Abstract
Studies of ecological stoichiometry typically emphasize the role of interspecific variation in body elemental content and the effects of species or family identity. Recent work suggests substantial variation in body stoichiometry can also exist within species. The importance of this variation will depend on insights into its origins and consequences at various ecological scales, including the distribution of elemental phenotypes across landscapes and their role in nutrient recycling. We investigated whether trophic divergence can produce predictable patterns of elemental phenotypes among populations of an invasive fish, the white perch (Morone americana), and whether elemental phenotypes predict nutrient excretion. White perch populations exhibited a gradient of trophic phenotypes associated with landscape-scale variation in lake trophic state. Perch body chemistry varied considerably among lakes (from 0.09 for % C to 0.31-fold for % P) casting doubt on the assumption of homogenous elemental phenotypes. This variation was correlated with divergence in fish body shape and other trophic traits. Elemental phenotypes covaried (r 2 up to 0.84) with lake trophic state. This covariation likely arose in contemporary time since many of these perch populations were introduced in the last century and the trophic state in many of the lakes has changed in the past few decades. Nutrient excretion varied extensively among populations, but was not readily related to fish body chemistry or lake trophic state. This suggests that predictable patterns of fish body composition can arise quickly through trophic specialization to lake conditions, but such elemental phenotypes may not translate to altered nutrient recycling by fish.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Emphasis on the role of animals as drivers of aquatic ecosystem dynamics is typically placed on variation in traits that aid in the capture and processing of prey, partly because trophic traits contribute to trophic cascades (Carpenter and Kitchell 1996; Post et al. 2008) and adaptive radiations (Schluter 2000). However, animals can also influence ecosystem dynamics by altering pools and fluxes of available nutrients indirectly and directly through bioturbation, translocation, nutrient sequestration, and enhanced cycling through excretion or egestion of waste (Meyer et al. 1983; Vanni 2002; Sereda et al. 2008; Knoll et al. 2009; Flecker et al. 2010; Vanni et al. 2013). Among these, consumer-driven nutrient recycling (CDNR) from excretion is one of the most direct ways animals influence the availability and flux of nitrogen (N) and phosphorus (P) (Vanni 2002), potentially altering nutrient limitation of primary production (Elser et al. 1988; Vanni et al. 2006; Knoll et al. 2009). The magnitude and chemical content of CDNR is dictated by a consumer’s ability to assimilate nutrients (i.e., assimilation efficiency), the balance between body and food elemental content, and consumption and growth rates (Elser et al. 2000; Sterner and Elser 2002; Vanni et al. 2002). Consequently, there has been considerable interest in characterizing stoichiometric variation in consumer elemental content and excretion rates and these studies have revealed considerable variation among species as a consequence of divergence in consumer body structure (Vanni et al. 2002; McIntyre and Flecker 2010). Recent work has revealed considerable variation in body elemental content among populations of fishes (El-Sabaawi et al. 2012a, b, 2016; Boros et al. 2015), but its origins and implications remain unclear. One important implication is that stoichiometric features of fish populations could serve as pathways for eco-evolutionary dynamics where recent evolutionary changes in elemental phenotypes might lead to a feedback on ecosystem conditions through processes like CDNR (Palkovacs et al. 2009; Jeyasingh et al. 2014). Here, we consider how consumer body elemental content varies across the landscape, how this variation may be tied to recent trophic-mediated divergence driven by the lake trophic state, and what the consequences might be for CDNR and eco-evolutionary dynamics.
The relationship between excretion and body elemental content follows from mass balance accounting where excretion is positively related to nutrient ingestion and negatively related to body N or P, which reflects demand if a consumer is growing (Sterner and Elser 2002; Vanni et al. 2002). Thus, as relative body content of an element increases, its loss through excretion should decline, assuming constant diet (Sterner and Elser 2002). Excretion is also constrained by physiological rates leading to excretion scaling with body size at approximately 3/4 following metabolic theory (Brown et al. 2004; Allgeier et al. 2015). Homeostatic regulation of carbon (C), N, and P body content is typically assumed over limited timescales. Following consumption of chemically varying food, assimilated resources are used for growth or exploited during metabolic reactions resulting in nutrient excretion, but the elemental composition of tissues remain consistent. However, recognition of the full scope of stoichiometric variation among vertebrate taxa is increasing and some findings suggest the elemental composition of aquatic consumers can be quite variable, even approaching interspecific levels (Hendrixson et al. 2007; Vrede et al. 2011; El-Sabaawi et al. 2012a, b, 2016; Boros et al. 2015).
A dichotomy has been suggested where fish body N and P are dictated by either environmental (nutrients, predation pressure) or organismal trait (body size, shape) determinants (El-Sabaawi et al. 2012a). Support for organismal trait determinants has been found, especially for the effects of body size, shape, and structural variation among taxa, which may alter investment in scales, bone and muscle tissues (Tanner et al. 2000; Hendrixson et al. 2007; Vrede et al. 2011; Boros et al. 2015; El-Sabaawi et al. 2016). These tissues may have predictable elemental signatures, where carbohydrates and lipids are high in C, protein is high in N and DNA, RNA and hydroxyapatite of bone are high in P. Other studies have found weak support for trait determinants; instead, environmental conditions such as ecosystem and resource nutrient concentration or predation environment were more important (El-Sabaawi et al. 2012a, b, 2014). It is unlikely that environmental and organismal determinants are mutually exclusive. Environmental variation such as available habitat and the overall input and availability of nutrients can strongly affect organismal traits such as body size, shape, growth rate and prey selectivity in fishes (Svanbäck and Eklöv 2002; Turesson and Brönmark 2007; Tuckett et al. 2013), perhaps influencing consumer stoichiometry.
Trophic state is one of the most important axes of environmental variation for lentic ecosystems which integrates biotic (e.g., plankton communities) and abiotic (e.g., nutrients, lake depth, littoral area) factors ultimately affecting fish traits and ecology (Tuckett et al. 2013). Fish also influence trophic state variables via morphological and behavioral adaptations, representing a possible reciprocal feedback between biotic trophic state factors and organismal traits (Harmon et al. 2009; Bassar et al. 2010). Animals distributed across trophic state gradients may be useful study systems for examining eco-evolutionary feedbacks, including those mediated through the relationship between environment-driven organismal trait change and stoichiometry. One such study system is the white perch (Morone americana) in North American lakes. White perch are native to eastern North America where their range has been expanded through deliberate and accidental introductions dating back to the 1800s (Hergenrader and Bliss 1971; Busch et al. 1977; Stanley and Danie 1983; Halliwell 2005). In prior work, we showed that introduced populations of white perch exhibit contemporary divergence in a suite of trophic traits (e.g., streamlined versus robust body shape, narrow versus broad gill raker spacing, etc.) related to lake trophic state (Tuckett et al. 2013), despite recent changes in lake trophic state due to cultural eutrophication.
