Abstract
The evolution of defensive traits is driven both by benefits gained from protection against enemies and by costs of defence production. We tested the hypothesis that specialisation of herbivores on toxic host plants, accompanied by the ability to acquire plant defensive compounds for herbivore defence, is favoured by the lower costs of sequestration compared to de novo synthesis of defensive compounds. We measured physiological costs of chemical defence as a reduction in larval performance in response to repeated removal of secretions (simulating predator attack) and compared these costs between five species synthesising defences de novo and three species sequestering salicylic glucosides (SGs) from their host plants. Experiments simulating low predator pressure revealed no physiological costs in terms of survival, weight and duration of development in any of study species. However, simulation of high predation caused reduction in relative growth rate in Chrysomela lapponica larvae producing autogenous defences more frequently, than in larvae sequestering SGs. Still meta-analysis of combined data showed no overall difference in costs of autogenous and sequestered defences. However, larvae synthesising their defences de novo demonstrated secretion-conserving behaviour, produced smaller amounts of secretions, replenished them at considerably lower rates and employed other types of defences (regurgitation, evasion) more frequently when compared to sequestering larvae. These latter results provide indirect evidence for biosynthetic constraints for amounts of defensive secretions produced de novo, resulting in low defence effectiveness. Lifting these constraints by sequestration may have driven some leaf beetle lineages toward sequestration of plant allelochemicals as the main defensive strategy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemical defences against natural enemies are extremely widespread among animals (Blum 1981; Ruxton et al. 2004). Components of chemical defences vary widely and are frequently synthesised de novo (Eisner et al. 2007). However, some plant-feeding insects sequester various plant secondary metabolites (allelochemicals) and use them as a defence against natural enemies (Nishida 2002; Opitz and Müller 2009), and this ability is tightly linked with the evolution of host-plant specialisation in herbivores (Price et al. 1980; Bernays and Graham 1988).
Plant secondary metabolites, which supposedly evolved as anti-herbivore defences, have toxic or deterrent properties which usually lead to their avoidance by generalist herbivores (Bernays and Chapman 1987; Nishida 2002). Therefore, specialisation on toxic plants allows insects to escape from interspecific competition (Futuyma and Moreno 1988; Loxdale et al. 2011). On the other hand, specialisation on toxic plants has been suggested to provide herbivores, which use plant allelochemicals for defence against predators, with ‘enemy-free space’ (Jeffries and Lawton 1984; Stamp 2001); thus, generalist enemies may contribute to the narrowing of the host plant range of herbivores (Price et al. 1980; Bernays and Graham 1988).
The evolution of defensive traits is commonly considered in terms of trade-offs between the benefits gained from protection against enemies and the costs of defence production (Bowers 1992; Camara 1997). Thus, sequestration would be advantageous if more effective and/or less expensive defence was obtained with plant-derived chemicals than with compounds synthesised de novo. The effectiveness of defences of different origin and of various chemical compositions has been directly compared only rarely, but some studies have demonstrated that leaf beetle larvae using sequestered defence compounds obtained better protection from predators compared to larvae using autogenously produced compounds (Rowell-Rahier et al. 1995; Zvereva et al. 2010a). However, a recent meta-analysis (Zvereva and Kozlov 2016) found no differences in the effectiveness of defences of different origin across a variety of prey-predator systems, thereby suggesting the potential importance of the costs of defence production. These costs can determine the evolutionary path taken by any particular insect species (Camara 1997); therefore, quantitative evaluation and comparison of the costs between the two defensive strategies of leaf beetles—sequestration of plant toxins and autogenous synthesis of anti-predator defences—could provide a key to understanding the evolution of host plant specialisation in herbivorous insects (Bowers 1992; Nishida 2002).
Costs associated with possessing chemical defence, or constitutive costs, are paid by all individuals and therefore they are hard to measure (Cogni et al. 2012). Among costs associated with defence functioning, physiological costs (also called metabolic or energetic costs in animals) are studied most frequently. These involve allocation of limited resources to the production, maintenance and operation of a defence at the expense of other functions of an organism. The physiological costs may be measured as trade-offs between investments in defence and in some other components of fitness, such as growth, survival or fecundity (Camara 1997; Ruxton et al. 2004). The published studies have provided contradictory evidence about the physiological costs of chemical defence, and meta-analysis has demonstrated an overall absence of these costs in herbivorous insects (Zvereva and Kozlov 2016). However, a great majority of these studies explored the costs of sequestration, whereas the costs of autogenous defences for herbivorous insects were reported only in a single study (Rowell-Rahier and Pasteels 1986), leaving a considerable research gap (Zvereva and Kozlov 2016).
The research bias towards the costs of sequestration is critical, because the costs of de novo synthesis of defensive chemicals and the costs of sequestration have different origins, being based on two different biochemical strategies of defence production (Bowers 1992; Ruxton et al. 2004). The costs of de novo synthesis may arise due to the use of chemical precursors (e.g. amino acids) for the biosynthesis of defensive compounds that could otherwise be used for other organism functions and due to the maintenance of the complex biochemical machinery necessary for de novo synthesis and transportation of compounds (Bowers 1992). On the other hand, the costs of sequestration are associated with transportation, concentration and biotransformation of chemicals acquired from plants (Ruxton et al. 2004). In general, de novo synthesis of defensive compounds requires more complex and multistep chemical reactions than sequestering ready compounds from host plants (Boland 2015); in other words, biosynthetic costs of production of defences de novo should exceed biotransformation costs of sequestration. Therefore, in spite of scarce experimental evidence, de novo synthesis is frequently believed to have a higher cost than that incurred by sequestration of plant allelochemicals (e.g. Fürstenberg-Hägg et al. 2014), and this presumption is sometimes used to explain the wide distribution of sequestration among plant-feeding insects (Bowers 1992; Nishida 2002).
Leaf beetles (Coleoptera: Chrysomelidae) demonstrate a variety of defensive strategies and are therefore suitable subjects for studying the role of chemical anti-predator defences in insect specialisation on toxic host plants. Some leaf beetle species have retained their ancestral autogenous synthesis of defences, but some lineages—for example, the subfamily Chrysomelinae—have evolved the ability to sequester from their host plants salicyl glucosides (SGs) (Termonia et al. 2001), which are toxic for generalist herbivores (Rowell-Rahier and Pasteels 1986). Specialised beetles transform SGs to salicylaldehyde, which serves as a defence against natural enemies (Pasteels et al. 1988; Termonia et al. 2001). Some of these specialised beetles have entirely lost the ability to produce defences de novo, but others, e.g. the Chrysomela interrupta group, utilise a dual defence strategy involving both sequestered and autogenous defensive compounds (Termonia et al. 2001). C. lapponica, the best studied species of this group, demonstrates a geographic variation in host plant use and in the composition of defensive secretions, providing further opportunities to explore the relative costs of sequestration and de novo synthesis and their relationship to diet breadth.
