Abstract
Ecological traits that reflect movement potential are often used as proxies for measured dispersal distances. Whether such traits reflect actual dispersal is often untested. Such tests are important because maximum dispersal distances may not be achieved and many dispersal events may be unsuccessful (without reproduction). For insects, many habitat patches harbour ‘resident’ species that are present as larvae (sedentary) and adults (winged and dispersing), and ‘itinerant’ species present only as adults that have dispersed from elsewhere and fail to reproduce. We tested whether itinerancy patterns were temporally consistent, and whether itinerant and resident species differed in wing morphology, a strong correlate of flight capability. Over 3 years and at multiple locations in a 22 km stream length, we sampled larvae and adults of caddisflies in the genus Ecnomus to categorize species as residents or itinerants. Flight capacity was measured using wing size (length and area) and shape parameters (aspect ratio and the second moment of wing area). Three species of Ecnomus were residents and three species were itinerants, and patterns were consistent over 3 years. On average, itinerant species had larger wings, suggesting a greater capacity to fly long distances. Wing shape differed between species, but did not differ systematically between residents and itinerants. Wing morphology was associated with actual but not effective dispersal of some species of Ecnomus. Morphological traits may have weak explanatory power for hypotheses regarding the demographic connectedness of populations, unless accompanied by data demonstrating which dispersers contribute new individuals to populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The spatial distribution of organisms across the landscape is a function of the distribution, size and relative abundance of suitable habitat patches, coupled with the capability of organisms to disperse and colonize patches. Knowing how far and how often organisms disperse is necessary to answer many ecological questions, for example in the contexts of metapopulations, metacommunities, invasion ecology and biogeography. It is equally important, for many questions, to know whether and when dispersal results in populations that are connected demographically, i.e. when dispersal is accompanied by successful reproduction. Despite the obvious importance of dispersal, there is a paucity of information on dispersal rates, distances and the demographic outcomes of dispersal for most species. This constrains our ability to test many hypotheses directly. In the context of demography, dispersal can be defined broadly as the tendency of an organism to reproduce away from its birth place (Levin et al. 2003), or the movement of an organism from its place of origin to a place where it reproduces or would reproduce if it survived and conditions were suitable for reproduction (e.g. presence of mates, nesting or egg-laying sites). ‘Actual dispersal’ describes movement of individuals irrespective of whether reproduction occurs (e.g. inter-patch movement), whereas ‘effective dispersal’, a subset of actual dispersal, describes successful reproduction of an individual that has dispersed (i.e. recruitment). Distinguishing between the two is important ecologically. In the context of community assembly, for example, the set of actual dispersers defines a regional or geographical species pool, whereas effective dispersers define the local species pool, i.e. the observable community (Zobel 1992; Belyea and Lancaster 1999). The difference between the two defines the set of potential colonists that have been excluded from the local species pool by environmental or biotic constraints.
Species with individuals that disperse to some locations without reproducing we call ‘itinerants’. In contrast, ‘residents’ are species that occur at the same locations that reproduce successfully, and that may comprise both dispersing and non-dispersing individuals. Note that our focus is on the occurrence of dispersers at times and habitat patches where reproduction or recruitment could occur; we omit species, often referred to as itinerants, occurring at non-breeding times or locations for other activities such as migratory birds foraging at over-wintering grounds (e.g. Morrison et al. 2013). Additionally, we distinguish between itinerant and vagrant species in this study: vagrant individuals are typically outside their normal range and occur only rarely and in very low numbers, whereas itinerants are often numerous and occur frequently or regularly at potential breeding sites, but where they do not reproduce. Following these definitions, itinerants are species where some individuals routinely move between habitat patches, but fail to colonize some locations. Thus, in any habitat patch there may be some dispersing individuals that originate from local breeding populations (residents) and some from distant populations (itinerants). Numerous studies have documented species belonging to these categories across a range of organisms and ecosystems, including insects (McCauley 2006), birds (Schoener et al. 2005) and freshwater fish (Humphries et al. 2008).
When considering the potential role of dispersal in population and community dynamics, one approach is to explore the differences between species that make some more likely to colonize new habitat patches than others (e.g. Sakai et al. 2001). For example, an association between the morphology and dispersal potential of wind-dispersed plant seeds is well documented (Vittoz and Engler 2007). In this study, we tested whether dispersing individuals of resident and itinerant species have different morphological traits related to dispersal potential, which we define as the capacity to travel long distances. At any particular location, determining dispersal distances of resident species is difficult because dispersing individuals could arise locally (i.e. very short travel distances) or from distant populations. Itinerants, however, must have travelled from elsewhere, and thus are likely, on an average, to have travelled longer distances than most residents; it follows that itinerant individuals should, on an average, have greater dispersal capabilities than residents which should be reflected in differences in dispersal-related morphology. However, this finding would also show that traits associated with strong dispersal potential may not be associated with demographic outcomes. If correct, this suggests that between-patch dispersal is not necessarily evidence of demographic connectedness. Such an outcome is ecologically important because many studies that compare species based on their dispersal traits assume implicitly that dispersal capability can be used to infer connected populations (review: Lowe and McPeek 2014).