We used white perch to examine the stoichiometric response to invasion and adaptation along this same gradient of lake trophic state and the potential for correlated divergence in trophic/elemental phenotypes to suppress/promote nutrient stability and eco-evolutionary feedbacks. We hypothesize that lability in trophic traits contributes to divergence in elemental phenotypes. Addressing this hypotheses requires that we build upon our previous studies showing how trophic state dictates trophic phenotypes (Tuckett et al. 2013) and the importance of body elemental content to excretion (Tuckett et al. 2015). First, we attempt to establish a relationship between trophic state and elemental phenotypes. Second, we examine whether trophic/elemental phenotypes represent a suite of correlated traits. Finally, with respect to potential eco-evolutionary feedbacks, we examine whether feedbacks might be suppressed if eutrophic environments lead to benthic phenotypes and greater fish body N or P and, alternately, whether feedbacks might be enhanced if eutrophication leads to greater nutrient excretion regardless of stoichiometric considerations.
Methods
Study sites
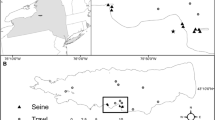
We quantified morphological and elemental phenotypes of nine white perch populations inhabiting freshwater lakes spanning a range of trophic state in Maine, U.S.A (Table 1; see Tuckett et al. 2013 for a detailed description of fish communities and study lakes). Fish were also collected at Merrymeeting Bay, a largely freshwater tidal bay, serving as an ancestral estuarine reference condition. We used archived (2004–2007) and new data (2008) regarding lake chemistry and physical attributes to quantify environmental drivers and lake trophic status. Secchi depth, chlorophyll-a, total phosphorus (TP) were collected under U.S. Environmental Protection Agency guidelines and archived by the Maine Department of Environmental Protection and the Volunteer Lake Management Program (lakesofmaine.org). Triplicate water samples used for analysis of chlorophyll-a (chl-a) and total nutrients were taken using a 5-cm-diameter plastic tube sampled and composited through the epilimnion. Samples were transported to the lab on ice and at least one liter of lake water was filtered onto 47 mm filters (GF/F) and subsequently frozen; filters were then ground and chl-a was acetone extracted and absorbance was recorded using a spectrophotometer and phaeophytin corrections (APHA 2005). Water samples for analysis of total dissolved nitrogen and total dissolved phosphorus were stored in acid-washed bottles, frozen upon laboratory arrival and dual digestion procedures were used. Unlike TP which was represented by a 5-year average, total dissolved nitrogen was unavailable for years before 2008; thus, results will represent the average of 3 replicates from a single sampling date. Merrymeeting Bay indicators are represented by a single sampling date in 2008. In most analyses we use the 5-year average Secchi depth as an integrative indicator of lake trophic state because of its correlation with TN, TP, mean lake depth, and littoral area in these lakes (Tuckett et al. 2013).
Fish capture and processing
We collected between 27 and 32 perch at each lake and measured body morphology, elemental content, growth rate, and excretion of N and P. Fish were captured between 19:00 and 23:00 h in littoral habitats from June through August 2008 using boat electrofishing. Following capture, fish were held to measure nutrient excretion rates (described below), euthanized, and then frozen at −20 °C for 3–6 months. Fish were later photographed on the left side with a uniform background at fixed focal length (60 cm), measured for standard length (±1 mm), and weighed (±0.1 g). Fish traits typically associated with trophic-mediated divergence were analyzed from digital photographs and caliper measurements, including overall fish body shape, gill raker length and spacing, width of the closed mouth between the maxillary bones, open mouth gape size, and caudal, pectoral, pelvic fin lengths using approaches described in Tuckett et al. (2013).
Two-dimensional white perch shape variation was analyzed with geometric morphometric procedures using 21 homologous landmarks placed on randomly ordered digital images (8.1 mps Canon Eos Rebel) using the program tpsDig Version 2.0 (Rohlf 2004). Landmarks represent the outline of the body and also the location of the snout, eye, opercula bone, and fin insertions. Sliding landmarks were included at the narrowest location (dorsal and ventral) of the caudal peduncle. Digitized x- and y-coordinate data from the 21 landmarks were scaled, rotated and translated using tpsRelw, version 1.42 (Rohlf 2005). Variation in white perch shape was summarized with 36 relative warp scores from orthogonal summary of the partial warps.
Raker morphology was analyzed following removal of the most anterior, left gill arch from each fish; digital images were then taken at fixed focal length using a dissecting microscope (Leica EZ4). On the digital photographs, we measured raker length and spacing on the first three rakers ventral to the apex using ImageJ software. Mouth dimensions were analyzed as the width of the closed mouth (±0.05 cm) between the maxillary bones and by inserting into the mouth a scaled wooden cone (36.8 mm base diameter by 75.9 height) following Nilsson and Brönmark (2000). Pectoral, pelvic and caudal fin length was analyzed from digital images using the Pythagorean Theorem.
We estimated fish growth using calcified scales and the Fraser–Lee method to estimate growth using back-calculated length at age with the assumption that growth in calcified scales is proportional to growth in length (Ricker 1992). Scales were photographed with a digital microscope (Leica EZ4) and distance between annuli was measured from images using ImageJ software (Abràmoff et al. 2004). Growth in length was calculated as the proportional increase in length between the two most recent annuli. A subsample of fish analyzed for trophic phenotypes by Tuckett et al. (2013) was also used in the present study.
