Abstract
Life-history trade-offs and the costs of reproduction are central concepts in evolution and ecology. Episodic climatic events such as drought and extreme temperatures provide strong selective pressures that can change the balance of these costs and trade-offs. We used size-structured matrix models parameterized from field and laboratory studies to examine the effect of periodic drought on two species of aquatic salamanders (greater siren, Siren lacertina; lesser siren, Siren intermedia) that differ in size at reproduction and maximum body size. Post-drought body size distributions of the larger species (S. lacertina) are consistent with size-dependent mortality. Smaller individuals were extirpated from the population during each drought while large animals persisted, a pattern that contrasted with that seen in several ectotherms. This appears to be largely explained by estivation proficiency and a positive relationship between body size and estivation potential. Increased body size, however, may come at the cost of fecundity and maturation rate compared to a closely related congener. The cost of somatic allocation in this case may manifest itself via reduced per-capita competitive ability, which (at least in simulation studies) allows the smaller, fast-maturing species to outcompete the larger, slow-maturing species when drought is minimal or nonexistent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Animal body size is under strong selection because it determines several life-history attributes and is shaped by divergent and contrasting evolutionary pressures (e.g., reproduction and survival, Blueweiss et al. 1978; Peters 1983; Wikelski and Trillmich 1997; Nagel and Schluter 1998; Winne et al. 2010). Because reproductive output rather than somatic growth maximizes fitness (Gibbons et al. 1981; Brown and Sibly 2006), increased body size at maturity is costly unless it confers additional benefits, (e.g., increased survival, fecundity, or resistance to environmental extremes; Peters 1983; Kingsolver and Pfennig 2004).
Trade-offs between reproduction and future survival (Williams 1966) ultimately shape life-history variables such as growth, maturity, and fecundity in response to habitat suitability and predictability. In temporally varying environments, optimal life-history strategies are especially sensitive to conditions affecting age-specific or size-specific growth or mortality (e.g., Werner 1986; Taborsky et al. 2003). In fluctuating environments, the relative strengths of size-dependent and size-independent mortality can lead to antagonistic selection pressures between early maturation and large body size through delayed maturation (Stearns 1976; Roff 1992; Brown and Sibly 2006). High size-independent mortality selects for early maturation because it minimizes the likelihood of failing to reach maturity prior to death, whereas negative size selection (selection for larger animals) would confer an advantage to animals that grow quickly to a minimum “refuge size” (e.g., Nakaoka 1998) at the cost of reproduction (Brown and Sibly 2006).
Severe climatic episodes offer temporally strong selection pressure on body size and affect cost of reproduction trade-offs (Brown and Brown 1998; Clutton-Brock and Pemberton 2004). Episodic selection events (e.g., drought) present chronic selection pressures and are expected to increase in severity and frequency in some freshwater systems under climate change (e.g., Overpeck and Udall 2010). Climate change and increased drought are expected to select against larger-bodied animals because of increased metabolic costs associated with elevated temperatures and drought-induced alteration of food chain structure (Sheridan and Bickford 2011; Brose et al. 2012; Woodward et al. 2012). However, body size interacts with other factors that determine survival under periodic perturbations (e.g., dispersal ability, estivation; Gehlbach et al. 1973; Willson et al. 2006; Bårdsen et al. 2011).
Aquatic and semi-aquatic animals that inhabit isolated wetlands undergoing drought emigrate or estivate with varying degrees of success (Gibbons et al. 1983; Withers 1993; Willson et al. 2006; Buhlmann et al. 2009; Luhring and Todd 2010; Luhring et al. 2011). For animals that are unable to disperse during times of drought (e.g., Schalk and Luhring 2010), the ability to withstand environmental extremes (environmental resistance) is at a premium. Isolated populations of animals experiencing periodic environmental extremes (e.g., Wikelski and Trillmich 1997; Winne et al. 2010) provide an especially powerful insight into within-population size-dependent mortality as a function of environmental extremes.
To investigate in greater mechanistic detail how trade-offs between body size and reproduction could affect the persistence of nondispersing animals under drought conditions, we conducted an analysis that combines empirical data with a species-specific size-structured demographic model of salamander population dynamics. For the empirical portion, we drew on demographic data from field studies and size-dependent estivation potential derived from laboratory studies (Etheridge 1990). These models simulate the relative success of divergent life-history strategies in two closely related and sympatric species across a continuum of hydrological conditions and competition scenarios. Differences in persistence rates between large and small species provide fundamental insights into interactions among life-history strategies, environmental stochasticity, and body size.