Many insects have larvae that are relatively sedentary and restricted to patches of suitable habitat, and dispersal occurs in the adult stage and involves flight between habitat patches. Thus, itinerant insects can be defined as species that are present as adults but not as larvae (i.e. no evidence of successful reproduction), whereas residents are present as both larvae and adults. Aquatic insects are model study organisms in this context because typically larvae are long-lived and restricted to the aquatic environment, whereas adults are short-lived, terrestrial, winged and the major dispersal stage. Larvae of lentic species (inhabiting standing waters such as ponds and lakes) have little potential to colonize different water bodies [except via zoochory (Bilton et al. 2001)], whereas larvae of lotic species (inhabiting running waters of streams and rivers) could—theoretically—disperse downstream by drifting with the current. Most genetic studies of dispersal in aquatic insect populations have shown, however, that flight is the major dispersal mechanism (e.g. Hughes 2007) and the aquatic stages of many taxa may drift only rarely or travel short distances (e.g. Schreiber 1995; Downes and Lancaster 2010; Lancaster et al. 2011).
Flight distances are difficult to quantify directly in natural environments, especially for insect taxa with small-bodied adults that are largely nocturnal or inhabit dense vegetation. Alternatively, morphologic characters of wings can provide proxy measures of flight capability, because wings are high-lift structures and the magnitude of lift varies with wing morphology. The diversity of wing morphology among insect taxa is matched by functional divergence in wing kinematics (wingbeat motions) and in the underlying aerodynamics of flight (Dudley 2000). The importance and suitability of wing size and shape for comparing flight capability among species has been recognized for decades (e.g. Weis-Fogh 1973; Ellington 1984a) even though many aspects of the aerodynamics of insect flight remain unresolved (Dudley 2000; Floreano et al. 2010; Hedrick et al. 2015). Wing morphology cannot capture all aspects of flight capability and species may differ in other traits (e.g. kinematics, physiology, behaviour) that can influence flight, especially if species are distantly related. Thus, it is prudent to focus on species within a narrow phylogenetic range and thereby minimize the possibility that unmeasured traits might confound interpretations based on wing morphology. Quantifying morphological parameters is more practicable than many other aspects of flight, and wing morphology has been used to test various ecological and evolutionary hypotheses regarding flight capability of diverse insects, including Lepidoptera (Betts and Wooton 1988), Odonata (Serrano-Meneses et al. 2008; Outomuro et al. 2013) and Diptera (Ribak et al. 2009). However, there are few empirical tests using field data that demonstrate an association between wing morphology and actual dispersal distances (but see Sakar 2012). Such field tests are difficult to devise, but are essential to determine the veracity of assumptions underpinning tests that use putative dispersal traits to test ecological hypotheses. For example, when considering the flight or dispersal capabilities of any organism, it is important to distinguish between the ‘dispersal distance’ and the ‘travel distance’. We define dispersal distance as the straight line or vector distance between a dispersing individual’s place of origin to a place where it reproduces or would reproduce if it survived and conditions were suitable for reproduction; travel distance is the total path length an individual travelled during a dispersal event, i.e. including all the twists and turns. These definitions make clear that an organism’s capability to travel long distances may not necessarily be associated with a tendency to disperse long distances.
The aims of this field study were to test whether morphological traits of some aquatic insects that are currently used to infer a capacity to fly long distances, differ between itinerant and resident species, i.e. between species known to have dispersed different average distances. If our results support this hypothesis, then we would have provided a field test confirming the oft-used assumption that dispersal traits (e.g. wing morphology) can be a proxy for travel and dispersal distances. Simultaneously, however, the same outcome would suggest that dispersal traits do not necessarily indicate whether populations are connected demographically, and this raises important questions about whether dispersal traits are suitable to address many ecological questions. In this study, measures of wing morphology comprised two gross parameters, wing area and length, and two shape parameters, wing aspect ratio and the second moment of wing area. These metrics reflect aspects of aerodynamic performance according to well-established models of insect flapping flight (Weis-Fogh 1973; Ellington 1984a, b). If itinerants are better dispersers than resident species (i.e. have the capability to fly longer distances) then, on an average, itinerants were expected to have larger wings and/or wing shapes better suited for long-distance flight. Before comparing wing morphologies, however, we must first identify species that classify as residents and itinerants, and evidence from multiple sites and times is required to demonstrate that itinerancy patterns are persistent (the absence of such evidence would suggest that itinerancy is rare or unimportant). Tests of our hypothesis do not require us to sample itinerants at locations where they are residents because we do not pose questions about the causes or evolutionary origin of any potential differences between species. In the text to follow, it is implicit that ‘resident species’ refers to adults collected at sites where larvae are present, ‘itinerant species’ refers to dispersing individuals found at sites where there is no recruitment.
Methods
Study species, site and sampling protocols
Our study focused on species within a single genus of Trichoptera, Ecnomus McLachalan (Ecnomidae). This genus is diverse and widespread throughout Australia and multiple species often co-occur (Cartwright 1990), thus maximizing the possibility that several closely related species would fit in each category, as required for hypothesis tests. Our preliminary observations suggested that both resident and itinerant species occurred in some locations, as observed for Trichoptera in other systems (e.g. Svensson 1974; Sode and Wiberg-Larsen 1993). Several species of Ecnomus co-occurred in the study stream, suggesting some similarities in habitat and resource requirements. All reliable records of larvae of these species are from running waters, suggesting that these species inhabit only lotic environments (Atlas of Living Australia http://www.ala.org.au/). The adults are small bodied (≈1 cm length), but large enough that flight occurs at high Reynolds numbers, Re ≫ 102 (flight is aerodynamically different at low Re). Ecnomid adults generally fly at night, but not during cold or windy conditions. Wing venation and articulation is almost identical for these species so there are unlikely to be differences in wing movement, deformation and bending. The net-spinning larvae of Ecnomus are omnivorous but prey primarily upon invertebrates that become entangled in the silken threads of the net (Chessman 1986; Lancaster et al. 2009). In the study stream, larvae occur throughout the year, the adult flight period is approximately 6 months (November–April) and oviposition occurs throughout (see also Macqueen and Downes 2015). These observations suggest that these species may be bi- or multivoltine, have weakly synchronized cohorts with long emergence periods, overlapping generations, and perhaps relatively long-lived adults (e.g. up to 2 weeks).