Allocation costs on pre-imaginal stages are frequently measured as changes in larval performance in response to the increased defence production by simulating predator attack (e.g. Rowell-Rahier and Pasteels 1986; Bowers 2003; Higginson et al. 2011). Current models predict that growth rate always decreases in response to predation due to investments in the defences, which reduces energy available for growth (Higginson and Ruxton 2010). However, timing of metamorphosis is plastic, and models of optimal defence generally predict that in response to predation risk prey may reduce age and body size at metamorphosis to avoid predators, but in certain conditions metamorphosis at a larger size and later time is likely to be optimal (Higginson and Ruxton 2010 and references therein). We test these predictions for two different strategies of chemical defence used by leaf beetle larvae: sequestration of plant allelochemicals and synthesis of compounds de novo.
We estimated changes in the performance in response to simulated predation accompanied by defence depletion in eight leaf beetle species that use different defence strategies to test the hypothesis that the evolutionary shift to sequestration of plant allelochemicals observed in several lineages of leaf beetles was favoured by lower cost/benefit ratio of sequestration relative to de novo synthesis of defensive compounds. We also compared species with different strategies of chemical defence by the replenishment rates of secretions as a measure of production intensity, and recorded the larval defensive behaviour, as it was predicted to interact with other types of defences (Steiner and Pfeiffer 2007; Higginson and Ruxton 2009a).
Materials and methods
Study species
We studied eight leaf beetle species (Online resource, Table S1) whose larvae externalise defensive secretions from specialised glands to repel potential enemies. Five of these species synthesise secretions of different compositions de novo, whereas another three species sequester SGs from their host plants and produce salicylaldehyde as the major defensive component of the secretions (Online resource, Table S1). Two of the three sequestering species, C. tremulae and Phratora vitelline, have lost the ability to produce defensive compounds de novo and totally rely on sequestration. The third species, C. lapponica, uses a dual defence strategy. Some populations of C. lapponica are specialised to Salicaceae rich in SGs or to SG-free Betulaceae (Hilker and Schulz 1994; Termonia et al. 2001; Gross et al. 2004), while other populations are non-specialised and use salicaceous host plants that vary in SG content—ranging from species totally lacking SGs to those extremely rich in SGs (Zvereva et al. 2010b). In these latter populations, the origin of their larval defensive secretions (almost exclusively sequestered or almost exclusively autogenous) depends on the host plant used (Geiselhardt et al. 2015). We used C. lapponica from four geographically distinct populations (Online resource, Table S1). Larvae from three populations (Belarus, Baikal and Ural) autogenously produce butyric esters when feeding on host plants with low concentrations of SGs, but start sequestering SGs when they are moved to SG-rich host plants, adding salicylaldehyde to de novo components of their defensive secretions. Larvae of the Monche population, which is specialised to SG-rich hosts, mostly sequester SGs from their host plants, but they also produce small amounts of butyric esters. These esters become the major defensive chemicals when larvae of the Monche population feed on SG-poor hosts (for a detailed description of the secretion composition, consult Geiselhardt et al. 2015).
Experimental design
Physiological costs are generally measured by either of two methods. The first method quantifies the costs of defence production by correlating herbivore performance indices with the concentrations of defensive chemicals in the insect body (Bowers 1992). If defence production is costly, then this correlation should be negative. This method is well suited to estimate the costs of sequestration by rearing insects on diets with varying concentrations of allelochemicals, but it is not suitable to study the costs of autogenously produced defences, because amounts of these defences are hard to manipulate. Moreover, it is methodologically difficult to separate the costs of sequestration from the other effects of plant allelochemicals on a herbivore (Bowers 1992; Higginson et al. 2011).
The second method measures changes in herbivore performance in response to intensified production of secretions caused by their removal. This method simulates predator attacks, which usually cause a release of defensive secretions. The released secretions are largely sampled by the predator, but part of the secretions can be resorbed back into the glands (Bowers 1993); for example, when secretion is not collected artificially, larvae of sequestering C. lapponica require on average 13 disturbances until their gland reservoirs are completely depleted (Zvereva et al. 2016). The method of defence depletion has previously been used to study the costs of defences released as regurgitants (Björkman and Larsson 1991; Bowers 2003; Higginson et al. 2011) or defensive secretions (Rowell-Rahier and Pasteels 1986; Kearsley and Whitham 1992) and is suitable for measuring the costs of externalised defences of both autogenous and sequestered origin.
We used the second method to compare the costs of defences produced by different leaf beetle species and having different chemistry and origin. We repeatedly depleted the defensive glands of the experimental larvae by slightly pressing the tip of their abdomina with soft forceps until secretions were released and then removing the secretions with a small piece of filter paper. The ‘control’ larvae were disturbed in the same way as experimental larvae, but the released secretions were not removed and allowed to be absorbed back into defensive glands. The ‘undisturbed control’ larvae were never disturbed, to account for possible effects of the disturbance itself.
To measure physiological costs, we conducted two kinds of experiment. Long-term experiments involved secretion removal once per day during larval development, thus simulating low but permanent predation pressure; these experiments yielded such fitness characteristics as pre-imaginal survival, duration of development and weight of newly emerged beetles. To estimate long-term fitness of the larvae, we used a composite measure suggested by Higginson and Ruxton (2009a), which includes larval survival, mass at maturation (which is proportional to reproductive potential), and development time as a proxy of survival related with predation risk.
In short-term experiments secretion was removed three times during 24 h, thus simulating short-term but intensive predation pressure. These experiments yielded data on relative growth rate, consumption rate and efficiency of conversion of ingested food.
In addition, we measured the rates of secretion recovery after complete depletion and recorded defensive behaviour of larvae in response to disturbance.
Long-term experiments
Long-term experiments were conducted with all species (except for C. lapponica) in Turku, Finland in June–July of 2015 at room temperature (20 °C) and under natural illumination in a common laboratory environment. The larvae that hatched from each field-collected egg batch (‘family’ hereafter) were reared on leaves of their primary host plants in 50 mL vials for 1–3 days until they reached the second instar.