Body elemental content
Following evacuation of the foregut, the digestive system was rinsed and macerated with the body in a Waring (CB15) commercial blender. Macerated tissue was dried at 65 °C, passed through a Wiley Mill with a 20 mesh screen, ground to a fine powder with a mortar and pestle and stored at −20 °C. Prior to elemental analysis, powdered material was re-dried at 65 °C. A subsample was ashed at 550 °C for 5 h, acidified with 50 % HCl, and analyzed for P and calcium (Ca) content using inductively coupled plasma atomic emission spectroscopy (ICP-AES; TJA Model 975, Waltham, MA, USA). Phosphorus was measured for all fish while Ca was measured on 33 %–100 % of each population. A second subsample was used to measure C and N content using an elemental analyzer (Leco CN-2000, St. Joseph, MI, USA). Relative percent error (among subsamples) was 1.0 % for C and N, 3.7 % for P and 5.7 % for Ca.
Nutrient excretion
Rates of N and P excretion were estimated by measuring accumulation of ammonia-N, and total dissolved N (TDN) and P (TDP) in tubs containing individual fish (Schaus et al. 1997). Following collection, fish were held in a net pen for less than 30 min then transferred into covered, dark, plastic tubs containing 15 L of pre-filtered (Whatman GF/F) lake water. Adult fish spanning a size gradient from each site were incubated individually for 40–60 min to minimize handling and fasting effects (Whiles et al. 2009). Tubs were aerated and partially submerged in the lake to maintain ambient temperature. Water samples were collected before fish addition and 5, 15, and 40 min after adding fish, filtered (Whatman GF/F) into acid-washed bottles and frozen until analysis. Water samples were analyzed for ammonia-N, TDN and TDP using a Lachat QuikChem 8500 flow injection analyzer (Lachat Instruments, Hach Co. Loveland, CO, USA) following standard methods (APHA 2005). Individual excretion rates were estimated by regression of nutrient accumulation over incubation time. Only regressions significant at P < 0.100 were used in subsequent data analysis.
Statistical analyses
To account for variation among individuals and populations in body size we employed analyses of covariance (ANCOVA) using centroid size, standard length, or dry mass in initial analyses of individual body shape, proportional growth rates (g wet mass g−1), and body elemental content. ANCOVA models use log (10) transformed data to meet the requirements of normality and homoscedasticity. Non-significant interaction terms (P > 0.050) were removed from ANCOVA models and reanalyzed. Resulting ANCOVA-derived least-squares (LS) means or allometric slopes for the respective populations were used subsequently in model selection (AIC) or Pearson correlations to assess potential associations with lake conditions (e.g., Secchi depths) at the landscape scale, as well as the relative role of trophic divergence in explaining elemental phenotypes. The Merrymeeting Bay population was not formally included in such regressions due to its estuarine nature, but is noted in figures and results for the sake of comparison. The alpha for among-population regressions was set at 0.100 in deference to the lower statistical power (df = 7). The contribution of bone (% Ca used as a proxy) to body % P was evaluated using ordinary least-squares (OLS) regression with data pooled across populations (Hendrixson et al. 2007).
Individual excretion of ammonia-N, TDN, TDP and N:P among populations was similarly evaluated with ANCOVA, but with two covariates, body elemental content (N, P or N:P) and dry mass (g). Interaction terms were removed when not significant (P ≥ 0.05). The influence of lake trophic state indicators (Secchi, TN and TP) on LS mean population excretion was again evaluated with linear regression. Mass-normalized excretion was used to account for variable size–structure among populations (Brown et al. 2002, sensu Torres and Vanni 2007). Subsequent statistical analyses were performed in JMP (V. 10.0, SAS Institute Inc., Cary, NC).
Results
Lake trophic state
For the nine study lakes, 5-year summer mean Secchi depth, chl-a, TP, and TN varied broadly (Table 1). Secchi depth was strongly correlated with nutrient enrichment (TN: R = −0.82, P = 0.007; TP: R = −0.76, P = 0.017).
Organismal traits
Fish wet weight ranged from 8 to 523 g and varied among populations (F 9, 288 = 5.25, P < 0.001), but was unrelated to Secchi depth (F 1, 7 = 0.75, P = 0.416). Trophic traits, gill raker length and spacing, width of the closed mouth between the maxillary bones, open mouth gape size and caudal, pectoral, pelvic fin lengths typically varied among populations, were always positively related to body size, and often exhibited significant size × population interaction (Table 2). Proportional yearly growth ranged from 0.15 to 0.29 among populations, negatively scaled with wet weight, and exhibited a significant weight × population interaction (Table 2). Trophic-elemental phenotype correlations are discussed below.
DFA of 36 relative warps (geometric morphometrics) produced 4 canonical axes (CA) explaining 80 % of the variation in body shape (Wilks Λ < 0.001; Approximate F 324, 2218 = 9.32, P < 0.001). CA 1 explained 31 % of total shape variation and clearly integrated many of the common trophic adaptations (pelagic/limnetic versus littoral/benthic) seen in perch and other fishes (Skulason and Smith 1995; Taylor 1999; Schluter 2000; Tuckett et al. 2013), and was thus the focus of further analyses. This CA describes variation in body depth (− streamlined, + gibbose), mouth position (− terminal, + subterminal), length and depth of the caudal peduncle (− shallow/long, + deep/short), and rotation of the opercle bone (− cranial; + caudal). CA 1 differed among populations, increased with fish centroid size, and exhibited a size × population interaction (Table 2). LS means of CA 1 strongly declined with increasing Secchi depth (F 1, 7 = 99.80, r 2 = 0.93, P < 0.001) while slopes increased (F 1, 7 = 5.06 r 2 = 0.42, P = 0.059). In other words, perch populations in productive lakes were more gibbose/benthic overall and became increasingly so with size whereas populations from unproductive lakes retain the more juvenile, streamlined/pelagic shape even at larger sizes.
Elemental phenotypes
Across populations, body elemental content ranged between 1.9 and 10.1 % for Ca, 34.7 and 48.4 % for C, 8.6 and 11.7 % for N, 2.5 and 5.6 % for P, and 4.0 and 8.0 for N:P. Elemental contents and molar ratios (C:N, C:P, N:P) varied among populations and typically exhibited significant size × population interactions (Table 3). Stoichiometric variation strongly depended on population source; partial r 2 for the effect of population source varied from 0.24 to 0.47 and was greater than size or population × size interaction terms for all elements and ratios. As lakes became more eutrophic (i.e., declining Secchi depth), body % P increased (Fig. 1a) while body % N (Fig. 1b) and N:P (Fig. 1c) declined. Body % C (Fig. 1d) and % Ca (F 1, 7 = 1.89, r 2 = 0.21, P = 0.211) were not clearly related to Secchi depth but 73 % of variation in individual % P in our dataset is explained by % Ca (F 1, 121 = 333.85, P < 0.001) suggesting that bone content is a major determinant.