The study was carried out in 22 km length in the headwaters of Hughes Creek, a sandy-bed stream in central Victoria, south-eastern Australia. There were no major tributaries along this length. Sample sites were in the upper reaches (36° 59′S; 145° 21′E) where the stream runs off the granite batholith of the Strathbogie Ranges and before reaching the floodplain of the Goulburn River. There were 12 sample sites (each site a 40 m channel length), at altitudes ranging from 355 to 242 m ASL, and spaced on average 1.6 km apart (range 0.6–3.7 km) along the study length. Sampling multiple locations minimizes the risk that results are unduly influenced by locations that are suitable for adults but not larvae, and vice versa. Above our study length, Hughes Creek becomes narrow and swampy and at its most upstream area becomes a series of spring-fed pools (>6 km from our most upstream sample site). The distance between our most upstream site and the headwaters of the nearest creek (Seven Creeks) is ≈18 km in a straight line and >60 km if dispersing individuals follow stream corridors. The nearest at least semi-permanent creek to our most downstream site on Hughes Creek is ≈16 km away in a direct line (Creightons Creek). Detailed information on channel morphology, physicochemistry, vegetation cover, etc., is available elsewhere (e.g. Lancaster et al. 2009; Downes et al. 2011; Lancaster and Downes 2015; Downes et al. 2017). Longitudinal environmental gradients along the study length included an increase in water temperature accompanying increasing channel width, decreasing water depth and reduced shade from a dwindling riparian zone. The most upstream sites were located in areas with relatively intact riparian vegetation and in a moderately well-treed landscape, and within a few km of other creek headwaters and freshwater springs. With distance downstream, stream populations become increasingly isolated as the valley in which the stream lies becomes incised and the land is increasingly altered for grazing (e.g. loss of tree cover, decreased riparian zone). Nevertheless, these environmental gradients limit the distribution of only a few species in Hughes Creek (Lancaster and Downes 2017; Downes et al. 2017).
All sites were sampled in summer (January or February) and during the breeding season in three consecutive years (2013, 2014, 2015) to determine the relative abundance of larval and adult Ecnomus, and to categorize species as residents or itinerants. We have sampled benthic and adult insects from this stream over multiple years and in multiple seasons (references above and unpublished data), and have observed no seasonal turnover in the presence/absence of species as larvae and no species-specific variations in flight period. Thus, we are confident that sampling larvae and adults only in summer (middle of the flight period) is adequate to describe the assemblage of Ecnomus spp. in this stream. In this study, larvae and adults were collected contemporaneously and within 1 week in the first 2 years; in 2015 larvae were sampled 3 weeks later than adults, but this time lag is unlikely to influence hypothesis tests. On each occasion, larvae were collected with a Surber sampler (0.09 m2, 250 µm mesh): 10 samples per site in 2013, 15 samples per site in 2014 and 2015. Samples were located within each of the 12 sites according to a random stratified design, with roughly one quarter of the samples located within each 10 m segment of the 40 m site. Samples were composited and then subsampled to provide a single estimate of larval densities per site. Composited samples of invertebrates were split into 100 aliquots using a sample splitter (Marchant 1988), and 20 aliquots were selected at random for enumeration. Invertebrates were sorted under a stereomicroscope and all third to fifth instar larval Ecnomus were identified to species (early instars cannot be identified to species with confidence) (Cartwright 1997). Adult caddisflies active locally at each site were sampled using light traps placed at the water’s edge and within the flight boundary layer, where flight is intentionally directed and wind-assisted dispersal is rare (Dudley 2000). All individuals were sexed and identified to species (Neboiss 1986; Cartwright 1990). All 12 sites were sampled in 2013 and 2014; only eight sites were sampled in 2015 (see “Results”). Light traps comprised a white, plastic tray (28 × 22 × 5 cm) with 70% ethanol to a depth of approximately 1.5 cm. A fluorescent, ultraviolet blacklight (6 Watt, 12 V, 225-mm long tube) was laid across the top of the tray, which was placed inside a black plastic tub (diameter = 39 cm; height = 32 cm). This ensured that light did not spill sideways but was directed upwards to attract only insects flying nearby (Collier and Smith 1998). Because the efficacy of light traps is sensitive to insect responses to daily weather variations, the number of traps deployed and number of trapping nights required to collect adequate numbers of insects varied between sites. In 2013, three traps were deployed at each site for 2 h, beginning 30 min before sunset. In 2014 and 2015, trapping intensity was increased as required by the weather (more traps or more nights per site) to ensure large sample sizes. Because comparisons of abundance data across years and sample sites focused on species relative abundances, differences in the number of specimens collected are unimportant.