Following model construction, we used hydrologic and demographic data from past and ongoing research at the Savannah River Ecology Laboratory in Aiken, South Carolina to examine the effects of three droughts (1989–1990, 2001–2002, and 2011–2013) on aquatic salamander population demographics. We used field data from 1993, 2006–2011, and 2014 to evaluate the effects of these droughts on subsequent size-class distributions and to compare model outputs to the post-drought size distributions of wild populations.
Materials and methods
The SIREN model (see the Electronic supplementary material for the corresponding Matlab source code) simulates two well-mixed salamander populations (differing in size at maturation) in an isolated wetland over a 5000-year period. It is a discrete-time model (with a 1-year time step), with size-specific survival, growth, and fecundity modeled through a size-structured Leslie matrix in Matlab (R2009a, The MathWorks, Natick, MA, USA). Drought severity and species-specific competition strength between the two populations are varied systematically (described further below) to test effects on population persistence caused by differences in life-history strategies. A subsequent set of single-species simulations was run with varying demographic parameters to test model output sensitivity to differing values of mass, annual survivorship, and fecundity.
At each time step, a random drought length was drawn from an exponential distribution with mean h (in years). Values of h ranged between 0 and 0.3—an upper limit that led to deterministic extinction for each species. Drought length was then converted to days and discretized into three drought severity classes (no drought—demographic parameters unaffected; short drought—some mortality, growth and reproduction unaffected; and long—no growth, no reproduction), each with its own transition matrix. Drought severity class-specific transition matrices (Eqs. 1, 2, 3, 4, 5, 6) were constructed for six (S. lacertina) or four (S. intermedia) size classes and used according to the drought severity class (discussed below) for each time step:
Species matrices (L: S. lacertina; l: S. intermedia) with subscripts n, sd, and ld represent transition matrices for non-drought, short-drought, and long-drought years, respectively (Eqs. 1, 2, 3, 4, 5, 6). P i and p i denote non-drought survival,Pd i and pd i denote size-dependent drought survival, and F i and f i denote fecundity for size class i (capitalized and lowercase letters for S. lacertina and S. intermedia, respectively). Because short drying episodes are common in isolated wetlands and generally do not kill yearlings (Luhring and Todd 2010), no drought-induced size-dependent survivorship occurs during drying events shorter than 60 days (Eqs. 1, 4). For short droughts lasting between 60 and 250 days (wetland holds water long enough for growth), drought survival is assumed to be size dependent, and recruitment only occurs when the drought is short enough for size-class 0 juveniles to survive and grow into the next size class (Eqs. 2 and 5, where j(t) is a binomial indicator of whether juveniles can survive into the next year). We used the intercept of the body-mass-dependent drought survival equation (see Eq. 9 below), equal to 145 days, as the maximum length survivable by size class 0 juveniles. For long droughts (i.e., wetland fills for a very short period, or not at all), drought survival was assumed to be size-dependent, and no recruitment or growth into the next size class occurred (Eqs. 3, 6).
As body size (not age) determines estivation potential (Etheridge 1990) and thus ability to survive droughts, we grouped animals by size (although age and size classes are equal in some cases). We added a density-dependent term G(t) for S. lacertina or g(t) for S. intermedia to the fecundity terms (Caswell 2001), given by:
Here, N(t) and n(t) are equal to the total abundances of non-hatchling lesser and greater sirens, respectively, at time t (Eqs. 7a, 7b). The variable B is a system-wide constant that determines the strength of density dependence, selected to prevent unchecked exponential population growth. Competition coefficients (CC) convert the number of heterospecifics into effective conspecific competitors (CC12: lesser into greater sirens; CC21: greater into lesser sirens) for simulations that included competition (Eqs. 7a, 7b; discussed further in “Competition models”).
There are few age/size-specific estimates for survival or fecundity for sirenids. We estimated survival values that mimic a type-III survivorship curve, typical of animals (such as amphibians) that experience high levels of mortality in early life stages (Pearl and Miner 1935; Wells 2007; Table 1). Once individuals reached the largest size class, they were assumed to remain in that size class in subsequent years (minus annual death rate or drought extirpations). Fecundity estimates were based on estimates from populations in the Upper Coastal Plain of South Carolina (lesser siren: Sever et al. 1996; greater siren: Luhring, unpublished data).