Each long-term experiment involved five to ten families. The experimental unit consisted of a Petri dish 85 mm in diameter with the bottom covered by wet filter paper. Five sibling larvae were placed in each dish and provided ad libitum with fresh leaves of the desired host plant; leaves were changed every day as long as the larvae continued feeding. Two dishes with larvae from the same family (or a family by host plant combination for C. lapponica) were randomly attributed to depletion and control treatments. When the numbers of larvae allowed, the larvae in the third dish were left undisturbed; this was possible for five of the eight species. We depleted the secretions once per day, always at the same time, beginning on the day after the larvae were transferred to the Petri dishes, and we terminated this treatment when larvae stopped feeding and were preparing to pupate.
We recorded the number of pupated larvae (‘survival’ hereafter) and the duration of development from egg hatching to beetle hatching, and we weighed the newly hatched beetles (to the nearest 0.1 mg). The larvae of Agelastica alni failed to pupate, presumably because they needed soil to dig into; therefore, survival could not be estimated in this species and the weights of the prepupae were recorded instead of beetle weight.
Short-term experiments
We further explored the links between the costs of chemical defences and host plant specialisation by measuring the effects of depletion of defensive secretions on the relative growth rate (RGR) of the last-instar larvae. Nine experiments were conducted in 2003–2015 with larvae from four populations of C. lapponica differing in host plant use and one experiment with Plagiosterna aenea (‘short-term experiments’ hereafter; Online resource, Fig. S1).
The larvae that hatched from each family were divided into two groups and reared in the same way as in the long-term experiment. One group fed on leaves from a SG-rich host (Populus suaveolens for the experiment conducted in Baikalsk and S. myrsinifolia for all other experiments), and the other group fed on leaves from a SG-poor host (S. glauca for the Ural population and for one of experiments with Monche population; S. caprea for all other experiments). When the larvae reached the final instar, 20 larvae feeding on the SG-rich host and 20 larvae feeding on the SG-poor host, all of similar weights (10–15 mg), were weighed to the nearest 0.1 mg, placed individually in Petri dishes (85 mm in diameter) and provided with fresh food ad libitum. Ten larvae from each host plant were randomly assigned to the treatment group (disturbed, secretions removed) and another ten larvae were assigned to the control group (disturbed, secretions resorbed). We removed the secretions from the larvae of the treatment group three times at 8-h intervals in the same way as described for the long-term experiments. After 24 h, the larvae were weighed again and their RGR was calculated as the difference between the final and initial dry weights divided by the initial dry weight of the larva. Dry weights (DW) were estimated from fresh weights (FW) of the larvae, as follows: DW = (0.262 × FW) − 0.690.
In addition, in two experiments (Baikal population, 2014 and Monche population, 2015) we scanned leaves damaged by the larvae, measured the consumed areas using Photoshop 3.0 and calculated the specific leaf weight (SLW) from the weight of discs 4.5 mm in diameter cut from the same leaves and dried at 85 °C for 24 h. We used these values to calculate the efficiency of conversion of ingested food (ECI) as the larval dry weight gain divided by the dry weight of consumed food (product of leaf area and SLW) and the relative consumption rate (RCR) as the dry weight of consumed food divided by the average larval dry weight.
Replenishment of secretions
The experiment measuring the rate of secretion replenishment was conducted with species producing large (measurable) amounts of secretions: P. aenea (producing autogenous secretion), C. tremulae (producing sequestered secretion) and C. lapponica (the Monche and Baikal populations; producing either autogenous or sequestered secretion). P. aenea and C. tremulae were fed their primary host plants (Alnus incana and Populus tremula, respectively), while C. lapponica were fed different host plants (SG-rich: Salix myrsinifolia for Monche population and P. suaveolens or S. myrsinifolia for Baikal population; SG-poor: S. glauca and S. caprea for both populations) to force the larvae to produce either sequestered or autogenous secretions, respectively.
The experiment with the Baikal population was conducted in Baikalsk in 2014 and the experiment with the Monche population was conducted in Apatity in 2015. In Baikalsk, we collected final instar C. lapponica larvae that had naturally developed on the selected host plant species (P. suaveolens, S. glauca or S. caprea), transferred them to the laboratory and continued feeding them the same host plant. In Apatity, larvae only rarely fed on SG-poor willows, so we reared larvae from eggs on both S. glauca and S. caprea.
In another experiment (conducted in 2014 in Turku), we reared larvae from both the Baikal and Monche populations on the same host plants. Larvae used in this experiment were hatched from field-collected egg batches and were reared in a common laboratory environment in 50 mL vials containing leaves of either SG-rich S. myrsinifolia or SG-poor S. caprea.
Ten final instar larvae of similar size from each host plant species × population combination were placed individually in Petri dishes (85 mm in diameter) and provided with leaves of the same host plant species as they had been feeding on before. The larvae were left for 24 h to recover from the disturbance associated with their transfer to Petri dishes. Then the secretions of each larva were carefully collected with a calibrated capillary and the volume of each secretion sample was calculated by multiplying the distance between menisci measured under a dissecting microscope and internal diameter of a capillary. The sampling was repeated after 6 h, and the volume of the replenished secretions was compared to the initial volume.
Defensive behaviour of larvae
During the secretion removal and disturbance treatments, we recorded the defensive behaviour of the last instar larvae. We used a three-grade scale to estimate the strength and duration of the disturbances that were required to cause release of secretions and trigger escape behaviour (i.e. active crawling around Petri dish). We also recorded whether the larvae regurgitated in response to disturbance, i.e. externalised semi-digested food and gut material, and whether the larvae curled up in response to the disturbance. Curling up was classified as an escape behaviour, because in natural conditions this causes the larva to drop from the plant.
Data analysis
The effects of secretion removal on leaf beetle performance in both long-term and short-term experiments were explored using a mixed model analysis of variance (ANOVA). The duration of development was log-transformed prior to the analyses. Survival was analysed using logistic regression and the events/trials syntax. In this case, a trial was the number of larvae placed in each Petri dish and an event was the number of pupated larvae. A composite fitness measure was calculated as the product of survival, beetle weight and exp[−0.1 × (duration of development)] (Higginson and Ruxton 2009a). In all these analyses, the fixed factors were the treatment and either leaf beetle species (in among-species comparisons) or the level of SGs in a host (high vs. low; in within-population comparisons of C. lapponica) and their interaction. In among-species comparisons, the family and the interaction between the family and treatment were considered random factors. In the experiments with C. lapponica, random factors were the population and its interactions with the level of SGs in a host and with the treatment. The least-square means were compared by Tukey–Kramer method, adjusted for multiple comparisons (procedure GLIMMIX; SAS Institute 2009). The significance of the random factors in these analyses was evaluated by calculating the likelihood ratio and testing it against χ 2 distribution (as described in Littell et al. 2006).
The volumes of secretions collected from the same larva with a 6-h interval were compared with a paired t test.