Morphological measurements
Analyses of comparative wing morphology focused on two gross parameters or first-order descriptions of morphology, wing area and wing length (or wing span), and on two shape parameters or second order descriptions, wing aspect ratio (AR) and the non-dimensional radius of the second moment of wing area, \(\hat{r}_{2} (S)\). In general, lift forces (and hence flight capability) increase with wing size (span; area). In terms of wing shape, high AR reflects slender wing shapes, which are associated with power economy and extended flight, whereas broad wings have a low AR, which favours slow, agile flight (Betts and Wooton 1988; Dudley 2000). Values of \(\hat{r}_{2} (S)\) are low for wings that have broad bases and narrow tips and values increase as the broadest part of the wing shifts towards the tip. Wings with very broad tips and high \(\hat{r}_{2} (S)\) may confer agility and maneuverability, but also increase the energetic power required for flight (Ellington 1984b). Conversely, wings with lower values of \(\hat{r}_{2} (S)\) (broad bases, or leading and trailing edges that are approximately parallel) may be better suited for extended or long-distance flight. These parameters have all been used successfully to compare flight capability among various insect species (references above). We did not measure wing loading because this parameter is more closely related to flight speed not flight distance (Dudley 2000). Furthermore, interpreting wing loading in terms of species’ relative flight capacity can be difficult without information on the relative contribution of different tissues to total body mass (e.g. flight muscle, fat body, cuticle).
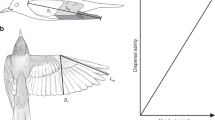
One pair of fore and hind wings were removed from each insect, mounted on a microscope slide and a digital image produced. Wings were oriented so that wing span or maximum wing length was horizontal and perpendicular to the longitudinal axis of the insect body (Fig. 1) and the hind wing was oriented in the coupled position (Stocks 2010). Wing measurements were carried out on digital images of coupled wing pairs in planform (the orientation of wings during the down stroke and the generation of lift forces) and using the software ImageJ 1.49 s (Rasband 1997–2012). There were five replicates for each species/sex combination, except measurements of wing length where N = 12. Replicate specimens were selected from a wide range of year/site combinations to avoid inadvertently selecting closely related individuals. Wing length, R, is the distance from the wing base to the tip of the fore wing. Wing area, S, was measured directly in ImageJ and multiplied by 2 to account for both pairs of wings.
Outline of a coupled wing pair showing variables measured and used to measure wing shape. R is wing span (forewing length), r i is the wing radius or distance from the wing base to the chord c i , which is perpendicular to R and measured as the distance from the leading to the trailing edge of the coupled wings
Wing aspect ratio (AR) is a non-dimensional representation of wing shape describing the wing length relative to its width, and is calculated as:
The moments of wing area indicate how the area is distributed along the wing length, or the shape of the wing in planform. The second moment of wing area and its non-dimensional radius was calculated following Ellington (1984a). The kth moment of wing area, S k , requires measurements of the wing chord, c, at various distances or radii, r, along the wing span, R, (Fig. 1) and is described by the equation:
For a given wing span and area, the moments of area depend only on the distribution of chord lengths along the wing. For each coupled wing pair, 50 measures of r and c, spaced evenly along R, were used to calculate moments of wing area, using the equation above. The non-dimensional radii of the moments of wing area provide parameters of shape that can be compared between taxa and are calculated as:
According to Weis-Fogh (1973), in a quasi-steady model of flight the second moment of wing area, \(\hat{r}_{2} (S)\), is proportional to the mean lift force of the wings, and the third moment, \(\hat{r}_{2} (S)\), is proportional to the mean profile power. Because the first three moments of wing area (k = 1, 2, 3) are strongly correlated (Ellington 1984a), it is sufficient to focus on one moment of area for the purpose of species comparisons, and we focus on \(\hat{r}_{2} (S)\).
Statistical analyses
Differences between species and sexes in wing size and shape were tested using two-way ANOVA (species and sexes as orthogonal fixed factors). These tests were followed by a priori, pair-wise comparisons (Sokal and Rohlf 1981) testing for differences between groups of species that were categorized as residents and itinerants. All species were sexually dimorphic (see “Results”) so pair-wise comparisons were conducted separately for each sex and within the interaction (i.e. Species × Sex) term. These comparisons thus used the mean square error to create the tests—this is appropriate because species is a fixed (not random) factor. Although samples were collected over multiple sites and years, site and year are not factors of interest to our hypothesis tests and were not included in the analyses.
For each species and year, sex ratios were calculated using specimens pooled over all sites, and differences between species were tested using one-way ANOVA with years as replicates. This test was followed by a priori, pair-wise comparisons testing for differences between groups of species that were categorized as residents and itinerants. Data were arcsine square-root transformed before analysis, as is appropriate for data that are proportions (Sokal and Rohlf 1981).
Results
Seven species of Ecnomus were collected in the 22 km length of Hughes Creek; three residents, three itinerants and one vagrant species. Relative abundances varied along the longitudinal stream gradient and patterns were broadly the same in each year (Table 1). The three resident species, E. continentalis Ulmer, E. pansus Neboiss and E. cygnitus Neboiss, were present as both larvae and adults. Ecnomus continentalis was numerically dominant at most sites; E. cygnitus was more abundant at upstream sites, whereas E. pansus was more abundant downstream. These patterns are consistent with previous research on Hughes Creek and another nearby river (Seven Creeks), which also showed an association between larval and adult abundances for E. continentalis and E. pansus (no information on E. cygnitis: Downes et al. 2017). The three itinerants, E. russellius Neboiss, E. tillyardi Mosely and E. turgidus Neboiss, were present as adults, but never as larvae. The only exception was E. russellius where one larva was found in each of 2013 and 2014, and three larvae in 2015. In contrast, adults of this species were collected every year, in multiple locations and often in large numbers. Thus, E. russellius may very occasionally colonize upstream sites, but recruitment appears to be exceedingly rare. Itinerants were most abundant at upstream locations where they could comprise over 50% of the adult assemblage. However, itinerants were present at all sites and occasionally were plentiful at sites that were most distant from headwaters. Ecnomus tillyardi was the most abundant itinerant species. Only one adult specimen of E. myallensis Cartwright was collected over the 3 years (Site 12, 2014) and it appears to be a true vagrant.