We derived size classes for lesser sirens from a population in Texas (Gehlbach and Kennedy 1978). We used estimated greater siren size classes (in mm snout-vent length; SVL) from a well-studied population of marked S. lacertina (Luhring 2008, 2009) and converted these values to mass (in grams) via a regression equation relating mass to SVL (Eq. 8):
The mass of the 6th size class (540 g) is an underestimate of the maximum size of greater sirens (23 % of animals captured during the study were larger). However, larger animals are generally male (Luhring, unpublished data), and the growth of individually marked animals is largely curtailed after reaching the 6th size class (Luhring 2008).
We derived size-dependent drought survival values (P d or p d), which dictate the maximum drought length that a given size class can survive, from body mass M, based on data from laboratory-induced estivation experiments (Etheridge 1990):
We assumed a fixed probability of immigration (0.01) via flooding in non-drought years at each time step, and used it to generate a Bernoulli random deviate to determine if immigration occurred in a given year. Sirenids are poor overland dispersers (Schalk and Luhring 2010), and likely rely on temporary waterways to form during heavy rains in order to colonize isolated wetlands (Snodgrass, unpublished data). For time steps in which stochastic immigration did occur, a total of 1–50 immigrants (drawn randomly from a uniform distribution) were added to each size class. Immigration allowed our simulated wetlands to act as open populations and prevented periodic extinctions from causing permanent extirpation (enabling average persistence estimates across several extinction and colonization events). Because the effects of emigration would be small relative to the total population size, it was assumed that emigration and death were both captured in class-specific mortality rates. At the end of each time step, the total population size of non-hatchlings was recorded in the N or n matrices for greater and lesser sirens, respectively. We used the mean number of years with a nonzero population as a proxy for persistence probability.
Competition models
We added competition to the model by converting the heterospecific population into effective conspecifics via a CC. For example, a greater siren competition coefficient (CC21) of 0.5 means that 50 % of the greater siren population size is added to the total lesser siren population prior to calculating density for that time step. A lesser siren competition coefficient (CC12) of 1.0 would indicate that lesser sirens have a per-capita competition effect on greater sirens equal to that of other greater sirens. Asymmetric competition occurred when competition coefficients differed between species. Potential competition between these species is a realistic assumption as they have broadly overlapping diets, physiologically similar feeding mechanisms, and overlap in body size during the first 2 years of their lives.
We first ran the model with both siren types co-occurring in the simulated wetland, with varying CC and mean drought severity (h). We examined the combined effects of competition coefficients (0, 0.5 and 1.0) and mean drought severities (0, 0.05, 0.01… 0.30) on population persistence for each species. We ran each possible combination of greater siren CC, lesser siren CC, and h (total of 63 unique combinations) 100 times for 5000-year simulations with both species present.
Single-species sensitivity analysis
In addition to competition models, we performed a global sensitivity analysis on size-class demographic parameters (mass, survivorship, fecundity) to assess their effects on model output and the robustness of our predictions. We ran 1000 simulations of 5000-year increments for a single species at a fixed value of mean drought severity (h). We varied parameter values at the inflection point of persistence versus mean drought severity in the absence of competition (e.g., Fig. 1a). Persistence was most sensitive to the change in model parameter values at the inflection point and we wanted to explore model robustness in response to uncertainty in our parameter estimates. We screened model simulation outputs for runs in which parameters violated biological assumptions (e.g., survival > 1.0, fecundity < 0) and removed them prior to sensitivity analyses (1–2 % of runs). We regressed the response variable (persistence) on the parameter values (size-specific mass, survival, fecundity) using linear regressions conducted in R v. 2.7.2 (R Development Core Team 2008) to generate adjusted-R 2 values and regression slopes as metrics of parameter influence (Holdo et al. 2011). We conducted all simulations in Matlab® v. 7.8.0.347 (R2009a, The MathWorks).
