in the nutritional quality of attached algae. Nitrogen-fixing diatoms and cyanobacteria dominate the Lake Tanganyika attached algal community with small contributions of green algae (Munubi 2015). Diatoms are rich in EPA, whereas cyanobacteria and other bacteria do not produce long chain EFAs. The replacement of EPA and DHA by SAFA with depth suggests a gradient of biofilm composition with greater abundance of diatoms near the shore and increasing contribution of cyanobacteria and other bacteria with depth (Strandberg et al. 2015). Essential fatty acids and P are critical for the growth and development of fish (Ahlgren et al. 2009). Thus, it is not surprising that T. brichardi aggregated in shallow waters where both algal quality and productivity are highest.
The preference of these algivorous fish for shallow water suggests that they are responding primarily to foraging opportunities, rather than avoiding predators. It was beyond the scope of this study to quantify predation risk. However, our extensive field observations suggest that the primary threats are birds (kingfishers, cormorants). Exposure to these diving predators is highest near the shoreline (e.g., Power 1984c, Schlosser 1987). We rarely see piscivorous fish large enough to consume adult Tropheus in our study area, but they occur at greater depths. The fact that T. brichardi prefer shallow habitats (Fig. 2) and spend > 90% of their time actively foraging (Munubi 2015) is inconsistent with strong predation threat. Responses to other non-resource factors, including temperature are not supported by the data. Based on our PAM fluorometer data and in situ loggers, water temperature did not vary spatially over the depth range where T. brichardi occurred.
We interpret the depth distribution of T. brichardi and Petrochromis spp. as a behavioral response to food availability, not predation threat. This same distribution of algivores occurs in other parts of Lake Tanganyika (Takeuchi et al. 2010), again supporting a general response of algivores to resource availability. We have repeatedly observed markedly increased feeding activity by both T. brichardi and Petrochromis during the increased wave activity caused by onshore winds each afternoon. The waves briefly increase the water depth over the shallowest rocks at the lake’s edge, and both species ‘surf’ into consuming the previously inaccessible algae. Additionally, when we conduct in situ small-scale exclusion experiments, we have to fight off the grazing fish in the time between removing the exclosure and taking our measurements. These behaviors demonstrate the avidity with which these algivores track their food resource.
Consumer–resource relationships at a landscape scale
Inhospitable sandy habitats separate our sites, impeding among-site movement by rock-dwelling fishes (Sefc et al. 2007; Wagner and McCune 2009). Thus, population densities of T. brichardi are independent across sites, and density at a given site reflects resource availability, reproductive success and predation pressure at the site. We have observed over ~ 15 years of monitoring fish assemblages, that some sites consistently support higher densities and higher diversity than degraded sites, especially Site 7. Attached algal primary productivity is the best index of food availability for algivores, but there was no relationship between area-specific algal productivity and algivore density among sites, regardless of whether we considered the density of T. brichardi only or of all algivores in the fish assemblage. Moreover, our fish surveys showed a neutral or positive relationship between densities of piscivores and T. brichardi across sites, primarily because the most pristine sites with intact riparian zones support higher fish densities of all trophic guilds. The aquarium trade occasionally targets Tropheus brichardi, but the species is not highly desirable for food. Thus, there is little evidence to invoke top-down constraints on T. brichardi populations.
The pattern of population densities across a dozen sites echoes our local-scale results with respect to food quality; densities of T. brichardi correlate positively with algal nutrient content (Fig. 3b). The effective isolation of rocky sites in Lake Tanganyika precludes the possibility that algivorous fish aggregated at sites in the landscape with the highest food quality. The two most plausible explanations for the positive relationship between grazer density and algal nutrient content are a demographic response to inherent differences in nutrient availability between sites (bottom-up control of consumer densities), or a density-dependent effect of grazers on algal quality (top-down control of resource quality). The influence of algal quality on the growth rate of primary consumers is well-documented for individual animals (Sterner and Elser 2002; Bracken et al. 2012), yet is rarely studied in the context of consumer demography in natural ecosystems (Pagès et al. 2014). Food quality could determine algivore carrying capacity if the nutritional benefits of high-nutrient algae reduce the amount of primary production necessary to support an individual fish. There was no relationship between condition factor at a site and algal quality, which might have been indicative of a positive effect of algal quality on individual growth. Among-site differences in nutrient availability to algae could be extrinsically determined by differential upwelling of nutrients during partial mixing events (Corman et al. 2010; Menge and Menge 2013). However, our years of monitoring hydrodynamic proxies at all study sites currently offers little support for consistent differences in upwelling that might drive variation in algal quality.