Linear relationships between body elemental content and lake trophic state (Secchi depth). Values are population LS Means (±1 SE) of body % P (a), % N (b), molar N:P (c) and % C (d) for 10 populations of white perch. Closed circles represent lentic populations (n = 9), the estuarine population (n = 1) is the closed triangle. The estuarine population is not included in regressions
Allometry of body chemistry also varied among populations. Six of 10 populations exhibited significant mass-element scaling relationships with fish size for % C, albeit with variable slope directionality. Population-specific scaling relationships were also observed for % N (5 populations), % P (6 populations), % Ca (4 populations) and N:P (8 populations). These allometries often correlated with lake conditions. As lakes became more eutrophic (Secchi decreased), allometric slopes of body % P transitioned from more negative to more positive values; the opposite was observed for % C and N:P ratios (Fig. 2a, c, d). There was no relationship between Secchi and allometric scaling for % N (Fig. 2b) and % Ca (F 1, 2 = 2.61, r 2 = 0.57; P = 0.247).
Linear relationships between allometric slopes of body stoichiometry and lake trophic state (Secchi depth). Values are ANCOVA-derived slopes of % P (a), % N (b), molar N:P (c) and % C (d) with log dry mass as a covariate for 10 populations of white perch. Closed circles are lake populations exhibiting statistically significant slopes (% P, n = 5; % N, n = 5; N:P, n = 7; % C, n = 5), open circles are lake populations with insignificant slopes and the lone estuarine population is represented by a closed triangle. A significant regression is indicated by a solid line. The estuarine population is not included in regressions
Significant correlations between body elemental content and both organismal trait and environmental determinants were common (Table 4). As found above, Secchi depth and overall lake nutrient stoichiometry were often correlated with body elemental content, especially body % P and N:P. However, these environmental drivers were weaker and less consistently related to body stoichiometry than were organismal traits. Correlations between organismal traits were strongest for body % P and N:P (Table 4). Many trophic traits were especially highly correlated with body % P (R = 0.57–0.78) and N:P (R = −0.33 to −0.89) wherein populations exhibiting low body N:P and high P content exhibited more robust body shape (larger CA 1; Fig. 3), longer fins, larger mouths, and higher growth rate, consistent with putative benthic phenotypes.
Distribution of % C (a), % N (b), % P (log dry mass (g) (c) and N:P (log molar (d) originating from 10 study sites across body shape (canonical axis 1). Points (n = 293) are coded by study site. Large open (study lakes; n = 9) and closed (Merrymeeting Bay; n = 1) circles are population means. Fish images on the x-axis are consensus representations of fish shape from geometric morphometrics and the discriminant function analysis and correspond to the axis end points (6, −6)
Nutrient excretion
Across populations, individual nutrient excretion rates ranged from 32.1 to 947.3 µmol h−1 (coefficient of variation = 0.73) for ammonia-N, 15.1–562.0 (0.59) for TDN, 1.8–101.5 (0.84) for TDP and 6.3–294.8 (1.01) for N:P (Fig. 4). Average ammonia-N contributed 44 % of the N to TDN but ranged broadly (7–91 %) across individuals. Excretion rate scaled positively with wet mass for ammonia-N (F 1, 270 = 461.98, r 2 = 0.63, P < 0.001), TDN (F 1, 198 = 114.45, r 2 = 0.37, P < 0.001), TDP (F 1, 186 = 27.51, r 2 = 0.12, P < 0.001) and N:P (F 1, 153 = 5.14, r 2 = 0.03, P < 0.025), but only ammonia-N scaled close to ¾ power (Fig. 4).
Allometry of log (10)-transformed ammonia-N (n = 272) (a), TDN (n = 200) (b), TDP (n = 188) (c) excretion and molar ratio of excreted N:P (n = 155) (d) for individual white perch from 10 populations. Closed circles are excretion estimates which exhibited significant regressions with time (P < 0.10); open circles are non-significant (P > 0.10) and were excluded from regression with log wet weight
ANCOVA models used to evaluate individual excretion (N, P and N:P) revealed significant variation among populations, a consistent relationship with body size and occasional relationships with body elemental content (Table 5). Model interaction terms involving population source were often significant. Population source was significant in all models but explained little variation in excretion rates or N:P ratio (partial r 2 < 0.169). Body size was significant for all excretion rates and N:P, with the exception of TDP. Size explained substantial variation in ammonia-N excretion (partial r 2 < 0.606) but little variation (partial r 2 < 0.114) in TDN or N:P. Body elemental chemistry did not explain variation in ammonia-N or TDN excretion and a small portion of variation in TDP (partial r 2 = 0.035) and N:P (partial r 2 = 0.037).
Mean mass-normalized excretion rates were related in a few cases to ecosystem stoichiometry (nutrient conditions), but not to Secchi depth (P > 0.112). Both mean TDN and ammonia-N excretion increased as lake TN increased (ammonia-N: F 1, 8 = 5.08, r 2 = 0.39, P = 0.054; TDN: F 1, 8 = 6.91, r 2 = 0.46, P = 0.030). Mean TDP excretion was unrelated to lake TP (F 1, 8 = 0.54, r 2 = 0.06, P = 0.483). Excretion N:P increased with increasing lake TN (F 1, 8 = 3.57, r 2 = 0.31, P = 0.095), but was unrelated to lake N:P (F 1, 8 < 0.01, r 2 < 0.01, P = 0.970) or TP (F 1, 8 = 1.36, r 2 = 0.15, P = 0.277).