We searched for the general pattern in the outcomes of all our experiments and explored sources of variation on our findings using meta-analysis. We calculated the Hedges’ d measure of the effect size (ES) as the difference between the means of the experimental and control groups divided by the pooled standard deviation and weighted by sample size. We changed the sign of ES for the duration of development to associate negative ESs in all performance indices with the existence of costs. All analyses were performed using the random effects categorical models in the MetaWin 2.0 program, assuming that studies differ not only by sampling error, but also by a random component in ESs (Rosenberg et al. 2000). The costs of defences were considered as statistically significant if the 95 % confidence interval of the mean ES (CI95) did not overlap zero. The variation in the ES values within and among the classes of categorical variables was explored by calculating the heterogeneity indices (Q T and Q B, respectively) and testing these against the χ 2 distribution (Koricheva et al. 2013). Power of meta-analysis was calculated after Lajeunesse (2013) against the hypotheses on small (0.2), medium (0.5) and large (0.8) differences in costs of defence production between species with autogenous and sequestered secretions.
Results
Defensive behaviour of larvae
Strong, sometimes repeated, disturbance was required to cause secretion release in all autogenously defended species, whereas all sequestering species required only a slight touch or even nearby movements (e.g. the opening of a Petri dish) to stimulate the larvae to expel abundant secretions. At the same time, autogenously defended larvae required weaker disturbances to trigger escape behaviour (crawling away or curling up) when compared to sequestering species (Table 1). The exception is P. aenea, which remained motionless even after strong disturbances. The larvae of four of the five autogenously defended species responded to disturbance by regurgitation in addition to secretion release, whereas none of the larvae of the sequestering species displayed regurgitation (Table 1).
Long-term experiments
The removal of secretions in long-term experiments did not affect any of the measured performance indices in species that either sequestered or autogenously produced their defences (Fig. 1a; Online resource, Table S2). Similarly, secretion removal did not affect the performance of C. lapponica larvae, irrespective of the population and SG content of the plants on which the larvae were reared (Fig. 1b; Online resource, Table S3). Consistently, the composite performance index did not respond to secretion depletion (data not shown). The effect of disturbance per se, i.e. the difference between the treatment, in which the larvae were disturbed but secretion was allowed to resorb, and undisturbed control was not significant for either of the performance indices (survival: F 1,58 = 0.01, P = 0.92; beetle weight: F 1,59 = 0.30, P = 0.59; duration of development: F 1,51 = 0.01, P = 0.94).
Effects of secretion removal on the duration of larval plus pupal development (least-square means + SE) of (a) leaf beetle species that either sequester (Pv, Phratora vitelline; Ct, Chrysomela tremulae) or autogenously produce (Gv, Gonioctena viminalis; Gd, Gonioctena decemnotata; Pa, Plagiosterna aenea; Pl, Plagiodera versicolora) their defensive secretions and (b) of Chrysomela lapponica from three geographic populations on host plants contrasting in SG content (SG-rich: Salix myrsinifolia; SG-poor: S. caprea). The least-square means are based on family-specific values; the numbers of families are shown in parentheses (a) or was 5 (b). All pairwise differences between control and secretion removal treatments were not significant at P = 0.05 (Tukey–Kramer test)
Short-term experiments
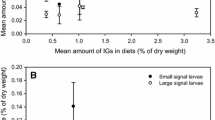
The level of SGs in the host plant strongly affected the ECI and marginally significantly affected the RGR, but did not affect the RCR of C. lapponica larvae (Online resource, Table S4). The effect of treatment was not significant across the combined data (Online resource, Table S4), but the Monche population showed an effect of secretion removal that depended on the host plant, as indicated by the significant (for RCR) and marginally significant (for RGR) interactions between treatment and host plant (Fig. 2).
Effects of secretion removal on larval growth and feeding efficiency (least-square means + SE) of Chrysomela lapponica from a specialist Monche population and a generalist Baikal population feeding on local host plants contrasting in SG content (SG-rich: Salix myrsinifolia for Monche population and Populus suaveolens for Baikal population; SG-poor: S. caprea for both populations): a, b relative growth rate (RGR); c, d relative consumption rate (RCR); e, f efficiency of conversion of ingested food (ECI). Least-square means are each based on 7–10 individual values. Statistics refers to interaction between depletion treatment and SG content in host plant within each population (mixed model ANOVA). All pairwise differences between control and secretion removal treatments were not significant at P = 0.05 (Tukey–Kramer test)
Other experiments that measured only RGR produced variable outcomes, even when they were conducted with larvae from the same population (Online resource, Fig. S1). For example, one of three experiments conducted with the Monche population revealed a cost of production of autogenous secretions, while another experiment detected a cost for sequestration (Online resource, Fig. S1), and a third experiment indicated no costs for either type of secretions (Fig. 2a). Across nine short-term experiments with four populations of C. lapponica, costs of de novo synthesis of secretions were revealed in three experiments, and costs of sequestration in two experiments (Figs. 2; Online resource, S1).
Meta-analysis of experimental data
Meta-analysis did not reveal the physiological costs of defences across the studied leaf beetle species and populations (Online resource, Table S5; d = −0.03, CI95 = −0.16…0.10), and the heterogeneity of effect sizes among our experiments was not significant (Q T = 64.3, df = 70, P = 0.67). The costs of either autogenous or sequestered defences did not differ either from zero or from each other, irrespective of the analysed performance trait (Fig. 3). The analysis of a composite fitness measure either did not reveal differences in responses of larvae using these two strategies of defence production to secretion depletion (Q B = 0.41, df = 1, P = 0.52).
Costs of sequestered and autogenously produced defences: meta-analysis combining the results of all experiments across study species and populations (for the data, consult Table S5). Dots indicate mean Hedge’s d effect sizes (measured as changes in performance in response to secretion removal); horizontal lines denote 95 % confidence intervals; sample sizes are shown in parentheses. All costs are non-significant, because all 95 % confidence intervals overlap zero. Non-significant Q B values indicate the absence of between-group heterogeneity
However, larvae producing autogenous defence showed some reduction of RGR in response to secretion depletion (Fig. 3). This reduction was significant in populations of C. lapponica specialised on SG-rich host plants (d = −0.60, CI95 = −0.86…−0.39, N = 5), in contrast to non-specialised populations of the same species (d = −0.02, CI95 = −0.54…0.38, N = 5).
The power of meta-analysis combining all our data was sufficient (95–99 %) to detect medium or large difference in costs of defence production between species with autogenous and sequestered secretions, but too low (32 %) to detect a small difference. The power of meta-analyses of individual performance traits was sufficient to detect large differences in these costs.