An alternative explanation for the correlation between algal quality and T. brichardi is that when grazing pressure is low, fixed carbon from photosynthesis accumulates, diluting algal P and N content (Evans-White and Lamberti 2006). This well-established pattern in grasslands and benthic algal communities emerges because grazing pressure accelerates the turnover of carbon relative to N and P (Liess and Hillebrand 2004; Evans-White and Lamberti 2006). Grazers necessarily remineralize dietary nutrients even when their food resources are nutrient-poor (Vanni and McIntyre 2016). Grazing fish can both suppress carbon stocks and accelerate P regeneration, making top-down control of algal quality quite likely. It is unfortunate that we counted only T. brichardi in 2013. However, based on T. brichardi abundance relative to other algivores and its correlation with Petrochromis, T. brichardi densities are a robust index of grazing pressure. The ten-fold differences in algal standing crop are negatively correlated with T. brichardi densities across sites (Fig. 5). These patterns are consistent with strong top-down control on attached algae (Steinman 1996; Rosemond et al. 1993; McIntyre et al. 2006). In situ grazing experiments and behavioral observations (McIntyre et al. 2006; Munubi 2015; Vadeboncoeur unpublished data) support the conclusion that the among-site variation in algal biomass and biomass-specific productivity (Fig. 5) is driven by differences in grazing pressure.
Anthropogenic sediment negatively affected T. brichardi densities (Fig. 4). Sediment accumulation has reduced biodiversity along many parts of the shoreline of Lake Tanganyika (Cohen et al. 1993; Alin et al. 1999; McIntyre et al. 2005), and algivores are the most impacted guild of fish (Donohue et al. 2003). More than 25% of the cichlid species in Lake Tanganyika consume attached algae (Vadeboncoeur et al. 2011), and algivore densities are declining relative to other trophic guilds (Takeuchi et al. 2010). Our data suggest that a temporal decline in algivore condition (this study) and densities (McIntyre unpublished data) is occurring in the Kigoma region, though the mechanism is unclear. The incorporation of sediment into algal biofilms attenuates the nutritional value of algae. Algivorous fish must either reduce their foraging efficiency to avoid ingesting sediment or incur a reduction in digestive efficiency if sediment is consumed along with algae (Power 1984b; McIntyre et al. 2005). Although T. brichardi is more tolerant of sediments than its congeners (Konings 2015), behavioral data demonstrate an inhibitory effect of sediment on its feeding rate (Munubi 2015). The plasticity of gut length in response to sediment deposition in the littoral zone (Fig. 4; Wagner et al. 2009) highlights the physiological cost to ingesting sediments. The combination of reduced grazer densities and altered grazer morphology suggests that sedimentation is altering the structure of the ecosystem by limiting the strength of algivory, which subsequently leads to higher biomass of attached algae (Fig. 5).
It is notable that despite the high inorganic sediment content of the algal scrubs, algivore densities were high at site 13. The land around site 13 is well-vegetated with Miombo, the native forest. Site 13 is exposed to more waves, winds and currents than the other sites. Each afternoon during our study wave action increased substantially, and this caused colloidal polymers derived from bacteria and phytoplankton (marine snow) to precipitate in the water column. Marine snow was noticeably more abundant at site 13. We speculate that inorganic sediment at site 13 originated from the lake, rather than the land, and contained a substantial amount of associated phytoplankton detritus. Site 13 was not an outlier with respect to the correlation between algivore densities and algal quality (Fig. 3b).
Conclusions
Algivorous fish structure tropical marine and freshwater ecosystems (Power 1984a; Flecker and Taylor 2004; Burkepile and Hay 2006) and efficiently shunt algal carbon and nutrients to higher trophic levels (Power 1984a; Cebrian et al. 2009). The high food quality and rapid turnover of microalgae are essential to producing the inverted biomass pyramids typical of high-light, low-nutrient aquatic ecosystems (Vadeboncoeur and Power 2017). Algivorous fish densities in Lake Tanganyika closely track both algal quality and productivity at the local scale, but not at the landscape scale. Rather, strong top-down effects of grazing on algal biomass and nutrient content were evident across sites. Terrigenous sediments appeared to affect fish densities negatively, reducing the ability of fish to control attached algal biomass. Disentangling the direct and indirect effects of algal productivity and nutrient content on consumer growth rate and densities is the first step to managing these highly productive, low-nutrient littoral ecosystems. The long-term decline in algivores in Lake Tanganyika’s littoral zone (Takeuchi et al. 2010) is likely to lead to a shift in littoral zone structure in which higher sediment loads lead to greater accumulation of algal biomass in rocky habitats.