Discussion
Variation in elemental phenotypes
White perch C, N, P, and Ca content was highly variable among populations, spanning a large portion of the range reported across fish species (McIntyre and Flecker 2010). For white perch, the range in % P (2.5–5.6 %) was 70 % of the range reported for 100 species representing 31 fish families; the % N range for perch (8.6–11.7 %) was 48 % of the range among species. This is consistent with an emerging picture that there is substantial intraspecific variation in body elemental content, casting considerable doubt on the assumption of homeostatic elemental phenotypes within a species. The variation we found was comparable to the few other species studied to date, including among morphs of three-spine stickleback (Gasterosteus aculeatus; 2.5–6.1 % P) (El-Sabaawi et al. 2016), small-bodied Trinidadian guppies (Poecilia reticulata; 2.1–5.4 % P) (El-Sabaawi et al. 2012b), and bluegill sunfish (Lepomis macrochirus; 2.7– 4.4 % P) (Hendrixson et al. 2007). In this study of white perch and also with guppies, much of the variation in elemental content was attributed to population/site differences (El-Sabaawi et al. 2014); for white perch, the partial r 2 for population exceeded the direct or interactive effects of body size (Table 3). Further, population means spanned 35 % of the total individual range for % P (3.43–4.50), 29 % for % N (9.90–10.80) and 39 % for N:P (4.97–6.51). The landscape variation shown here and elsewhere may shift how stoichiometry is considered, where emphasis is often placed on the ecological consequences of species or family identity (Vanni et al. 2002; McIntyre et al. 2008).
Environmental versus trait determinants
One of our major objectives was to examine the causes of variation in elemental phenotypes and whether they can be linked to environmental or trait drivers which could promote/suppress eco-evolutionary feedbacks. To date, variation in elemental phenotypes have been tentatively linked to site-specific environmental factors such as biofilm nutrient content (El-Sabaawi et al. 2012b) and also to organismal traits such as body size, body shape, and investment in structural material (Sterner and George 2000; Tanner et al. 2000; Hendrixson et al. 2007; McIntyre and Flecker 2010; El-Sabaawi et al. 2016), which may be constrained by species identity (Hendrixson et al. 2007) and ontogenetic trajectories (Boros et al. 2015). In perch, significant regressions between body stoichiometry and our index of lake trophic state (Secchi depth) suggests environmental factors, particularly ecosystem trophic state, are important in shaping white perch stoichiometry. However, comporting to our predictions, variation in perch body stoichiometry was often better explained by trophic traits that diverged quickly in response to lake conditions. Thus, in perch, the greatest role for environmental factors in producing consistent patterns of body stoichiometry may be via their ultimate effects on population trophic traits compared to their proximal effects on individuals. This was shown by the particularly strong correlations between a suite of trophic traits and body stoichiometry.
Trophic state is strongly related to nutrient availability and also mean depth in lakes, which influences community and ecosystem processes that govern resource availability and the distribution and profitability of prey (Fox 2007). In unproductive lakes perch were streamlined, had narrow-spaced gill rakers and shorter fins, consistent with adaptation to foraging in pelagic habitats. As lakes became more eutrophic, perch body shape became more robust and deeper with wider spaced gill rakers and longer fins with pronounced fin rays; this is consistent with traits associated with greater benthic foraging and, possibly, predator defense (El-Sabaawi et al. 2016). In our previous work, we showed that Secchi depth, an indicator of trophic state, was a much better predictor of these phenotypes than any one biotic or abiotic factor, including mean lake depth and littoral area (Tuckett et al. 2013). The relationship between feeding and locomotor traits is well known and represents a suite of correlated traits, where a streamlined body shape is more efficient at foraging in open water for spatially distributed prey and a robust body shape with larger fins is more efficient at foraging in complex habitats like the littoral zone of lakes (Webb 1984; Robinson et al. 1993; Blake 2004). Ultimately, these patterns of morphology are consistent with our prior observations of these populations and support the hypothesis that populations can diverge in traits expected to shape body stoichiometry and excretion (Tuckett et al. 2013).
McIntyre and Flecker (2010) hypothesized that elemental phenotypes in fish are also driven by tradeoffs between investment in bone (high P) and muscle (high N) due to sedentary (low N:P) versus mobile (high N:P) lifestyles. Support for this hypothesis in our data can be found not only in the strong mean trophic trait–element correlations, but also in observed patterns of allometric scaling of N, P, and N:P. Typical of lentic fishes, white perch juveniles occupy pelagic habitats, feeding on rotifers and switching to larger zooplankton and benthic invertebrates as they mature (Stanley and Danie 1983). Allometric niche shifts are mirrored in body shape changes as perch transition from streamlined to rounded body shape, more typical of a benthic or littoral lifestyle (Webb 1978, 1984). In more oligotrophic lakes, perch are slower to undergo this transition and retain the streamlined body shape and other trophic features (e.g., gill rakers) adapted for cruising the water column for pelagic prey (Tuckett et al. 2013), including zooplankton and eventually fish. These putative pelagic-cruising fish had higher mean body N:P and shallow or negative allometric slopes in body chemistry which might indicate neoteny (i.e., juvenile traits in adults) and retention of pelagic trophic morphology. Conversely, we would expect productive lakes to favor a more sedentary benthic lifestyle as fish mature; this is also consistent with our results. This correlated stoichiometric and trophic divergence may have a contemporary origin because body shape closely tracks lake Secchi depth (r 2 = 0.93) and several lakes in our study have undergone eutrophication with increased nutrients and large declines in Secchi depth over the past few decades (Tuckett et al. 2013).
Although we did not directly include Merrymeeting Bay fish in many analyses due to their unique habitat, they may be an exception that proves the rule when it comes to stoichiometric associations with body shape and mobility. These fish exhibited benthic trophic morphology more extreme than fishes originating from the most productive source lakes (see also Tuckett et al. 2013). This is expected as estuarine fish are commonly more gibbose and exhibit greater benthic feeding than their lentic counterparts (Berner et al. 2008). Here, estuarine perch exhibited high body % P and low % N and N:P, similar to fish from eutrophic lakes. However, allometric scaling for body shape and % P for these estuarine fish were opposite to those of fish from productive lakes (i.e., fish became more streamlined and lower in % P), which may be expected for estuarine fishes that transition from tidal creeks and near-shore habitats as juveniles to deep, swift estuarine channels and piscivores as adults (Able and Fahay 1998). Ultimately, we were unable to identify the exact proximate reason for lower % P in streamlined individuals and populations; yet, it could be related to all or a combination of several factors, including reduced fins, less slab-sided morphology and even reduced investment in bone and defensive traits (Tanner et al. 2000; Hendrixson et al. 2007; Vrede et al. 2011; El-Sabaawi et al. 2016), as Ca, a proxy for bone, explained 73 % of the individual variation in body P.