Volume and replenishment of secretions
Larvae of the three sequestering species produced high volumes of secretions, while larvae of the five species with autogenous secretions produced moderate to low volumes of secretions; in three of these five species, the amount of secretion was so low that it was impossible to measure even when using a capillary with a 0.15 mm internal diameter (Table 1).
The sequestering larvae of C. tremulae produced considerably greater volumes of secretions than were produced by similarly sized larvae of P. aenea, which synthesise their defences autogenously (Fig. 4; F 1,17 = 65.2, P < 0.0001). The larvae of C. lapponica from the specialist Monche population produced lower volumes of secretions when feeding on SG-poor than on SG-rich hosts (Fig. 5a: F 1,45 = 86.0, P < 0.0001; Fig. 5c, F 1,15 = 14.8, P = 0.002), while the larvae from the generalist Baikal population produced similar amounts of secretions on either SG-rich or SG-poor host plants (Fig. 5b: F 1,18 = 2.44, P = 0.14; Fig. 5d: F 1,18 = 3.58, P = 0.08).
Six hours after removal, the secretions were replenished completely in C. tremulae, but only to 10 % of the initial volume in P. aenea (Fig. 4). Similarly, when larvae of C. lapponica from both the Monche and Baikal populations sequestered their defensive compounds from SG-rich host plants, they totally replenished their defensive glands after 6 h (Fig. 5). However, when fed on SG-poor host plants, larvae from the specialist Monche population replenished only 15–24 % of the original volume of secretions, whereas larvae from the generalist Baikal population replenished their secretions almost completely (Fig. 5).
Volume of defensive secretions (means + SE, each based on 10 larvae) released by larvae of species with sequestered (Ct, Chrysomela tremulae) and autogenous (Pa, Plagiosterna aenea) secretions at the first sampling and 6 h after secretion depletion. An asterisk indicates a significant (P < 0.05) difference between these volumes (paired t test)
Volume of defensive secretions (means + SE, each based on 10 larvae) released by Chrysomela lapponica larvae from Monche (a, b) and Baikal (c, d) populations feeding on SG-rich and SG-poor host species at the first disturbance and 6 h after secretion removal. An asterisk indicates a significant (P < 0.05) difference between these volumes (paired t test) (a, c plants from native localities; b, d plants from foreign locality). SG-rich: myr, Salix myrsinifolia; pop, Populus suaveolens; SG-poor: gla, S. glauca; cap, S. caprea)
Discussion
Physiological costs of defensive secretions
We designed our study to compare the physiological costs of chemical defences among leaf beetle species that use different strategies of defence production, expecting that the costs of sequestration would be lower than the costs of de novo synthesis of defensive compounds. Contrary to our expectations, we found no physiological costs in terms of survival, weight or duration of development in any of the eight studied leaf beetle species, regardless of their defensive strategy or the types of chemicals used for defence.
Our results therefore do not support the hypothesis regarding the existence of trade-offs between growth and defence; however, they are in line with a number of previous case studies. In particular, as many as 19 of 33 publications providing data that allowed quantification of the physiological costs of chemical defences in insect herbivores (reviewed by Zvereva and Kozlov 2016) did not demonstrate the existence of these costs. Consequently, our meta-analysis (Zvereva and Kozlov 2016) did not reveal any physiological costs of defence, because those individual cases that supported the existence of physiological costs were balanced by cases where this hypothesis was not supported. Importantly, no costs of defence were detected in five studies (reporting 14 measurements of performance traits in eight insect species) that used the same method for cost measurements (i.e. depletion of released defences) as we did in the current study. At the same time, this meta-analysis revealed a considerable shortage of data regarding the costs of autogenous defences (only three measurements); thus, the reported overall lack of costs may have reflected the fact that most of the available studies explored the costs of sequestration. We endeavoured to fill this research gap by measuring the costs of defence production in six leaf beetle species that synthesised various defensive compounds de novo. We used the same method to measure costs in three species that sequestered SGs from host plants, thereby avoiding the influence of the methodology, which was recently (Zvereva and Kozlov 2016) identified as the main source of variation in the expression of costs among individual studies.
Some of our short-term experiments with C. lapponica detected decreases in the RGR in response to secretion removal, whereas long-term experiments with this species did not show trade-offs between other performance indices (either alone or combined in a composite fitness measure) and defence production. This difference between the two types of experiments may be explained by the more intensive removal of secretions (three times during 24 h) in short-term experiments, simulating high predator pressure, than in other experiments (once per day over 6–15 days), which hints that only frequent depletion may impose physiological costs and only in some circumstances. This result is in line with the prediction that investment in defence will increase with increasing predator density (Higginson and Ruxton 2009a). On the other hand, growth rate may better suit to measure immediate physiological costs, because growth is predicted to be always decreased in response to predators, whereas both time to metamorphosis and weight at metamorphosis may change in different ways depending on certain conditions (Higginson and Ruxton 2010). Our study indicates that costs of de novo synthesis may be partly associated with host plant specialisation: significant (but still minor) reduction of RGR was observed in C. lapponica only when larvae from populations specialised to sequestration of SGs were fed on SG-poor host plants.
Although some of our experiments detected costs for secretion production (Online resource, Fig. S1), meta-analysis that combined the results of all our experiments did not reveal any physiological costs of chemical defences across the study species and populations. The absence of heterogeneity in our data indicates that variation in the costs of chemical anti-predator defences among experiments is random. In particular, we found no significant differences in the costs of production of sequestered versus autogenous defences; the power analysis suggests that these differences, if they exist, could be only minor.
The ability to use readily available toxic plant compounds at no cost was suggested to favour specialisation of leaf beetles on toxic host plants, which in several leaf beetle lineages was accompanied by evolutionary shifts from de novo synthesis of defences to sequestration of plant chemicals (Termonia et al. 2001). For example, sequestration of SGs from host plants to produce salicylaldehyde was suggested to be a no-cost defensive strategy because the energetic costs, should they exist, would be compensated by glucose released during the degradation of salicin (Rowell-Rahier and Pasteels 1986; Kearsley and Whitham 1992). In this specific case, an increase in growth due to the additional income of glucose was observed when salicylaldehyde production was intensified by secretion removal in P. vitellinae (Rowell-Rahier and Pasteels 1986). We assume that this process is also responsible for the slight (although non-significant) increases in larval growth rates observed in response to secretion depletion in some experiments with C. lapponica (Fig. 2a; Online resource, Fig. S1), which were associated with increased consumption rates (Fig. 2c) and, consequently, with the provision of extra resources for secretion replenishment.