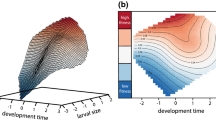
Predicted greater siren (filled circles) and lesser siren (open circles) persistence (mean years present during 100 simulations ± 1 SE) as a function of wetland drought length (years) and competition strength. Each graph represents simultaneous responses of both species to seven discrete simulated drought severities for a set combination of competition coefficients
Field data
We used individually measured greater siren body size data from two distinct trapping efforts at Dry Bay, an isolated wetland on the US Department of Energy’s Savannah River Site (SRS) in the Upper Coastal Plain of South Carolina, USA (1993, Tucker, unpublished data; 2006–2014, Luhring, unpublished data) to test the premise that prolonged drought events select against smaller body sizes in sirens. One of us (TML) used a variety of trap types (Luhring 2008; Luhring and Jennison 2008) to ensure consistent captures across size classes. Greater sirens, however, were not reliably captured until they reached the second size class (200 mm SVL). Dry Bay greater siren captures varied in their proximity to three severe droughts (1989–1990, 2001–2002, 2011–2013) 1.25–1.60 years in duration (McCleod, Lide, Luhring, unpublished data; Fig. 2a). To provide a common reference point of capture records across years, we plotted the body size of each capture as a function of time since the most recent severe drought. We used body-size distributions in June 2011 (Fig. 2b) as an approximation for pre-drought body-size distributions, as they were sampled 8.4 years after the most recent drought (2001–2002). We used growth rates from individually marked and recaptured sirens (Luhring 2008, 2009) and published young-of-year sizes (Goin 1947) to project the trajectories of size classes over time following drought (recruitment growth: dashed line; drought survivors: solid lines associated with numbered size-class circles; Fig. 2a). Size-class lines passing through a gap in recorded body sizes would indicate the loss of that size class during the previous drought (essentially back-casting growth rates to the most recent drought event). Histograms of size distributions across time were constructed with a reference line (dashed) to track the advancement of post-drought recruitment into the sixth size class (~6 years post-drought) and the eventual closure of size-class gaps in the absence of drought (Fig. 2b–g).
a Body sizes of greater sirens from a single population following severe droughts (three different droughts from 1989 to 2013). Horizontal dotted lines demarcate size classes, vertical dotted lines demarcate years since the last major drought. Numbered circles (a) correspond to lines projecting the growth of size classes following droughts (size-specific growth rates from marked individuals; Luhring 2008). Histograms (b–g) show body-size distributions of lettered boxes in main figure (a). The smallest two non-recruit size classes pass through the size-class gaps and indicate the loss of those size classes during each drought. Body-size distributions of animals prior to (b) and following (c–g) droughts show post-drought recruitment (vertical dashed lines indicate average projected size of post-drought recruits during sampling period) eventually closing the gap caused by drought-induced size-dependent mortality
Results
Competition models
Scenarios with any combination of CC of zero or intermediate (CC = 0.5) strength predicted no appreciable effect on species persistence (Fig. 1). When interspecific and heterospecific competition were equal (when CC = 1), however, the predicted patterns of extirpation changed. In all combinations of competition scenarios in which the smaller species had a CC equal to 1, it repeatedly extirpated the larger species at lower values of drought severity. When both species had CC’s equal to 1 (i.e., each species had an equal negative effect on the other that was equivalent to its own intraspecific competition), the smaller species frequently extirpated the larger species up to a certain value of drought severity, whereupon the larger species was predicted to be able to maintain a nonzero population in most years. The larger species was only able to exclude the smaller species at lower levels of drought severity when it exerted a disproportionately stronger and unidirectional competition effect (CC for larger species on smaller species = 1 and CC for smaller species on larger = 0).
Single-species sensitivity analysis
Sensitivity analyses on single-species models indicated that the maximum size reached had the strongest effect on model behavior at a fixed value of mean annual drought severity (h; Table 2). Other parameters with appreciable effects were related to life-history values of the largest size class for each species.
Field data
Body-size distributions following each of three severe droughts were consistent with the loss of the 1st and 2nd size classes (size-class lines pass through gaps in the data for 2014, 1993, and 2007; Fig. 2c–f, respectively) and are indicative of drought-induced size-specific mortality. The predicted growth of post-drought hatchlings following droughts (dashed line in Fig. 2a–g) corresponds with peaks in the lower range of size-class distributions (vertical dashed line in Fig. 2c–g), indicating that post-drought reproduction occurred and thus ruling out breeding failure as a potential cause of size-class gaps. At 4.0–5.5 years post-drought (Fig. 2g), recruits neared the sixth size class and size-class gaps were no longer evident.