Sex ratios differed between species with either equal numbers of males and females, or more females than males caught over the 3 years (Fig. 2). Differences between species were statistically significant (F 5,12 = 4.84, P = 0.012), but pair-wise contrasts revealed no difference in sex ratios between resident and itinerant species (F 1,12 = 1.96, P = 0.187).
All species were sexually dimorphic with respect to all measures of wing size and shape (Table 2; Figs. 3a, 4). Relative to males, females were generally larger (longer wings and larger wing area) and had slender wings with low lift force and energy-efficient flight (high AR, low \(\hat{r}_{2} (S)\)). There were significant differences between species in all measures of wing size and shape (Table 2; Figs. 3a, 4), but wing shapes of species within the genus Ecnomus were very similar to one another compared with caddisflies from other families (Fig. 3). Within each species/sex combination, coefficients of variation in shape, \(\hat{r}_{2} (S)\), were very low and typically <1%. Shape and size parameters were correlated across species/sex combinations suggesting that shape did not change independently of size (Table 3). The directions of these correlations indicate that changes in shape that facilitate long-distance flight were accompanied by an increase in wing size, which also increases flight capability. Comparing species groups, resident and itinerant species did not differ in wing shape (no difference in AR or \(\hat{r}_{2} (S)\) between groups), but did differ significantly in wing size (Table 2): itinerants had longer wings and larger wing areas, suggesting a capacity to fly longer distances than residents.
Outlines of coupled wings of various species of Trichoptera. a Overlain outlines of males (grey) and females (black) of two species of Ecnomus, drawn to scale, to illustrate differences in shape and size. Ecnomus russellius (solid colours) was the largest species and has potentially the strongest flight performance within this genus (female AR = 6.16, \(\hat{r}_{2} (S)\) = 0.533); E. cygnitus (striped colours) was the smallest species and has potentially the weakest flight performance (male AR = 5.64, \(\hat{r}_{2} (S)\) = 0.540). To contrast wing shapes of Ecnomus with other Trichoptera, outlines of coupled wings of males from two different families (not drawn to scale): b Triplectides ciuskus ciuskus (Leptoceridae) (AR = 5.74, \(\hat{r}_{2} (S)\) = 0.489) and c Asmicridea edwardsi (Hydropsychidae) (AR = 3.49, \(\hat{r}_{2} (S)\) = 0.509)
Mean (±SE) a wing aspect ratio, b the second moment of wing area, \(\hat{r}_{2} (S)\), c wing length and d area for coupled wing pairs of adult caddisflies of each species grouped according to sex and whether species were classified as residents or itinerants (see text for explanation). a, b and d were calculated for two coupled wing pairs with N = 5 for each species/sex combination; c measured for a single coupled wing pair with N = 12 for each species/sex combination. See Table 1 for summary of statistical analyses
Discussion
Itinerant species were more likely than residents to have morphological traits associated with a capacity for long-distance flight in congeneric species of caddisfly (genus Ecnomus). This is consistent with the notion that inter-patch movement may be common for itinerants, even though they fail to colonize some locations. Dispersal traits that describe flight capability appeared to be associated with actual dispersal distances for these species because itinerants would have had to travel and to disperse longer distances—on average—than residents. Many itinerants were found at our upstream sites even though the closest stream across the catchment boundary was 18 km away in a straight line or >60 km if adults fly along stream corridors. In contrast, adults of resident species that completed their larval life in Hughes Creek could access many suitable oviposition sites in the same stream (Macqueen and Downes 2015) with much shorter flight distances. Our results thus show that commonly used measures of dispersal potential were associated with individuals that, on average, had to have travelled longer distances. These differences were clear-cut (statistical tests all with P values <0.001) even though the necessity to use closely related species (see “Introduction”) resulted in fairly small sample sizes. This is an encouraging outcome because it demonstrates that wing morphology can be linked to dispersal capacity for some insects, including aquatic insects (see also Kovats et al. 1996). Such evidence is valuable because measuring actual flight distances in nature is difficult for most insect groups (although more tractable for some, such as the Lepidoptera, Stevens et al. 2010).
Dispersal events by itinerant species have no demographic outcomes in Hughes Creek and hence the morphological traits were not associated with effective dispersal in this system. Theoretically, some of these individuals may continue dispersing to other locations and reproduce successfully. This is the first study, to our knowledge, to demonstrate a link between itinerancy and dispersal potential. The implication is that it may be inappropriate to use dispersal traits to make inferences about whether insect populations are connected demographically, a matter that has concerned some researchers (Lowe and McPeek 2014), but data to illustrate the problem are scarce. Why do itinerants exist if individuals may be demographic dead ends? Itinerant individuals may have zero fitness, but in a life history context, some long-distance dispersers may be successful, allowing populations to exploit new habitats and maintain connectivity within metapopulations. For itinerants, many dispersal events may be unsuccessful or some individuals may visit multiple habitat patches before oviposition occurs (Svensson 1998; Conrad et al. 1999). Among aquatic insects at least, itinerancy may be associated with the rapid colonization of new or restored aquatic habitats (Miller et al. 2010).