References
Ahlgren G, Vrede T, Goedkoop W (2009) Fatty acid ratios in freshwater fish, zooplankton and zoobenthos—are there specific optima? In: Arts MT, Brett MT, Kainz M (eds) Lipids in aquatic ecosystems. Springer Verlag, New York, pp 147–178
Alin SR, Cohen AS, Bills R, Gashagaza MM, Michel E, Tiercelin J-J, Martens K, Coveliers P, Mboko SK, West K, Soreghan M, Kimbadi S, Ntakimazi G (1999) Effects of landscape disturbance on animal communities in Lake Tanganyika, East Africa. Conserv Biol 13:1017–1033
Anderson TM, Hopcraft JGC, Eby S, Ritchie M, Grace JB, Olff H (2010) Landscape-scale analyses suggest both nutrient and antipredator advantages to Serengeti herbivore hotspots. Ecology 91:1519–1529
Andre ER, Hecky RE, Duthie HC (2003) Nitrogen and phosphorus regeneration by cichlids in the littoral zone of Lake Malawi, Africa. J Great Lakes Res 29:190–201
Bates D, Machler M, Bolker BM, Walker SC (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Bracken MES, Menge BA, Foley MM, Sorte CJB, Lubchenco J, Schiel DR (2012) Mussel selectivity for high-quality food drives carbon inputs into open-coast intertidal ecosystems. Mar Ecol Prog Ser 459:53–62. https://doi.org/10.3354/meps09764
Burkepile DE, Hay ME (2006) Herbivore vs. nutrient control of marine primary producers: context-dependent effects. Ecology 87:3129–3139
Cebrian J (1999) Patterns in the fate of production in plant communities. Am Nat 154:449–468
Cebrian J, Shurin JB, Borer ET, Cardinale BJ, Ngai JT, Smith MD (2009) Producer nutritional quality controls ecosystem trophic structure. PLoS One 4:e4929
Cohen AS, Bills R, Cocquyt CZ, Caljon AG (1993) The impact of sediment pollution on biodiversity in Lake Tanganyika. Conserv Biol 7:667–677
Cohen AS, Gerguricha EL, Kraemer BM, McGluec MM, McIntyre PB, Russell JM, Simmonsa JD, Swarzenskie PW (2016) Climate warming reduces fish production and benthic habitat in Lake Tanganyika, one of the most biodiverse freshwater ecosystems. Proc Natl Acad Sci USA 113:9563–9568
Corman JR, McIntyre PB, Kuboja B, Mbemba W, Fink D, Wheeler CW, Flecker AS (2010) Upwelling couples chemical and biological dynamics across the littoral and pelagic zones of Lake Tanganyika, East Africa. Limnol Oceanogr 55:214–224
Crain CM, Kroeker K, Halpern BS (2008) Interactive and cumulative effects of multiple human stressors in marine systems. Ecol Lett 11:1304–1315
DeForest J, Vis M, Drerup SA (2016) Using fatty acids to fingerprint biofilm communities: a means to quickly and accurately assess stream quality. Environ Monit Assess 188:277. https://doi.org/10.1007/s.10661-016-5290-7
Devlin SP, Vander Zanden MJ, Vadeboncoeur Y (2016) Littoral-benthic primary production estimates: sensitivity to simplifications with respect to periphyton productivity and basin morphometry. Limnol Oceanogr Methods 14:138–149
Donohue I, Verheyen E, Irvine K (2003) In situ experiments on the effects of increased sediment loads on littoral rocky shore communities in Lake Tanganyika, East Africa. Freshw Biol 48:1603–1616. https://doi.org/10.1046/j.1365-2427.2003.01112.x
Elser JJ, Fagan WF, Denno RF, Dobberfuhl DR, Folarin A, Huberty A, Sterner RW (2000) Nutritional constraints in terrestrial and freshwater food webs. Nature 408:578–580
Evans-White MA, Lamberti GA (2006) Stoichiometry of consumer driven nutrient recycling across nutrient regimes in streams. Ecol Lett 9:1186–1197
Flecker AS, Taylor BW (2004) Tropical fishes as biological bulldozers: density effects on resource heterogeneity and species diversity. Ecology 85:2267–2278
Froese R (2006) Cube law, condition factor and weight–length relationships: history, meta-analysis and recommendations. J Appl Ichthyol 22:241–253
Gratwicke B, Speight MR (2005) The relationship between fish species richness, abundance and habitat complexity in a range of shallow tropical marine habitats. J Fish Biol 66:650–667. https://doi.org/10.1111/j.1095-8649.2005.00629.x