Discussion
Optimal life-history strategies in our simulated wetlands varied with drought and competition intensity because of divergent pressures on maturation and growth. Fitness was optimized through fast maturation when perturbations were mild and infrequent (sensu Gibbons et al. 1981; Brown and Sibly 2006), as our fast-maturing small species (S. intermedia mature in the 2nd year and reach 120 g; Davis and Knapp 1953; Frese et al. 2003) outcompeted our larger, late-maturing species (S. lacertina mature in the 4th year or later and reach >1.25 kg; Luhring 2008; Fig. 1). However, as drought severity increased in our simulations, early maturation and smaller size led to more frequent drought-driven extirpations of S. intermedia and the subsequent release of larger and more drought-resistant S. lacertina from competition. These patterns are borne out in landscape distributions, with S. lacertina mostly inhabiting isolated and less permanent wetlands while being largely absent from connected permanent wetlands where S. intermedia are most abundant (Snodgrass et al. 1999; Luhring and Jennison 2008).
In single-species models (no competition), species persistence through droughts was most sensitive to maximum size reached for each species (Table 2). However, persistence of S. lacertina populations was also highly sensitive to fecundity and survivorship of the largest size class; a pattern seen in long-lived vertebrates (sensu lato Congdon et al. 1994). Unlike long-lived species with low fecundity that depend on high juvenile survival for recruitment (e.g., turtles, Congdon et al. 1994), large adult S. lacertina are highly fecund and their size permits them to occupy a size refuge that protects them from drought-induced mortality. This high fecundity and increased drought resistance enable large adults to serve as population reservoirs that can quickly repopulate wetlands following prolonged droughts. This “reservoir effect” is seen in a wild S. lacertina population going through droughts in three sequential decades (1990s, 2000s, 2010s) where drought-induced mortality of smaller individuals (Fig. 2a) is followed by a wave of recruitment after the drought ends (dashed line in Fig. 2a–g).
Size-specific drought survival has been documented for three vertebrate species which estivate in or near wetlands during droughts on the SRS (Order Squamata: black swamp snake, Seminatrix. pygaea, Willson et al. 2006; Order Testudines: chicken turtle, Deirochelys reticularia, Buhlmann et al. 2009; Order Caudata: S. lacertina, this study). In contrast to greater sirens, black swamp snakes (the smallest aquatic snake in North America), and chicken turtles (with their relatively fast maturation) exemplify the small body size and quick maturation usually predicted for organisms in fluctuating environments (Stearns 1976; Roff 1992). Both species experience increased mortality of larger individuals (primarily large females) during droughts (Buhlmann et al. 2009; Winne et al. 2010). These patterns of contrasting size-dependent mortality and life-history strategies of sympatric vertebrates led us to examine the possible explanation for this divergence.
Large animals with high energy reserves are predicted to be more resistant to food shortages (Peters 1983; Lindstedt and Boyce 1985). This contrasts with predictions that larger animals, while having a lower mass-specific metabolic demand (Dobson and Headrick 1995), have an increased total metabolic cost and fare poorly when resources are scarce (e.g., Wikelski and Trillmich 1997; Winne et al. 2010). One key facet of this apparent discrepancy is the degree to which body size inhibits or augments survival in lean times as a function of body mass composition. Black swamp snakes estivate in wetlands as do sirenids, but the largest individuals are females with high reproductive investment (live-bearing) that reproduce shortly before the onset of seasonal droughts and are left with few energy reserves to resist environmental extremes (Winne et al. 2010). Larger chicken turtles (adult females) likewise experience higher mortality during droughts (Buhlmann et al. 2009), which may be a function of the increased metabolic demand of larger individuals. Sirenids allocate an increasing proportion of their body mass to lipid storage as they grow larger (Etheridge 1990), which may permit lipid storage rates to outpace the increased metabolic demand associated with increased total body mass.