Itinerancy may be more widespread and common than ecologist has appreciated hitherto and it appears to be common among aquatic insects (e.g. Waringer 1991; McCauley 2006). Within the Trichoptera, itinerancy is not unique to the family Ecnomidae [of the 68 species in 15 families of Trichoptera identified in Hughes Creek in 2013–2014, at least seven species across four families were itinerants (unpublished data)], and itinerancy has been observed in other taxonomically diverse caddisfly assemblages (Svensson 1974; Sode and Wiberg-Larsen 1993). Itinerancy patterns can be persistent: for species of Ecnomus we observed the same pattern in Hughes Creek over three consecutive years (the same species classifying as residents or itinerants) and the same pattern occurred 5 years earlier in Hughes Creek and a nearby stream (Downes et al. 2017). It is unclear why these itinerant species fail to recruit in this system and this requires a separate investigation, but we are confident that our samples would have collected their larvae had they been present.
Flight capability is a function of both wing size and shape so whether itinerants are capable of flying longer distances than residents depends on the relative contributions of these factors to flight. Size and shape variables were correlated in for these species of Ecnomus and the correlation directions indicated that increased wing size was generally accompanied by shape changes that also facilitate long distance flight capability. The magnitudes of interspecific differences were greater for wing size than shape. For example, comparing wing lengths of the two species with the longest and shortest wings revealed a 1.3× difference for males, and 1.15× for females. In contrast, differences in \(\hat{r}_{2} (S)\) were much smaller at 1.015× for males and 1.007× for females. As shown by Weis-Fogh (1973) and Ellington (1984b), the lift forces of wings increase in proportion to R 3 (the cubic power of wing length), but increase only linearly with shape parameters. Thus, within the genus Ecnomus, small changes in wing length may result in substantial changes in flight capability, relative to changes in shape parameters of similar magnitude. This may not be true for taxonomically more diverse groups of caddisflies where wing size and shape may not be correlated, wing shapes may be more diverse, and where other taxon-specific factors may influence flight capability (Ivanov 1986, 1989, 1990).
Sexual dimorphism and sex-biased dispersal is common among insects, but the nature of such sex-biases did not differ between itinerant and resident species in this study. Among aquatic insects, empirical evidence suggests that females disperse farther than males in some Ephemeroptera (Caudill 2003; Hughes 2007) and some Odonata (Beirinckx et al. 2006), whereas some male Plecoptera disperse farther than females (Kuusela and Huusko 1996). Based on wing morphology, our results suggest that female Ecnomus may have the potential to travel longer distances than males. Additionally, although females significantly outnumbered the males trapped for some species, sex ratios in the samples did not differ between resident and itinerant species, as expected if actual dispersal distances were greater for females. Female-biased samples of caddisflies at light traps occurs in other species and the possible explanations include sex-specific attraction to UV lights, reproductive behaviours, habitat use, or simply that females may live longer than males (Svensson 1974; Kovats et al. 1996; Petersen et al. 1999).
Evidence that a capacity to travel long distances is associated with actual dispersal distances is an important step forward in assessing the utility of morphological parameters as dispersal traits. However, other species-specific traits or behaviours may simultaneously influence dispersal distances in diverse ways and may be influenced by diverse selection gradients (Duputié and Massol 2013). Disentangling how various traits interact and the demographic consequences for certain trait combinations requires further research. For example, for caddisflies (and many other taxa), we do not know whether traits reflecting flight potential and flight direction are correlated. Many insects, including some caddisflies, can travel long distances during mating and swarming, but remain within a relatively small area (Gullefors and Petersson 1993), suggesting that travel and dispersal distances may not be correlated for some species. Thus, morphological traits may suggest strong dispersal potential for some species that actually have low rates of inter-patch movement and various selection gradients can lead to such behaviourally constrained dispersal (Murrell et al. 2002). Similarly, many insects travel primarily along stream corridors, whereas other are more likely to fly laterally away from river channels (Svensson 1974), provided that stream valleys are not deeply incised (Hughes et al. 1999). It is plausible that itinerants are more likely to disperse laterally away from streams and have high inter-patch movement rates, whereas species that strongly favour dispersal along river corridors are more likely to be classified as residents and rarely move between catchments or discretely different habitat patches. Among the resident species, E. continentalis had the strongest dispersal potential and was abundant throughout the 22 km length of the study stream. In contrast, the two residents with weaker flight capability, E. cygnitus and E. pansus, were restricted to shorter stream lengths. A field experiment also suggested that E. cygnitus tends to remain in upstream areas (Lancaster and Downes 2017). We do not know where larvae of itinerant Ecnomus occur in this landscape, and that requires a separate investigation.
Overall, our results suggest that morphological traits may be useful in determining the relative capacity of congeneric species to make inter-patch movements and hence the relative probability that species have the capacity to change spatial distribution or to colonize new or restored habitat patches. On their own, however, these morphological traits may mislead about the degree of demographic connectedness of populations. Stronger inferences may require that morphological parameters are coupled with other dispersal traits (e.g. flight direction) and with information on recruitment or reproductive success. Our data show that itinerants are not necessarily rare and may comprise a consistently high proportion of dispersing individuals. In such cases, dispersal traits coupled with numbers of individuals sampled in different locations are insufficient to deduce the exact role dispersal plays in connecting populations. Many studies of aquatic insect metacommunities are based on analyses of survey data of larvae coupled with putative dispersal traits of adults, and this approach is clearly problematic if traits do not reflect effective dispersal, i.e. populations that are not demographically connected (Verberk et al. 2013). It is necessary to collect complementary data that demonstrate which dispersers are successful at contributing individuals to habitat patches, and which are not. Only then will we be able to disentangle the roles that dispersal plays in metapopulations and metacommunities.