Variation in excretion and eco-evolutionary feedbacks
Much like body element content, excretion of N and P was highly variable across populations of white perch relative to variation among species. For example, individual ammonia-N excretion across perch populations was 95 % of the mean range reported for 47 species representing 7 trophic guilds (McIntyre et al. 2008). Population source explained 10–48 % of the variation in individual excretion rates, but excretion was not as clearly tied to environmental and organismal determinants as body stoichiometry. Previously, we showed that perch elemental phenotypes were correlated to trophic phenotypes which are dictated by landscape variation in ecosystem trophic state. Next, to examine a possible eco-evolutionary feedback, we examined the causes of variation in perch excretion and whether it was related to trophic state or elemental phenotypes. Perch N, but not P excretion increased with lake TN, somewhat consistent with a study showing fish excretion increases along a productivity gradient; however, the increase in fish excretion was attributed to altered population biomass (Vanni et al. 2006). In particular, the increase in body % P and decrease in body % N and N:P as lakes became more eutrophic should have led to decreased relative P excretion and increased N excretion. Indeed, body elemental content explained little variation in N and P excretion rates and ratios. Interestingly, among species, excretion is well correlated with body elemental content and body N:P has been shown to be inversely related to excretion N:P (Vanni et al. 2002).
From an organismal trait perspective, metabolic and ecological stoichiometry theory suggests nutrient excretion should be driven by size, growth rate, and body and diet elemental content (Sterner and Elser 2002; Brown et al. 2004; Allgeier et al. 2015). Excretion rate increased with body size as in other studies (Hall et al. 2007; McIntyre and Flecker 2010) with scaling less than 1, but not consistently with 3/4 power scaling predicted by metabolic theory. Given the large changes in body N and P in perch, we expected to see a commensurate shift in excretion rate and ratio, but this generally did not occur. Ultimately, as has been found in other studies (Allgeier et al. 2015; Tuckett et al. 2015), our data suggest, compared to body size, stoichiometric variation in body composition does not simply translate to altered recycling stoichiometry in this study system over shortened timescales. These body size considerations will be important moving forward because of its strong relationship to excretion and as climate change, size-selective fishing, and other anthropogenic activities alter the size–structure of fishes (Shackell et al. 2010; Layman et al. 2011; Allgeier et al. 2015; Tuckett et al. 2015).
Diet shifts are also associated with trophic divergence and may offset or mask body content effects. The lack of clear results may be due to the complexity of variables influencing excretion, some of which were not measured here (e.g., assimilation efficiency leading to egestion, diet stoichiometry, etc.). For example, Trinidadian guppies alter the balance of nutrients consumed, assimilated, and excreted due to predator cues (Dalton and Flecker 2014). Further, as we would expect, diet stoichiometry is strongly related to excretion (Moody et al. 2015). Here, we show that perch body stoichiometry is highly variable, contrary to assumptions of homeostatic regulation; thus, we cannot assume the same for dietary items which would also need to be analyzed for each lake (but see Frost et al. 2002). Ultimately, there may be difficulty in accurately quantifying diet in a way that matches the measurement of excretion where an animal is not likely to be excreting what is in the stomach, but instead excretes a metabolically averaged product of its assimilated past diet. Thus, laboratory studies may be more useful in examining the relationship between diet and excretion stoichiometry.
Implications
There is growing recognition that a diverse group of animals can influence biogeochemical processes in aquatic ecosystems via their roles in nutrient storage and CDNR (Post et al. 1998; McIntyre et al. 2008). Typically these studies consider the effects of interspecific variation, with a few notable exceptions for intraspecific body size and life history variation (Vanni et al. 2002; Hall et al. 2007; McIntyre et al. 2008; Post and Palkovacs 2009; Bassar et al. 2010), and usually consider only body composition or excretion in relation to traits or environmental features. Our study is unique in simultaneously assessing intraspecific variation in body elemental content and excretion to one another, to trait divergence and to landscape variation in environmental conditions. Moreover, we do so for populations wherein current patterns of phenotypic divergence have originated in contemporary time. Our data suggest population divergence in response to lake trophic state leads to coherent patterns in elemental phenotypes via trophic adaptations. Theoretically, this may set the stage for eco-evolutionary feedbacks such that contemporary trait change influences ecosystem processes through stoichiometric linkages (Palkovacs et al. 2009; Bassar et al. 2010). We found limited support for a potential eco-evolutionary linkage via CDNR effects of perch, given that perch excretion was not consistently predicted by lake trophic state or trophic trait variation. However, eco-evolutionary dynamics may in many cases be cryptic and their outcomes difficult to detect (Kinnison et al. 2015), perhaps even leading to stability as elevated trophic state leads to greater body P which suppressed P excretion. Regardless, other CDNR pathways may exist for future study, pathways that could alter the pool of available nutrients, including the potential for stoichiometrically divergent fish to act as nutrient sinks or prey. Still, we not only show that stoichiometric diversity can be large within a species, approaching interspecific scales, but also provide mechanistic support for how variation can arise quickly and predictably through the effects of landscape-scale environmental drivers on trophic divergence.
References
Able K, Fahay M (1998) First year in the life of estuarine fishes in the middle Atlantic Bight. Rutgers University Press, New Brunswick
Abràmoff MD, Magalhães PJ, Ram SJ (2004) Image processing with ImageJ. Biophotonics Int 11:36–42
Allgeier JE, Wenger SJ, Rosemond AD, Schindler DE, Layman CA (2015) Metabolic theory and taxonomic identity predict nutrient recycling in a diverse food web. Proc Natl Acad Sci 112:E2640–E2647
APHA (2005) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington, D.C.