Guo F, Kainz MJ, Valdez D, Sheldon F, Bunn SE (2016) The effect of light and nutrients on algal food quality and their consequent effect on grazer growth in subtropical streams. Freshw Sci 35:1202–1212
Higgins SN, Hecky RE, Taylor WD (2001) Epilithic nitrogen fixation in the rocky littoral zones of Lake Malawi, Africa. Limnol Oceanogr 46:976–982
Hill WR, Smith JG, Stewart AJ (2010) Light, nutrients and herbivore growth in oligotrophic streams. Ecology 91:518–527
Hillebrand H, Sommer U (1999) The nutrient stoichiometry of benthic microalgal growth: redfield proportions are optimal. Limnol Oceanogr 44:440–446
Izagirre O, Serra A, Guasch H, Elosegi A (2009) Effects of sediment deposition on periphytic biomass, photosynthetic activity and algal community structure. Sci Total Environ 407:5694–5700
Karino K (1998) Depth-related differences in territory size and defense in the herbivorous cichlid, Neolamprologus moorii, in Lake Tanganyika. Ichthyol Res 45:89–94
Kelly BM, Mtiti M, McIntyre PB, Vadeboncoeur Y (2017) Stable isotopes reveal nitrogen loading to Lake Tanganyika from remote shoreline villages. Environ Manag 59:264–273
Konings A (2015) Tanganyika Cichlids in their Natural Habitat, 3rd edn. Cichlid Press, El Paso
Liess A, Hillebrand H (2004) Invited review: direct and indirect effects in herbivore-periphyton interactions. Arch Hydrobiol 159:433–453
Loeb SL (1981) An in situ method for measuring the primary productivity and standing crop of the epilithic periphyton community in lentic systems. Limnol Oceanogr 26:394–399
McCormick MI (1994) Comparison of field methods for measuring surface topography and their associations with a tropical reef fish assemblage. Mar Ecol Prog Ser 112:87–96
McIntyre PB, Michel E, France K, Rivers A, Hakizimana P, Cohen AS (2005) Individual- and assemblage-level effects of anthropogenic sedimentation on snails in Lake Tanganyika. Conserv Biol 19:171–181
McIntyre PB, Michel E, Olsgard M (2006) Top-down and bottom-up controls on periphyton biomass and productivity in Lake Tanganyika. Limnol Oceanogr 51:1514–1523
McIntyre PB, Jones LE, Flecker AS, Vanni MJ (2007) Fish extinctions alter nutrient recycling in tropical freshwaters. Proc Natl Acad Sci USA 104:4461–4466
McIntyre PB, Flecker AS, Vanni MJ, Hood JM, Taylor BW, Thomas SA (2008) Fish distributions and nutrient cycling in streams: can fish create biogeochemical hotspots? Ecology 89:2335–2346
Menge BA, Menge DNL (2013) Dynamics of coastal meta-ecosystems: the intermittent upwelling hypothesis and a test in rocky intertidal regions. Ecol Monogr 83:283–310
Munubi RN (2015) Algal Quality Controls the Distribution, Behavior and Growth of Algivorous Cichlids in Lake Tanganyika. Ph.D. Dissertation, Wright State University, Dayton, Ohio, USA
Pagès JF, Gera A, Romero J, Alcoverro T (2014) Matrix composition and patch edges influence plant herbivore interactions in marine landscapes. Funct Ecol 28:1440–1448
Perkins RG, Kromkamp JC, Serȏdio J, Lavaud J, Jesus B, Mouget JL, Lefebvre S, Forster RM (2011) The application of variable chlorophyll fluorescence to microphytobenthic biofilms. In: Suggett DJ (ed) Chorophyll a fluorescence in aquatic sciences: methods and applications, developments in applied phycology, vol 4. Springer, Dordrecht. https://doi.org/10.1007/978-90-481-9268-7_12
Power ME (1984a) Habitat quality and the distribution of algae-grazing catfish in a Panamanian stream. J Anim Ecol 53:357–374
Power ME (1984b) The importance of sediment in the grazing ecology and size class interactions of an armored catfish, Ancistrus spinosus. Environ Biol Fish 10:173–181
Power ME (1984c) Depth-distribution of armored catfish: predator-induced resource avoidance? Ecology 65:523–528
Power ME, Stewart AJ, Matthews WJ (1988) Grazer control of algae in an Ozark mountain stream: effects of short-term exclusion. Ecology 69:1894–1898
Rosemond AD, Mulholland PJ, Elwood JW (1993) Top-down and bottom-up control of stream periphyton: effects of nutrients and herbivores. Ecology 74:1264–1280
Schlosser IJ (1987) The role of predation in age- and size-related habitat use by stream fishes. Ecology 68:651–659. https://doi.org/10.2307/1938470