References
Beirinckx K, Van Gossum H, J Lajeunesse M, R Forbes M (2006) Sex biases in dispersal and philopatry: insights from a meta-analysis based on capture–mark–recapture studies of damselflies. Oikos 113:539–547
Belyea LR, Lancaster J (1999) Assembly rules within a contingent ecology. Oikos 86:402–417
Betts CR, Wooton RJ (1988) Wing shape and flight behaviour in butterflies (Lepidoptera: Papilionoidea and Hesperioidea): a preliminary analysis. J Exp Biol 138:271–288
Bilton DT, Freeland JR, Okamura B (2001) Dispersal in freshwater invertebrates: mechanisms and consequences. Ann Rev Ecol Syst 32:159–181
Cartwright DI (1990) The Australian species of Ecnomus McLachlan (Trichoptera: Ecnomidae). Mem Mus Vic 51:1–48
Cartwright DI (1997) Preliminary guide to the identification of late instar larvae of Australian Ecnomidae, Philopotamidae and Tasmiidae (Insecta: Trichoptera). Cooperative Research Centre for Freshwater Ecology, Albury
Caudill CC (2003) Measuring dispersal in a metapopulation using stable isotope enrichment: high rates of sex-biased dispersal between patches in a mayfly metapopulation. Oikos 101:624–630
Chessman BC (1986) Dietary studies of aquatic insects from two Victorian rivers. Aust J Mar Freshwat Res 37:129–146
Collier KJ, Smith BJ (1998) Dispersal of adult caddisflies (Trichoptera) into forests alongside three New Zealand streams. Hydrobiologia 361:53–65
Conrad KF, Willson KH, Harvey IF, Thomas CJ, Sherratt TN (1999) Dispersal characteristics of seven odonate species in an agricultural landscape. Ecography 22:524–531
Downes BJ, Lancaster J (2010) Does dispersal control population densities in advection-dominated systems? A fresh look at critical assumptions and a direct test. J Anim Ecol 79:235–248
Downes BJ, Lancaster J, Hale R, Glaister A, Bovill W (2011) Plastic and unpredictable responses of stream invertebrates to leaf pack patches across sandy-bottomed streams. Mar Freshw Res 62:394–403
Downes BJ, Lancaster J, Glaister A, Bovill W (2017) A fresh approach reveals how dispersal shapes metacommunity structure in a human-altered landscape. J Appl Ecol 54:588–598
Dudley R (2000) The biomechanics of insect flight: form, function, evolution. Princeton University Press, Princeton
Duputié A, Massol F (2013) An empiricist’s guide to theoretical predictions on the evolution of dispersal. Interface Focus 3:20130028
Ellington CP (1984a) The aerodynamics of hovering insect flight. II. Morphological parameters. Phil Trans Roy Soc Lond B 305:17–40
Ellington CP (1984b) The aerodynamics of hovering insect flight. VI. Lift and power requirements. Phil Trans Roy Soc Lond B 305:145–181
Floreano D, Zufferey J-C, Srinivasan MV, Ellington CP (eds) (2010) Flying insects and robots. Springer, Heidelberg
Gullefors B, Petersson E (1993) Sexual dimorphism in relation to swarming and pair formation patterns in leptocerid caddisflies (Trichoptera, Leptoceridae). J Insect Behav 6:563–577
Hedrick TL, Combes SA, Miller LA (2015) Recent developments in the study of insect flight. Can J Zool 93:925–943
Hughes JM (2007) Constraints on recovery: using molecular methods to study connectivity of aquatic biota in rivers and streams. Freshw Biol 52:616–631
Hughes JM, Mather PB, Sheldon AL, Allendorf FW (1999) Genetic structure of the stonefly, Yoraperla brevis, populations: the extent of gene flow among adjacent montane streams. Freshw Biol 41:63–72
Humphries P, Brown P, Douglas J, Pickworth A, Strongman R, Hall K, Serafini L (2008) Flow-related patterns in abundance and composition of the fish fauna of a degraded Australian lowland river. Freshw Biol 53:789–813
Ivanov VD (1986) Comparative analysis of wing kinematics in caddis flies (Trichoptera). Entomol Rev 65:60–71
Ivanov VD (1989) Action of wing articulations of caddis-flies (Trichoptera) in flight. Entomol Rev 68:119–129
Ivanov VD (1990) Comparative analysis of the aerodynamics of flight of caddisflies (Insecta: Trichoptera). Entomol Rev 69:51–66
Kovats ZE, Ciborowski JJH, Corkum LD (1996) Inland dispersal of adult aquatic insects. Freshw Biol 36:265–276
Kuusela K, Huusko A (1996) Post-emergence migration of stoneflies (Plecoptera) into the nearby forest. Ecol Entomol 21:171–177
Lancaster J, Downes BJ (2015) Population densities and density-area relationships in a community with advective dispersal and variable mosaics of resource patches. Oecologia 176:985–996
Lancaster J, Downes BJ (2017) A landscape-scale field experiment reveals the importance of dispersal in a resource-limited metacommunity. Ecology 98:565–575
Lancaster J, Downes BJ, Glaister A (2009) Interacting environmental gradients, trade-offs and reversals in the abundance–environment relationships of stream insects: when flow is unimportant. Mar Freshw Res 60:259–270
Lancaster J, Downes BJ, Arnold A (2011) Lasting effects of maternal behaviour on the distribution of a dispersive stream insect. J Anim Ecol 80:1061–1069
Levin SA, Muller-Landau HC, Nathan R, Chave J (2003) The ecology and evolution of seed dispersal: a theoretical perspective. Annu Rev Ecol Evol S 34:575–604
Lowe WH, McPeek MA (2014) Is dispersal neutral? Trends Ecol Evolut 29:444–450