In contrast to sequestration of salicylic glucosides, the synthesis of defensive compounds de novo includes complex multi-stage biochemical processes, which are described for most of the species used in our study (for references, consult Online resource, Table S1). These processes require substantial investment of resources and would be expected to incur high costs (Bowers 1992; Rowell-Rahier and Pasteels 1986); thus, a lack of costs for species synthesising their defences de novo is especially unexpected. Nevertheless, our study is not the only one which has failed to detect physiological costs for the synthesis of toxic compounds in animals. For example, production of autogenously synthesised alkaloids in the two-spotted ladybird beetle, Adalia bipunctata, showed mostly positive genetic covariances with fitness traits (Holloway et al. 1993), indicating that this species enjoyed cost-free chemical defence. Similarly, several other studies have demonstrated the absence of energetic costs of venom production in snakes (Pintor et al. 2010; Smith et al. 2014). However, in the experiments with frequent depletion of secretion, costs were detected more frequently for C. lapponica larvae producing autogenous defence, than for larvae sequestering SGs. As the result, our meta-analysis showed minor effects of secretion removal on RGR of larvae producing secretion autogenously, while the effects on sequestering larvae approached zero (Fig. 3). This result indicates that in natural conditions, detectable costs of de novo synthesis of secretion may be expressed under extremely high enemy pressure, whereas sequestering populations or species are able to sustain higher densities of natural enemies without additional investments in defence.
In contrast to physiological costs of defences, expressed in reduction in fitness, some costs of defence cannot be measured directly, such as the fixed constitutive costs associated with the possession of defence mechanisms, and some of our data provide indirect evidence for higher constitutive costs of de novo synthesis than for sequestration.
Volume and replenishment of secretions
The volume of secretions that can be released upon disturbance is of great ecological importance because it may determine whether prey is attacked by a predator and/or survives a predator’s attack (Bowers 1992). In general, higher amounts of defensive chemicals would provide more effective protection against enemies. For example, sawfly larvae that regurgitated greater volumes of fluid better survived ant attacks (Codella and Raffa 1995).
Our study provides the first demonstration that the amounts of secretions produced by leaf beetle larvae were, on average, smaller in those species that synthesise secretions de novo than in species sequestering defensive compounds from their host plants. This pattern suggests that, among the study species, larvae with sequestered defences would show a better survival rate following an encounter with an enemy. This suggestion is partly supported by observations on wood ant predation on the same leaf beetle species (Zvereva et al. 2016).
The only exception from the pattern outlined above is the generalist Baikal population of C. lapponica, whose larvae produced high volumes of secretions irrespective of the origin of the defensive compounds. However, in this case, the high volume of secretions does not mean a high level of protection, because this secretion contained 200-fold lower concentrations of defensive compounds (mostly butyric esters) when compared to salicylaldehyde-containing secretions (Geiselhardt et al. 2015). The importance of the toxin concentration in secretions was demonstrated in experiments with wood ants. These experiments revealed a lower anti-predator effectiveness for the de novo synthesised butyrate-containing defences relative to sequestered salicylaldehyde-containing secretions in the Belarus population of C. lapponica, although their volumes were similar (Zvereva et al. 2010a) and the repellence of the pure compounds did not differ (Hilker and Schulz 1994). A similar pattern was reported earlier in Heliconius butterflies: their larvae had higher concentrations of cyanide when it was sequestered from plants than when it was synthesised de novo (Engler-Chaouat and Gilbert 2007). Thus, higher concentrations of sequestered defence compounds relative to those produced de novo could be a general phenomenon.
Moreover, we have demonstrated that autogenous secretions replenished much more slowly when compared to sequestered secretions. The rate of defence replenishment has important ecological implications because it determines how long the prey remains unprotected after it survives an unsuccessful predator attack. Both a low volume and a slow recovery of secretions may indicate a generally lower efficiency of the synthesis of de novo compounds when compared to the sequestration of compounds already synthesised by plants. We suggest that this lower efficiency may have resulted from the high constitutive costs of maintenance of the complex machinery needed for the de novo biosynthesis of defensive compounds, which impose limitations on the production of secretions.
Defensive behaviour of larvae
The observations on larval anti-predatory behaviour, in line with our results on secretions volumes and replenishment rates, also provided indirect evidence for differences in costs associated with two defensive strategies. Sequestered salicylaldehyde-containing secretions are expelled more easily than those produced de novo and require a smaller disturbance for their release: even as minor a disturbance as opening the Petri dish or air movement resulted in eversion of the defensive glands. This fast response is beneficial, because it can prevent attack or force the predator to release the prey before it is harmed. In contrast, larvae of all species with autogenous defences were very reluctant to secrete defensive substances: pressing their body by forceps, sometimes several times, was required to cause expelling of secretions. This may indicate that the latter species try to conserve secretions, releasing them only in the event of a severe attack by a predator. This result is in line with the prediction that deployment of responsive defences to multiple enemy attacks should be smaller when costs of defences are higher (Higginson and Ruxton 2009b). On the other hand, secretion conservation may serve as indirect evidence of costs (Ruxton et al. 2004), and we suggest that these costs are constitutive costs of possessing complex biosynthetic machinery that could not be detected by measuring growth-defence trade-offs.
We report, for the first time, that larvae of several leaf beetle species combine two types of chemical defence: expelling defensive secretions from glands and regurgitation of gut contents containing semi-digested plant material, which is usually aversive to attacking predators (Larsson et al. 1986; Bowers 1993). Regurgitation is less effective against predators than expelling of either sequestered or autogenously produced chemicals (Zvereva and Kozlov 2016), but it is a common behaviour in many insects, including lepidopteran caterpillars (Bowers 1993; Grant 2006) and sawfly larvae (Larsson et al. 1986). Our observations showed that the last instar larvae of four of five autogenously defended leaf beetle species, but none of the sequestering species, regurgitate in response to disturbance, i.e. a combination of two defensive responses is more frequently used by species that synthesise their defensive secretions de novo. The use of regurgitation to complement the release of secretions, despite the costs caused by the loss of semi-digested food, fluids and gut material (Bowers 2003; Higginson et al. 2011), may indicate either low effectiveness (e.g. due to low volumes) of autogenously produced defences or the existence of some hidden (constitutive) costs of these defences. Interestingly, the disturbance threshold is lower for regurgitation in the last instar larvae than for release of secretions (EZ, personal observation); thus, in nature, a secretion release would be triggered only if regurgitation did not repel the predator upon first attack, which again suggests a conservation of secretions.