Although larger body size is also associated with increased fecundity in ectotherms, the fitness advantage conferred by increased fecundity through growth is only advantageous if lifetime fecundity likewise increases (Shine 1988). Because juvenile greater sirens are unlikely to survive a severe drought, female greater sirens would increase their lifetime fecundity and minimize the risk of complete reproductive failure by distributing reproductive activity across multiple years. Greater sirens maximize interannual survival in drought-prone habitats by growing rapidly to a size that protects them from drought mortality. However, growth at smaller sizes may come at the cost of fecundity. While an exceptionally large adult female in Alabama (Hanlin and Mount 1978) was estimated to have 1400 ova, greater sirens in their first years of reproduction (the smallest three greater sirens in Fig. 3) produce nearly half as many oocytes (74.3 vs. 140.3) as fully mature lesser sirens on the SRS, despite being nearly twice as long (273.7 vs 150.2 mm) (Sever et al. 1996; Fig. 3). While larger animals may indeed be more fecund, growth and reproduction trade-offs seen in sirenids appear to be driven by increased environmental resistance at the cost of relative fecundity.
Number of oocytes relative to body length (mm SVL) in mature female lesser sirens (open circles; N = 6; Sever et al. 1996) and greater sirens (filled circles; N = 4; Luhring, unpublished data)
Increasingly frequent and extreme floods and droughts resulting from global climate change are expected to have wide-ranging effects on amphibians (Walls et al. 2013 and references therein). Flood and drought regimes determine landscape-scale distributions of aquatic salamanders by affecting connectivity and species persistence (Snodgrass et al. 1999; Schalk and Luhring 2010, this study). Increased flooding would promote landscape connectivity of wetlands (Schalk and Luhring 2010) and enable the smaller species to colonize wetlands that previously would have been more isolated. This could potentially lead to local depression or extirpation of the larger species. However, in non-flood years, these newly colonized wetlands could serve as ecological traps for the smaller species under conditions of increased drought frequency and severity. Greater sirens may be able to resist increasingly severe droughts through delayed reproductive investment. However, delayed reproduction would increase vulnerability to competition from colonization waves (which would be more frequent with increased flooding) of the smaller species. Additionally, delayed reproduction would make recruitment increasingly dependent on successive years of suitable conditions to allow juveniles to reach size-based resistance to drought conditions.
Author contribution statement
TML conducted field work, conceived, designed, and executed the study and demographic models, and wrote the manuscript; RMH designed and advised on the use of quantitative analyses and edited the manuscript.
References
Bårdsen BJ, Henden JA, Fauchald P, Tveraa T, Stien A (2011) Plastic reproductive allocation as a buffer against environmental stochasticity–linking life history and population dynamics to climate. Oikos 120:245–257. doi:10.1111/j.1600-0706.2010.18597.x
Blueweiss L, Fox H, Kudzma V, Nakashima D, Peters R, Sams S (1978) Relationships between body size and some life history parameters. Oecologia 37:257–272. doi:10.1007/bf00344996
Brose U, Dunne JA, Montoya JM, Petchey OL, Schneider FD, Jacob U (2012) Climate change in size-structured ecosystems. Philos Trans R Soc B Biol Sci 367:2903–2912. doi:10.1098/rstb.2012.0232
Brown CR, Brown MB (1998) Intense natural selection on body size and wing and tail asymmetry in cliff swallows during severe weather. Evolution 52:1461–1475. doi:10.2307/2411315
Brown JH, Sibly RM (2006) Life-history evolution under a production constraint. Proc Natl Acad Sci USA 103:17595–17599. doi:10.1073/pnas.0608522103
Buhlmann KA, Congdon JD, Gibbons JW, Greene JL (2009) Ecology of chicken turtles (Deirochelys reticularia) in a seasonal wetland ecosystem: exploiting resource and refuge environments. Herpetologica 65:39–53. doi:10.1655/08-028R1.1