Macqueen A, Downes BJ (2015) Large-scale manipulations of oviposition substrata affects egg supply to populations of some stream-dwelling caddisflies. Freshw Biol 60:802–812
Marchant R (1988) A subsampler for samples of benthic invertebrates. Bull Aust Soc Limnol 12:49–52
McCauley SJ (2006) The effects of dispersal and recruitment limitation on community structure of odonates in artificial ponds. Ecography 29:585–595
Miller SW, Budy P, Schmidt JC (2010) Quantifying macroinvertebrate responses to in-stream habitat restoration: applications of meta-analysis to river restoration. Restor Ecol 18:8–19
Morrison CA, Robinson RA, Clark JA, Risley K, Gill JA (2013) Recent population declines in Afro-Palaearctic migratory birds: the influence of breeding and non-breeding seasons. Divers Distrib 19:1051–1058
Murrell DJ, Travis JMJ, Dytham C (2002) The evolution of dispersal distance in spatially-structured populations. Oikos 97:229–236
Neboiss A (1986) Atlas of Trichoptera of the SW Pacific–Australia region. Dr W. Junk, Dordrecht, The Netherlands
Outomuro D, Adams DC, Johansson F (2013) Wing shape allometry and aerodynamics in calopterygid damselflies: a comparative approach. BMC Evol Biol 13:118
Petersen I, Winterbottom JH, Orton S, Friberg N, Hildrew AG, Speirs DC, Gurney WSC (1999) Emergence and lateral dispersal of adult Plecoptera and Trichoptera from Broadstone stream, UK. Freshw Biol 42:401–416
Rasband WS (1997–2012) ImageJ. U.S. National Institutes of Health, Bethesda, Maryland, USA, http://imagej.nih.gov/ij. Accessed Feb 2013
Ribak G, Pitts ML, Wilkinson GS, Swallow JG (2009) Wing shape, wing size, and sexual dimorphism in eye-span in stalk-eyed flies (Diopsidae). Biol J Linn Soc 98:860–871
Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, Baughman S, Cabin RJ, Cohen JE, Ellstrand NC, McCauley DE, O’Neil P, Parker IM, Thompson JN, Weller SG (2001) The population biology of invasive species. Ann Rev Ecol Syst 32:305–332
Sakar S (2012) A meta-analysis of the traits affecting dispersal ability in butterflies: can wingspan be used as a proxy? J Anim Ecol 81:174–184
Schoener TW, Losos JB, Spiller DA (2005) Island biogeography of populations: an introduced species transforms survival patterns. Science 310:1807–1809
Schreiber ESG (1995) Long-term patterns of invertebrate stream drift in an Australian temperate stream. Freshw Biol 33:13–25
Serrano-Meneses MA, Córdoba-Aguilar A, Azpilicueta-Amorín M, González-Soriano E, Székeley T (2008) Sexual selection, sexual size dimorphism and Rensch’s rule in Odonata. J Evol Biol 21:1259–1273
Sode A, Wiberg-Larsen P (1993) Dispersal of adult Trichoptera at a Danish forest brook. Freshw Biol. 30:439–446
Sokal RR, Rohlf FJ (1981) Biometry, 2nd edn. Freeman, New York
Stevens VM, Turlure C, Baguette M (2010) A meta-analysis of dispersal in butterflies. Biol Rev 85:625–642
Stocks IC (2010) Comparative and functional morphology of wing coupling structures in Trichoptera: Annulipalpia. J Morphol 271:152–168
Svensson BW (1974) Population movements of adult Trichoptera in a South Swedish stream. Oikos 25:157–175
Svensson BW (1998) Local dispersal and its life-history consequences in a rock pool population of a gyrinid beetle. Oikos 82:111–122
Verberk WCEP, van Noordwijk CGE, Hildrew AG (2013) Delivering on a promise: integrating species traits to transform descriptive community ecology. Freshw Sci 32:531–547
Vittoz P, Engler R (2007) Seed dispersal distances: a typology based on dispersal modes and plant traits. Bot Helv 117:109–124
Waringer JA (1991) Phenology and the influence of meteorological parameters on the catching success of light-trapping for Trichoptera. Freshw Biol 25:307–319
Weis-Fogh T (1973) Quick estimates of flight fitness in hovering animals, including novel mechanisms for lift production. J Exp Biol 59:169–230
Zobel M (1992) Plant species coexistence—the role of historical, evolutionary and ecological factors. Oikos 65:314–320
Acknowledgements
We thank the many people who helped with field collections at various times, including Claire Allison, Wim Bovill, Alena Glaister, Steve Horn, Ashley Macqueen, Bobbi Peckarsky, Jared Polkinghorne, Bob Smith and Allyson Yarra. We are deeply indebted to Wim Bovill and Alena Glaister for their stellar assistance with identifications. This project was supported by a Discovery Grant from the Australian Research Council (DP120103145) awarded to JL and BJD. Adult sampling in 2015 was carried out in conjunction with an NSF Postdoctoral Extension awarded to R. Smith and hosted by the University of Melbourne.
Author information
Authors and Affiliations
Contributions
JL and BJD collected samples; JL measured wings; JL and BJD analysed the data and wrote the manuscript.
Corresponding author
Additional information
Communicated by Jamie M. Kneitel.
Rights and permissions
About this article
Cite this article
Lancaster, J., Downes, B.J. Dispersal traits may reflect dispersal distances, but dispersers may not connect populations demographically. Oecologia 184, 171–182 (2017). https://doi.org/10.1007/s00442-017-3856-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-017-3856-x