In our experiments, much weaker disturbance triggered escape by running in leaf beetle larvae that autogenously produced their chemical defences than in sequestering larvae, which remained motionless for considerably longer time (Table 1). Moreover, larvae of most of the species, which autogenously produced their defences, curled in response to disturbance; in natural conditions, they would release the substrate and drop from the plant. The latter strategy is the most common defence found across insect taxa (Gross 1993), but among our sequestering species, only the last instar larvae of C. tremulae sometimes released the substrate upon disturbance. Thus, on average, the sequestering species require a strong disturbance to trigger escape behaviour. At the same time, even a weak disturbance triggers escape in species autogenously producing their defences, although this behaviour leads to additional losses of energy, risks of not finding appropriate food (e.g. after dropping) and/or risks of not rejoining the aggregation. Moreover, some predators, e.g. ants, are attracted to moving prey (Howse 1987).
Thus, autogenous production of defensive chemicals is more frequently accompanied by various kinds of defensive behaviours, indicating that species using this strategy tend to release their secretions only when other lines of defence appear ineffective. The use of multiple anti-predator defences by species that synthesise their chemical defences de novo may serve as indirect evidence for a high constitutive biosynthetic cost of autogenous defences, which constrain their production. Moreover, the use of various defensive behaviours in addition to de novo production of defensive chemicals increases net costs of defences in insects using this defensive strategy.
The greater employment of defensive behaviour in addition to chemical defences found in leaf beetle species with autogenous defence production fits the prediction of models considering optimal anti-predator strategies (Steiner and Pfeiffer 2007; Higginson and Ruxton 2009a) that low effectiveness of morphological defence will result in higher investment in behavioural defence. Our findings are also in line with a number of empirical studies that show compensation effects of behavioural and morphological defences (cited by Steiner and Pfeiffer 2007), where less morphologically defended species exhibit stronger behavioural defence.
Conclusions
We detected physiological costs (in terms of trade-off with performance traits) for the production of sequestered or autogenous defences only in a few experiments with high levels of simulated predation. Although these costs were expressed more frequently for larvae synthesising defences de novo, than for larvae sequestering SGs, meta-analysis of outcomes of all our experiments failed to demonstrate costs across the studied species and populations of leaf beetles. However, a lack of physiological costs does not imply a complete lack of costs. The synthesis of defensive compounds de novo requires maintenance of specialised biochemical machinery, and our results hint at the existence of constitutive costs, which are likely to constrain the amounts of autogenous defences produced. Moreover, a low volume of defensive secretions and a slow rate of secretion replenishment not only indicate these constraints, but also lead to generally lower effectiveness of autogenously produced chemical defences relative to sequestered defences. This forces those species that possess ancestral strategy of synthesising defences de novo to complement release of defensive secretions with other defensive behaviours to enhance their protection against enemies. However, these behaviours also incur costs; thus, the autogenous synthesis of defensive compounds appears to be a generally more costly and less efficient strategy when compared to sequestration. In our opinion, the currently observed constraints in the de novo production of defensive secretions reflect the result of selection that had balanced the costs and benefits for the possessor in the past, thereby assuring an optimal trade-off between resource investments in growth and in defences. Therefore, sequestration could be favoured in the evolution of herbivorous insects as a strategy that allows to overcome these constraints and to enhance the effectiveness of anti-predator defences at a reduced cost.
References
Bernays E, Chapman R (1987) The evolution of deterrent responses in plant-feeding insects. In: Chapman RF, Bernays EA, Stoffolano JG Jr (eds) Perspectives in chemoreception and behavior. Springer, New York, pp 159–173
Bernays E, Graham M (1988) On the evolution of host specificity in phytophagous arthropods. Ecology 69:886–892
Björkman C, Larsson S (1991) Pine sawfly defense and variation in host plant resin acids: a trade-off with growth. Ecol Entomol 16:283–289
Blum MS (1981) Chemical defenses of arthropods. Academic, London
Boland W (2015) Sequestration of plant-derived glycosides by leaf beetles: a model system for evolution and adaptation. Perspect Sci 6:38–48
Bowers MD (1992) The evolution of unpalatability and the cost of chemical defense in insects. In: Roitberg BD, Isman MB (eds) Insect chemical ecology: an evolutionary approach. Chapman and Hall, New York, pp 216–244
Bowers MD (1993) Aposematic caterpillars: life styles of the warningly colored and unpalatable. In: Stamp NE, Casey TM (eds) Caterpillars: Ecological and evolutionary constraints of foraging. Chapman and Hall, London, pp 331–371
Bowers MD (2003) Host plant suitability and defensive chemistry of the catalpa sphinx, Ceratomia catalpae. J Chem Ecol 29:2359–2367
Camara MD (1997) Physiological mechanisms underlying the costs of chemical defence in Junonia coenia Hübner (Nymphalidae): a gravimetric and quantitative genetic analysis. Evol Ecol 11:451–469
Codella SG, Raffa KF (1995) Host plant influence on chemical defense in conifer sawflies (Hymenoptera: Diprionidae). Oecologia 104:1–11
Cogni R, Trigo JR, Futuyma DJ (2012) A free lunch? No cost for acquiring defensive plant pyrrolizidine alkaloids in a specialist arctiid moth (Utetheisa ornatrix). Mol Ecol 21:6152–6162
Eisner T, Eisner M, Siegler M (2007) Secret weapons: Defenses of insects, spiders, scorpions, and other many-legged creatures. Belknap, Cambridge
Engler-Chaouat HS, Gilbert LE (2007) De novo synthesis vs. sequestration: negatively correlated metabolic traits and the evolution of host plant specialization in cyanogenic butterflies. J Chem Ecol 33:25–42
Fürstenberg-Hägg J, Zagrobelny MC, Olsen E, Jørgensen K, Møller BL, Bak S (2014) Transcriptional regulation of de novo biosynthesis of cyanogenic glucosides throughout the life-cycle of the burnet moth Zygaena filipendulae (Lepidoptera). Insect Biochem Mol Biol 49:80–89