Caswell H (2001) Matrix population models, 2nd edn. Sinauer Associates, Sunderland
Clutton-Brock TH, Pemberton JM (2004) Soay sheep: dynamics and selection in an island population. Cambridge University Press, Cambridge. doi:10.1017/CBO9780511550669
Congdon JD, Dunham AE, Van Loben Sels RC (1994) Demographics of common snapping turtles (Chelydra serpentina): implications for conservation and management of long-lived organisms. Am Zool 34:397–408. doi:10.1093/icb/34.3.397
Davis WB, Knapp FT (1953) Notes on the salamander Siren intermedia. Copeia 1953:119–121. doi:10.2307/1440144
Dobson GP, Headrick JP (1995) Bioenergetic scaling: metabolic design and body-size constraints in mammals. Proc Natl Acad Sci USA 92:7317–7321. doi:10.1073/pnas.92.16.7317
Etheridge K (1990) The energetics of estivating sirenid salamanders (Siren lacertina and Pseudobranchus striatus). Herpetologica 46:407–414
Frese PW, Mathis A, Wilkinson R (2003) Population characteristics, growth, and spatial activity of Siren intermedia in an intensively managed wetland. Southwest Nat 48:534–542. doi: 10.1894/0038-4909(2003)048<0534:PCGASA>2.0.CO;2
Gehlbach FR, Kennedy SE (1978) Population ecology of a highly productive aquatic salamander (Siren intermedia). Southwest Nat 23:423–429. doi:10.2307/3670250
Gehlbach FR, Gordon R, Jordan JB (1973) Aestivation of the salamander, Siren intermedia. Am Midl Nat 89:455–463. doi:10.2307/2424051
Gibbons JW, Semlitsch RD, Greene JL, Schubauer JP (1981) Variation in age and size at maturity of the slider turtle (Pseudemys scripta). Am Nat 117:841–845. doi:10.1086/283774
Gibbons JW, Greene JL, Congdon JD (1983) Drought-related responses of aquatic turtle populations. J Herpetol 17:242–246. doi:10.2307/1563826
Goin CJ (1947) Notes on the eggs and early larvae of three Florida salamanders. Chic Acad Sci Nat Hist Misc 10:1–4
Hanlin HG, Mount RH (1978) Reproduction and activity of the greater siren, Siren lacertina (Amphibia: Sirenidae), in Alabama. J Ala Acad Sci 49:31–39
Holdo RM, Fryxell JM, Sinclair ARE, Dobson A, Holt RD (2011) Predicted impact of barriers to migration on the Serengeti wildebeest population. PLoS One 6:e16370. doi:10.1371/journal.pone.0016370
Kingsolver JG, Pfennig DW (2004) Individual-level selection as a cause of Cope’s rule of phyletic size increase. Evolution 58:1608–1612. doi:10.1554/04-003
Lindstedt SL, Boyce MS (1985) Seasonality, fasting, endurance, and body size in mammals. Am Nat 125:873–878. doi:10.1086/284385
Luhring TM (2008) Population ecology of greater siren, Siren lacertina. M.S. thesis. University of Georgia, Athens
Luhring TM (2009) Using PIT tags to evaluate non-individual-specific marks under field conditions: a case study with greater siren (Siren lacertina). Herpetol Rev 40:170–173
Luhring TM, Jennison CA (2008) A new stratified aquatic sampling technique for aquatic vertebrates. J Freshw Ecol 23:445–450. doi:10.1080/02705060.2008.9664222
Luhring TM, Todd BD (2010) Siren intermedia (lesser siren). Drought survival. Herpetol Rev 41:60
Luhring TM, Willson JD, Winne CT (2011) Nerodia fasciata (banded watersnake). Inter-wetland movement. Herpetol Rev 42:100–101
Nagel L, Schluter D (1998) Body size, natural selection, and speciation in sticklebacks. Evolution 52:209–218. doi:10.2307/2410936
Nakaoka M (1998) Optimal resource allocation of the marine bivalve Yoldia notabilis: the effects of size-limited reproductive capacity and size-dependent mortality. Evol Ecol 12:347–361
Overpeck J, Udall B (2010) Dry times ahead. Science 328:1642–1643. doi:10.1126/science.1186591
Pearl R, Miner JR (1935) Experimental studies on the duration of life. XIV. The comparative mortality of certain lower organisms. Q Rev Biol 10:60–79. doi:10.1086/394476
Peters RH (1983) The ecological implications of body size. Cambridge University Press, Cambridge. doi:10.1017/CBO9780511608551
Roff DA (1992) The evolution of life histories: theory and analysis. Chapman and Hall, New York
Schalk CM, Luhring TM (2010) Vagility of aquatic salamanders: implications for wetland connectivity. J Herpetol 44:104–109. doi:10.1670/08-312.1