Sefc KM, Baric S, Salzburger W, Sturmbauer C (2007) Species-specific population structure in rock-specialized sympatric cichlid species in Lake Tanganyika, East Africa. J Mol Evol 64:33–49
Stainton MJ, Capel MJ, Armstrong FAJ (1977) The chemical analysis of fresh water. Environ Can Freshw Inst Misc Spec Publ 25:67–69
Steinman AD (1996) Effects of grazers on freshwater benthic algae. In: Stevenson RJ, Bothwell ML, Lowe RL (eds) Algal ecology: freshwater benthic ecosystems. Aquatic ecology series. Academic Press, Boston, pp 341–373
Sterner RW, Elser JJ (2002) Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princeton University Press, Princeton
Sterner RW, Elser JJ, Fee EJ, Guildford SJ, Chrzanowski TH (1997) The light: nutrient ratio in lakes: the balance of energy and materials affects ecosystem structure and process. Am Nat 150:663–684
Strandberg U, Taipale SJ, Hiltunen MA, Galloway WE, Brett MT, Kankaala P (2015) Inferring phytoplankton community composition with a fatty acid mixing model. Ecosphere 6:16. https://doi.org/10.1890/ES14-00382.1
Takeuchi Y, Ochi H, Kohda M, Sinyinza D, Hori M (2010) A 20-year census of a rocky littoral fish community in Lake Tanganyika. Ecol Freshw Fish 19:239–248
R Core Team (2016) R: A language and environment for statistical computing. R foundation for Statistical Computing. Vienna, Austria. https://www.R-project.org. Accessed Mar 2016
Vadeboncoeur Y, Power ME (2017) Attached algae: the cryptic base of inverted trophic pyramids in fresh waters. Annu Rev Ecol Evol Syst 48:255–279
Vadeboncoeur Y, McIntyre PB, Vander Zanden MJ (2011) Borders of biodiversity: life at the edge of the world’s Great Lakes. Bioscience 61:526–537
Vadeboncoeur Y, Devlin SP, McIntyre PB, Vander Zanden MJ (2014) Is there light after depth? Distribution of periphyton chlorophyll and productivity in lake littoral zones. Freshw Sci 33:524–536
Vanni MJ, McIntyre PB (2016) Predicting nutrient excretion rates of aquatic animals using metabolic ecology and ecological stoichiometry: a global synthesis. Ecology 97:3460–3471
Wagenhoff A, Lange K, Townsend CR, Matthaei CD (2013) Patterns of benthic algae and cyanobacteria along twin-stressor gradients of nutrients and fine sediment: a stream mesocosm experiment. Freshw Biol 58:1849–1863
Wagner CE, McCune AR (2009) Contrasting patterns of spatial genetic structure in sympatric rock-dwelling cichlid fishes. Evolution 63:1312–1326
Wagner CE, McIntyre PB, Buels KS, Gilbert DM, Michel E (2009) Diet predicts intestine length in Lake Tanganyika’s cichlid fishes. Funct Ecol 23:1122–1131
Acknowledgements
We thank Dr. Rashid Tamatamah, Dr. Ismael Kimirei, and the Tanzanian Fisheries Research Institute for facilitating this research. We gratefully acknowledge the field help of George Kazumbe, Len Kenyon, Ryan Satchell, Erica Hile, Sam Drerup, Ellen Hamann and Leslie Kim. Funds were provided by the US National Science Foundation (DEB 0842253 to YV and DEB 1030242 to PBM) and Wright State University’s Environmental Sciences Ph.D. Program.
Author information
Authors and Affiliations
Contributions
RM conceived of and designed the study, collected and analyzed data, and wrote the manuscript. YV advised on study design, collected and analyzed productivity data and wrote the manuscript. PBM advised on study design, collected community fish data, and provided editorial contributions to the manuscript
Corresponding author
Ethics declarations
Statement of human and animal rights
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Additional information
Communicated by Joel Trexler.
Rights and permissions
About this article
Cite this article
Munubi, R.N., McIntyre, P.B. & Vadeboncoeur, Y. Do grazers respond to or control food quality? Cross-scale analysis of algivorous fish in littoral Lake Tanganyika. Oecologia 188, 889–900 (2018). https://doi.org/10.1007/s00442-018-4240-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-018-4240-1