Futuyma DJ, Moreno G (1988) The evolution of ecological specialization. Annu Rev Ecol Syst 19:207–233
Geiselhardt S, Hilker M, Müller F, Kozlov MV, Zvereva EL (2015) Inter- and intrapopulation variability in the composition of larval defensive secretions of willow-feeding populations of the leaf beetle Chrysomela lapponica. J Chem Ecol 41:276–286
Grant JB (2006) Diversification of gut morphology in caterpillars is associated with defensive behavior. J Exp Biol 209:3018–3024
Gross P (1993) Insect behavioral and morphological defenses. Annu Rev Entomol 38:251–273
Gross J, Fatouros NE, Hilker M (2004) The significance of bottom-up effects for host plant specialization in Chrysomela leaf beetles. Oikos 105:368–376
Higginson AD, Ruxton GD (2009a) Dynamic models allowing for flexibility in complex life histories accurately predict timing of metamorphosis and antipredator strategies of prey. Funct Ecol 23:1103–1113
Higginson AD, Ruxton GD (2009b) Dynamic state-dependent modelling predicts optimal usage patterns of responsive defences. Oecologia 160:399–410
Higginson AD, Ruxton GD (2010) Adaptive changes in size and age at metamorphosis can qualitatively vary with predator type and available defences. Ecology 91:2756–2768
Higginson AD, Delf J, Ruxton GD, Speed MP (2011) Growth and reproductive costs of larval defence in the aposematic lepidopteran Pieris brassicae. J Anim Ecol 80:384–392
Hilker M, Schulz S (1994) Composition of larval secretion of Chrysomela lapponica (Coleoptera, Chrysomelidae) and its dependence on host plant. J Chem Ecol 20:1075–1093
Holloway GJ, De Jong PW, Ottenheim M (1993) The genetics and cost of chemical defense in the two-spot ladybird (Adalia bipunctata). Evolution 47:1229–1339
Howse PE (1987) Temporal effects of chemical communication of alarm in ants. In: Pasteels JM, Deneubourg J-L (eds) From individual to collective behavior in social insects. Birkhäusen, Basel and Boston, pp 271–275
Jeffries MJ, Lawton JH (1984) Enemy free space and the structure of ecological communities. Biol J Linn Soc 23:269–286
Kearsley MJC, Whitham TG (1992) Guns and butter: a no cost defense against predation for Chrysomela confluens. Oecologia 92:556–562
Koricheva J, Gurevitch J, Mengersen K (eds) (2013) Handbook of meta-analysis in ecology and evolution. Princeton University Press, Princeton and Oxford
Lajeunesse MJ (2013) Power statistics for meta-analysis: test for mean effects and homogeneity. In: Koricheva J, Gurevitch J, Mengersen K (eds) Handbook of meta-analysis in ecology and evolution. Princeton University Press, Princeton, pp 348–363
Larsson S, Björkman C, Gref R (1986) Responses of Neodiprion sertifer (Hym., Diprionidae) larvae to variation in needle resin acid concentration in Scots pine. Oecologia 70:77–84
Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O (2006) SAS for mixed models, 2nd edn. SAS Institute Inc, Cary
Loxdale HD, Lushai G, Harvey JA (2011) The evolutionary improbability of ‘generalism’ in nature, with special reference to insects. Biol J Linn Soc 103:1–18
Nishida R (2002) Sequestration of defensive substances from plants by Lepidoptera. Annu Rev Entomol 47:57–92
Opitz SEW, Müller C (2009) Plant chemistry and insect sequestration. Chemoecology 19:117–154
Pasteels JM, Braekman J-C, Daloze D (1988) Chemical defense in the chrysomelidae. In: Solivet P, Petitpierre E, Hsiao TH (eds) Biology of chrysomelidae. Kluwer, Dordrecht, pp 233–252
Pintor AFV, Krockenberger AK, Seymour JE (2010) Costs of venom production in the common death adder (Acanthophis antarcticus). Toxicon 56:1035–1042
Price PW, Bouton CE, Gross P, Mcpheron BA, Thompson JN, Weis AE (1980) Interactions among three trophic levels: influence of plants on interactions between insect herbivores and natural enemies. Annu Rev Ecol Syst 11:41–65
Rosenberg MS, Adams DC, Gurevitch J (2000) MetaWin: Statistical software for meta-analysis, Version 2.0. Sinauer, Sunderland
Rowell-Rahier M, Pasteels JM (1986) Economics of chemical defense in Chrysomelinae. J Chem Ecol 12:1189–1203
Rowell-Rahier M, Pasteels JM, Alonso-Mejia A, Brower LP (1995) Relative unpalatability of leaf beetles with either biosynthesized or sequestered chemical defense. Anim Behav 49:709–714
Ruxton GD, Sherratt TN, Speed MP (2004) Avoiding attack: the evolutionary ecology of crypsis, warning signals, and mimicry. Oxford University Press, Oxford
SAS Institute (2009) SAS/Stat. User’s guide, Version 9.2. SAS Institute, Cary
Smith MT, Ortega J, Beaupre SJ (2014) Metabolic cost of venom replenishment by Prarie Rattlesnakes (Crotalus viridis viridis). Toxicon 86:1–7
Stamp N (2001) Enemy-free space via host plant chemistry and dispersion: assessing the influence of tri-trophic interactions. Oecologia 128:153–163
Steiner UK, Pfeiffer T (2007) Optimizing time and resource allocation trade-offs for investment into morphological and behavioural defense. Am Nat 169:118–129
Termonia A, Hsiao TH, Pasteels JM, Milinkovitch MC (2001) Feeding specialization and host-derived chemical defense in Chrysomeline leaf beetles did not lead to an evolutionary dead end. Proc Natl Acad Sci USA 98:3909–3914
Zvereva EL, Kozlov MV (2016) The costs and effectiveness of chemical defenses in herbivorous insects: a meta-analysis. Ecol Monogr 86:107–124
Zvereva EL, Kruglova OY, Kozlov MV (2010a) Drivers of host plant shifts in the leaf beetle Chrysomela lapponica: natural enemies or competition? Ecol Entomol 35:611–622
Zvereva EL, Kozlov MV, Hilker M (2010b) Evolutionary variations on a theme: host plant specialization in five geographical populations of the leaf beetle Chrysomela lapponica. Popul Ecol 52:389–396
Zvereva EL, Kozlov MV, Rank N (2016) Does ant predation favour leaf beetle specialization on toxic host plants? Biol J Linn Soc 119:201–212
Acknowledgments
We are grateful to M. Pentinsaari for help in collecting beetles and to A. D. Higginson and two anonymous reviewers for inspiring comments to an earlier version of the manuscript. The study was supported by the Academy of Finland (Project 268124 and researcher exchange grants to E. Zvereva and V. Zverev).
Author contribution statement
ELZ and MVK conceived and designed the experiments, analyzed the data and wrote the manuscript. ELZ, MVK, VZ and OYK performed the experiments and provided editorial advice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Roland A. Brandl.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zvereva, E.L., Zverev, V., Kruglova, O.Y. et al. Strategies of chemical anti-predator defences in leaf beetles: is sequestration of plant toxins less costly than de novo synthesis?. Oecologia 183, 93–106 (2017). https://doi.org/10.1007/s00442-016-3743-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-016-3743-x