Sever DM, Rania LC, Krenz JD (1996) Reproduction of the salamander Siren intermedia Le Conte with especial reference to oviducal anatomy and mode of fertilization. J Morphol 227:335–348. doi:10.1002/(SICI)1097-4687(199603)227:3<335:AID-JMOR5>3.0.CO;2-4
Sheridan JA, Bickford D (2011) Shrinking body size as an ecological response to climate change. Nat Clim Change 1:401–406. doi:10.1038/nclimate1259
Shine R (1988) The evolution of large body size in females: a critique of Darwin’s “fecundity advantage” model. Am Nat 131:124–131. doi:10.1086/284778
Snodgrass JW, Ackerman JW, Bryan ALJ, Burger J (1999) Influence of hydroperiod, isolation, and heterospecifics on the distribution of aquatic salamanders (Siren and Amphiuma) among depression wetlands. Copeia 1999:107–113. doi:10.2307/1447391
Stearns SC (1976) Life-history tactics: a review of the ideas. Q Rev Biol 51:3–47. doi:10.1086/409052
Taborsky B, Dieckmann U, Heino M (2003) Unexpected discontinuities in life-history evolution under size-dependent mortality. Proc R Soc Lond Ser B Biol Sci 270:713–721. doi:10.1098/rspb.2002.2255
Walls SC, Barichivich WJ, Brown ME (2013) Drought, deluge and declines: the impact of precipitation extremes on amphibians in a changing climate. Biology 2:399–418. doi:10.3390/biology2010399
Wells KD (2007) The ecology and behavior of amphibians. University of Chicago Press, Chicago. doi:10.7208/chicago/9780226893334.001.0001
Werner EE (1986) Amphibian metamorphosis: growth rate, predation risk, and the optimal size at transformation. Am Nat 128:319–341. doi:10.1086/284565
Wikelski M, Trillmich F (1997) Body size and sexual size dimorphism in marine iguanas fluctuate as a result of opposing natural and sexual selection: an island comparison. Evolution 51:922–936. doi:10.2307/2411166
Williams GC (1966) Natural selection costs of reproduction and a refinement of Lack’s principle. Am Nat 169:673–683. doi:10.1086/282461
Willson JD, Winne CT, Dorcas ME, Gibbons JW (2006) Post-drought responses of semi-aquatic snakes inhabiting an isolated wetland: insights on different strategies for persistence in a dynamic habitat. Wetlands 26:1071–1078. doi:10.1672/0277-5212(2006)26[1071:prossi]2.0.co;2
Winne CT, Willson JD, Gibbons JW (2010) Drought survival and reproduction impose contrasting selection pressures on maximum body size and sexual size dimorphism in a snake, Seminatrix pygaea. Oecologia 162:913–922. doi:10.1007/s00442-009-1513-8
Withers PC (1993) Metabolic depression during aestivation in the Australian frogs, Neobatrachus and Cyclorana. Aust J Zool 41:467–473. doi:10.1071/zo9930467
Woodward G, Brown LE, Edwards FK, Hudson LN, Milner AM, Reuman DC, Ledger ME (2012) Climate change impacts in multispecies systems: drought alters food web size structure in a field experiment. Philos Trans Roy Soc B Biol Sci 367:2990–2997. doi:10.1098/rstb.2012.0245
Acknowledgments
R. Semlitsch, S. Pittman, T. Meckley, and G. Connette provided helpful comments on earlier drafts of the manuscript. A. Tucker provided data from 1993 sampling of greater sirens. K. McCleod, and R. Lide provided wetland data. J. W. Gibbons, T. Tuberville, K. Buhlmann, S. Poppy, and the Savannah River Ecology Laboratory provided lab space, academic support, and materials used during parameter data collection. R. Cocroft and the IBM Model Class of 2010 at the University of Missouri provided suggestions and help during initial model development. The Trans-World Airlines Scholarship and Life Sciences Fellowship at the University of Missouri provided financial support to TML during the course of model development. This material is based on work supported by the American Museum of Natural History’s Theodore Roosevelt Memorial Fund (to TML), and by the Department of Energy under Award Number DE-FC09-07SR22506 to the University of Georgia Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Ross Andrew Alford.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Luhring, T.M., Holdo, R.M. Trade-offs between growth and maturation: the cost of reproduction for surviving environmental extremes. Oecologia 178, 723–732 (2015). https://doi.org/10.1007/s00442-015-3270-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-015-3270-1