Bassar RD, Marshall MC, López-Sepulcre A, Zandonà E, Auer SK, Travis J, Pringle CM, Flecker AS, Thomas SA, Fraser DF, Reznick DN (2010) Local adaptation in Trinidadian guppies alters ecosystem processes. Proc Natl Acad Sci USA 107:3616–3621
Berner D, Adams DC, Grandchamp A-C, Hendry AP (2008) Natural selection drives patterns of lake-stream divergence in stickleback foraging morphology. J Evol Biol 21:1653–1665
Blake R (2004) Fish functional design and swimming performance. J Fish Biol 65:1193–1222
Boros G, Sály P, Vanni MJ (2015) Ontogenetic variation in the body stoichiometry of two fish species. Oecologia 179:329–341
Brown JH, Gupta VK, Li B-L, Milne BT, Restrepo C, West GB (2002) The fractal nature of nature: power laws, ecological complexity and biodiversity. Philosophical transactions of the Royal Society of London. Ser B Biol Sci 357:619–626
Brown JH, Gillooly JF, Allen AP, Savage VM, West GB (2004) Toward a metabolic theory of ecology. Ecology 85:1771–1789
Busch W-DN, Davies DH, Nepszy SJ (1977) Establishment of White Perch, Morone americana, in Lake Erie. J Fish Res Board Can 34:1039–1041
Carpenter SR, Kitchell JF (1996) The trophic cascade in lakes. Cambridge University Press, New York
Dalton CM, Flecker AS (2014) Metabolic stoichiometry and the ecology of fear in Trinidadian guppies: consequences for life histories and stream ecosystems. Oecologia 176:691–701
El-Sabaawi RW, Kohler TJ, Zandoná E, Travis J, Marshall MC, Thomas SA, Reznick DN, Walsh M, Gilliam JF, Pringle C, Flecker AS (2012a) Environmental and organismal predictors of intraspecific variation in the stoichiometry of a neotropical freshwater fish. PLoS One 7:e32713
El-Sabaawi RW, Zandonà E, Kohler TJ, Marshall MC, Moslemi JM, Travis J, López-Sepulcre A, Ferriére R, Pringle CM, Thomas SA, Reznick DN, Flecker AS (2012b) Widespread intraspecific organismal stoichiometry among populations of the Trinidadian guppy. Funct Ecol 26:666–676
El-Sabaawi RW, Travis J, Zandonà E, McIntyre PB, Reznick DN, Flecker A (2014) Intraspecific variability modulates interspecific variability in animal organismal stoichiometry. Ecol Evol 4:1505–1515
El-Sabaawi RW, Warbanski ML, Rudman SM, Hovel R, Matthews B (2016) Investment in boney defensive traits alters organismal stoichiometry and excretion in fish. Oecologia 181:1209–1220
Elser J, Elser M, MacKay N, Carpenter S (1988) Zooplankton-mediated transitions between N-and P-limited algal growth. Limnol Oceanogr 33:1–14
Elser JJ, Sterner RW, Gorokhova E, Fagan WF, Markow TA, Cotner JB, Harrison JF, Hobbie SE, Odell GM, Weider LW (2000) Biological stoichiometry from genes to ecosystems. Ecol Lett 3:540–550
Flecker AS, McIntyre PB, Moore JW, Anderson JT, Taylor BW, Hall RO Jr (2010) Migratory fishes as material and process subsidies in riverine ecosystems. Am Fish Soc Symp 73:559–592
Fox JW (2007) The dynamics of top-down and bottom-up effects in food webs of varying prey diversity, composition, and productivity. Oikos 116:189–200
Frost PC, Stelzer RS, Lamberti GA, Elser JJ (2002) Ecological stoichiometry of trophic interactions in the benthos: understanding the role of C: N: P ratios in lentic and lotic habitats. J N Am Benthol Soc 21:515–528
Hall Jr RO, Koch BJ, Marshall MC, Taylor BW, Tronstad LM (2007) How body size mediates the role of animals in nutrient cycling in aquatic ecosystems. In: Hildrew AG, Edmonds-Brown R, Raffaelli D (eds) Body size: the structure and function of aquatic ecosystems. Cambridge University Press, New York, pp 286–305
Halliwell DB (2005) Introduced fish of maine. Maine Department of Environmental Protection, MAPB Series: Focus on Freshwater Biodiversity, Augusta
Harmon LJ, Matthews B, Des Roches S, Chase JM, Shurin JB, Schluter D (2009) Evolutionary diversification in stickleback affects ecosystem functioning. Nature 458:1167–1170
Hendrixson HA, Sterner RW, Kay AD (2007) Elemental stoichiometry of freshwater fishes in relation to phylogeny, allometry and ecology. J Fish Biol 70:121–140
Hergenrader GL, Bliss QP (1971) The white perch in Nebraska. Trans Am Fish Soc 100:734–738
Jeyasingh PD, Cothran RD, Tobler M (2014) Testing the ecological consequences of evolutionary change using elements. Ecol Evol 4:528–538
Kinnison MT, Hairston NG, Hendry AP (2015) Cryptic eco-evolutionary dynamics. Ann N Y Acad Sci 1360:120–144
Knoll LB, McIntyre PB, Vanni MJ, Flecker AS (2009) Feedbacks of consumer nutrient recycling on producer biomass and stoichiometry: separating direct and indirect effects. Oikos 118:1732–1742
Layman CA, Allgeier JE, Rosemond AD, Dahlgren C, Yeager LA (2011) Marine fisheries declines viewed upside down: human impacts on consumer-driven nutrient recycling. Ecol Appl 21:343–349
McIntyre PB, Flecker AS (2010) Ecological stoichiometry as an integrative framework in stream fish ecology. Am Fish Soc Symp 73:539–558
McIntyre PB, Flecker AS, Vanni MJ, Hood JM, Taylor BW, Thomas SA (2008) Fish distributions and nutrient cycling in streams: can fish create biogeochemical hotspots? Ecology 89:2335–2346
Meyer J, Schultz E, Helfman G (1983) Fish schools: an asset to corals. Science (New York, NY) 256:1–3
Moody EK, Corman JR, Elser JJ, Sabo JL (2015) Diet composition affects the rate and N:P ratio of fish excretion. Freshw Biol 60:456–465
Nilsson PA, Brönmark C (2000) Prey vulnerability to a gape-size limited predator: behavioural and morphological impacts on northern pike piscivory. Oikos 88:539–546
Palkovacs EP, Marshall MC, Lamphere BA, Lynch BR, Weese DJ, Fraser DF, Reznick DN, Pringle CM, Kinnison MT (2009) Experimental evaluation of evolution and coevolution as agents of ecosystem change in Trinidadian streams. Philosophical transactions of the Royal Society of London. Ser B Biol Sci 364:1617–1628
Post DM, Palkovacs EP (2009) Eco-evolutionary feedbacks in community and ecosystem ecology: interactions between the ecological theatre and the evolutionary play. Philosophical transactions of the Royal Society of London. Ser B Biol Sci 364:1629–1640
Post D, Taylor J, Kitchell J (1998) The role of migratory waterfowl as nutrient vectors in a managed wetland. Conserv Biol 12:910–920
Post DM, Palkovacs EP, Schielke EG, Dodson SI (2008) Intraspecific variation in a predator affects community structure and cascading trophic interactions. Ecology 89:2019–2032
Ricker W (1992) Back-calculation of fish lengths based on proportionality between scale and length increments. Can J Fish Aquat Sci 49:1018–1026
Robinson BW, Wilson DS, Margosian AS, Lotito PT (1993) Ecological and morphological differentiation of pumpkinseed sunfish in lakes without bluegill sunfish. Evol Ecol 7:451–464
Rohlf FJ (2004) TpsDig. Version 2.0. Available at: http://life.bio.sunysb.edu/ee/rohlf/software.html. Department of Ecology and Evolution, SUNY, New York
Rohlf FJJ (2005) TpsRelw. Version 1.31. Available at: http://life.bio.sunysb.edu/ee/rohlf/software.html. Department of Ecology and Evolution, SUNY, New York
Schaus M, Vanni M, Wissing T, Bremigan M, Garvey J, Stein R (1997) Nitrogen and phosphorus excretion by detritivorous gizzard shad in a reservoir ecosystem. Limnol Oceanogr 42:1386–1397
Schluter D (2000) The ecology of adaptive radiation. Oxford University Press, Oxford
Sereda JM, Hudson JJ, Taylor WD, Demers E (2008) Fish as sources and sinks of nutrients in lakes. Freshw Biol 53:278–289
Shackell NL, Frank KT, Fisher JAD, Petrie B, Leggett WC (2010) Decline in top predator body size and changing climate alter trophic structure in an oceanic ecosystem. Proc R Soc B Biol Sci 277:1353
Skulason S, Smith TB (1995) Resource polymorphisms in vertebrates. Trends Ecol Evol 10:366–370
Stanley JG, Danie DS (1983) Species profiles: life histories and environmental requirements of coastal fishes and invertebrates (North Atlantic)-white perch. FWS/OBS-82/II.7. National Coastal Ecosystems Team, Division of Biological Services, U.S. Fish and Wildlife Service, Washington, DC
Sterner RW, Elser JJ (2002) Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princeton University Press, Princeton
Sterner RW, George NB (2000) Carbon, nitrogen, and phosphorus stoichiometry of cyprinid fishes. Ecology 81:127–140
Svanbäck R, Eklöv P (2002) Effects of habitat and food resources on morphology and ontogenetic growth trajectories in perch. Oecologia 131:61–70
Tanner DK, Brazner JC, Brady VJ (2000) Factors influencing carbon, nitrogen, and phosphorus content of fish from a Lake Superior coastal wetland. Can J Fish Aquat Sci 57:1243–1251
Taylor EB (1999) Species pairs of north temperate freshwater fishes: evolution, taxonomy, and conservation. Rev Fish Biol Fish 9:299–324
Torres LE, Vanni MJ (2007) Stoichiometry of nutrient excretion by fish: interspecific variation in a hypereutrophic lake. Oikos 116:259–270
Tuckett QM, Simon KS, Saros JE, Halliwell DB, Kinnison MT (2013) Fish trophic divergence along a lake productivity gradient revealed by historic patterns of invasion and eutrophication. Freshw Biol 58:2517–2531
Tuckett QM, Simon KS, Saros JE, Coghlan SMJ, Kinnison MT (2015) Biomass versus biodiversity: the relative contribution of population attributes to consumer nutrient loading in aquatic systems. Evol Ecol Res 16:705–723
Turesson H, Brönmark C (2007) Predator-prey encounter rates in freshwater piscivores: effects of prey density and water transparency. Oecologia 153:281–290
Vanni MJ (2002) Nutrient cycling by animals in freshwater ecosystems. Annu Rev Ecol Syst 33:341–370
Vanni MJ, Flecker AS, Hood JM, Headworth JL (2002) Stoichiometry of nutrient recycling by vertebrates in a tropical stream: linking species identity and ecosystem processes. Ecol Lett 5:285–293
Vanni MJ, Bowling AM, Dickman EM, Hale RS, Higgins KA, Horgan MJ, Knoll LB, Renwick WH, Stein RA (2006) Nutrient cycling by fish supports relatively more primary production as lake productivity increases. Ecology 87:1696–1709
Vanni M, Boros G, McIntyre P (2013) When are fish sources vs. sinks of nutrients in lake ecosystems? Ecology 94:2195–2206
Vrede T, Drakare S, Eklöv P, Hein A, Liess A, Olsson J, Persson J, Quevedo M, Stabo HR, Svanbäck R (2011) Ecological stoichiometry of Eurasian perch—intraspecific variation due to size, habitat and diet. Oikos 120:886–896
Webb PW (1978) Fast-start performance and body form in seven species of teleost fish. J Exp Biol 74:311–326
Webb PW (1984) Body form, locomotion and foraging in aquatic vertebrates. Integr Comp Biol 24:107–120
Whiles MR, Huryn AD, Taylor BW, Reeve JD (2009) Influence of handling stress and fasting on estimates of ammonium excretion by tadpoles and fish: recommendations for designing excretion experiments. Limnol Oceanogr: Method 7:1–7
Acknowledgments
Many people contributed to this project including Dennis Anderson, Joe Dembeck, Joe Glowa, David Halliwell, John Reynolds and Wes Wright. This project was funded in part by the U.S. Environmental Protection Agency (EPA) under Section 319 of the Clean Water Act. This publication has not been formally reviewed by the EPA and the views expressed are solely those of the authors. Additional support was provided by the Maine Agricultural and Forest Experiment Station (Contribution # 3499) and the National Science Foundation through a Doctoral Dissertation Improvement Grant (DEB 1011267).
Author contribution statement
QMT, MTK, JES and KSS designed the experiments. QMT and KSS performed the experiments. QMT analyzed the data. QMT, MTK, JES and KSS wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Additional information
Communicated by Craig A. Layman.
Rights and permissions
About this article
Cite this article
Tuckett, Q.M., Kinnison, M.T., Saros, J.E. et al. Population divergence in fish elemental phenotypes associated with trophic phenotypes and lake trophic state. Oecologia 182, 765–778 (2016). https://doi.org/10.1007/s00442-016-3714-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-016-3714-2