Abstract
While the effects of drought and grazing are often studied separately, these disturbances co-occur in grasslands worldwide and interactively influence population, community, and ecosystem processes. The effects of drought and grazing on the belowground bud bank may dictate the trajectory of community recovery because new shoots arise from belowground buds after disturbance in perennial grasslands. We therefore investigated the separate and interactive effects of severe drought and grazing on the belowground bud bank and aboveground vegetation in the tallgrass prairie of northeast Kansas, USA. Contrary to our expectations, we observed changes in community structure and declines in species richness both above and below ground in response to drought and grazing. We also hypothesized that drought would reduce bud bank density of all taxonomic groups, but found that grass bud and shoot densities remained constant across all drought and grazing treatment combinations. While sedge and forb bud and shoot densities were reduced by drought, only sedge bud density declined to a greater extent when grazed under drought conditions. Live rhizome biomass did not vary by treatment and was highly correlated with bud bank density, suggesting that bud demography is tightly linked to the production and senescence of rhizomes. Despite the effects of drought and grazing on aboveground net primary productivity and community structure, our work suggests that grasses stabilize tallgrass prairie plant communities because their rhizomes and associated buds persist through co-occurring disturbances.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Grasslands and grass-dominated ecosystems cover nearly one-third of the earth’s surface, providing important habitat for a diverse flora and fauna, delivering important ecosystem services, and supporting a large human population (Curtin and Western 2008). However, much of the native grassland in central North America has been lost to agriculture (Samson and Knopf 1994), and remnant grasslands are being degraded by intensive cattle grazing (McNaughton 1993; Beschta et al. 2013). Humans have replaced many of the native grazers with domesticated grazers such as cattle, sheep, and goats, often altering not only the identity of the grazers but also their density, spatial distribution, and timing (McNaughton 1993; Frank and Groffman 1998). While grassland vegetation tolerates grazing because of the long, shared evolutionary history with grazers (Nilsson et al. 1996; Anderson 2006), altered grazing regimes may exacerbate damage from other co-occurring disturbances, such as extreme drought.
Drought severity is expected to increase in grasslands worldwide as a result of human activities (Min et al. 2011). Recent studies predict changes in temperature and precipitation amount and timing in the Great Plains of North America and globally (Patricola and Cook 2013). Therefore, a mechanistic understanding of vegetation response to extreme drought and grazing will allow land managers to adaptively manage grazers and promote long-term sustainability of grassland systems.
Both grazing and drought cause shifts in grassland vegetation. The effects of large ungulate grazers depend on grazing intensity, evolutionary history, and precipitation. In tallgrass prairie, moderate grazing intensity increases spatial heterogeneity and species diversity through selective herbivory, mechanical disturbance, redistribution of nutrients, and seed dispersal (Hartnett et al. 1996; Biondini et al. 1998). By preferentially feeding on grasses, large ungulate grazers in tallgrass prairie release unpalatable forbs and other subdominant plant species from competitive suppression by the dominant C4 grasses, resulting in increased forb diversity and abundance (Augustine and McNaughton 1998; Towne et al. 2005). Grazing in tallgrass prairie also reduces density of the bud bank (Dalgleish and Hartnett 2009), the source of most aboveground shoots in both undisturbed and disturbed areas of this grassland (Rogers and Hartnett 2001; Benson and Hartnett 2006) and an important component in plant response to herbivory (Nilsson et al. 1996).
Plant growth and survival during periods of drought is influenced by several key traits, including photosynthetic pathway, phenology, hydraulic architecture, morphology, and rooting depth (Weaver and Albertson 1936; Tucker et al. 2011). These traits influence changes in intra- and interspecific interactions during drought, mediating changes in community structure both during and after the drought event. Drought differentially reduced bud bank density of C4 grasses, C3 grasses, and forbs in a restored grassland, mediating shifts in community structure during drought and through 1 year of recovery (Carter et al. 2012).
Since aboveground plant structures of herbaceous plants in perennial grasslands are replaced annually, loss of this tissue may have fewer long-term implications for growth, survival and reproduction of plants than loss of belowground perennating organs (rhizomes and buds), which persist for multiple years (Hendrickson and Briske 1997; Ott and Hartnett 2012). Therefore, large changes in diversity and community structure aboveground may not be reflected belowground. We hypothesized that aboveground shifts in community structure and species richness would be greater than responses of the belowground bud bank to drought and grazing.
The effects of grazing on bud bank density during drought depend on disturbance intensity, timing, and duration. If plants senesce aboveground tissue to prevent desiccation and mortality during drought, grazing that removes dead aboveground tissue may have little additional effect on vegetation, including the belowground bud bank (Biondini et al. 1998). When plants senesce aboveground tissue in response to drought (Volaire et al. 2009), they translocate non-structural carbon from aboveground tissue to storage organs, facilitating future regrowth from belowground buds (Busso et al. 1989). Tucker et al. (2011) found that physiological drought tolerance did not change species responses to grazing at Konza Prairie. Furthermore, if plants form new belowground buds early in the growing season (Ott and Hartnett 2012), before drought becomes severe and before grazers are placed on the pasture, removal of aboveground biomass by grazers may have little effect on bud production.
Alternatively, grazing during severe drought may increase degradation of grassland relative to ungrazed areas (Illius and O’Connor 1999; Zwicke et al. 2013), perhaps by decreasing belowground carbon storage (McSherry and Ritchie 2013) and further reducing populations of belowground buds. Plants that are already stressed by drought may suffer greater mortality when grazed, reducing overall density of aboveground shoots (Koerner et al. 2013). If plants have not fully senesced their aboveground tissue, loss of this tissue to grazing may represent a significant loss of fixed carbon and nutrients. Given the results of these previous studies, we hypothesized that the synergistic effects of drought and grazing would degrade the bud bank to a greater extent than when either disturbance is applied alone.
Finally, we explored mechanisms controlling the bud bank response to drought and grazing. Previous work showed that grazing by large ungulate herbivores decreases bud bank density by increasing the rate at which buds transition to shoots and decreasing the ability of plants to replace those buds (Dalgleish and Hartnett 2009). Bud production is closely tied to the production of new rhizomes, so bud density will decline along with rhizome abundance if rhizome production is reduced by grazing or drought. The tight association between rhizome production and bud natality should also maintain the number of buds per unit rhizome biomass even when rates of rhizome production vary. However, bud abundance may decline more quickly than rhizome abundance if buds senesce at a greater rate than parent rhizomes. Previous work found that grazing and drought caused plants to senesce roots (Seastedt 1985; Hayes and Seastedt 1987), but rhizomes were maintained during drought conditions (Hayes and Seastedt 1987). We hypothesized that, if bud mortality is closely linked to rhizome mortality, bud density and live rhizome biomass would mirror each other and the number of buds per unit rhizome biomass would remain constant across drought or grazing treatments. However, if bud mortality is decoupled from rhizome mortality, bud density and rhizome biomass will differ, and the number of buds per unit rhizome biomass will vary in response to drought and grazing treatments.
In this research, we sought to compare above- and belowground vegetation responses to the separate and interactive effects of drought and grazing. We tested the hypotheses presented above by implementing a factorial treatment design which crossed two levels of precipitation (ambient precipitation and 80 % precipitation reduction, hereafter referred as ambient and drought, respectively) with two levels of simulated herbivory (unclipped and selective clipping of graminoids, hereafter referred as grazed and ungrazed treatments, respectively). We then documented above- and belowground vegetation responses to these treatments.
Materials and methods
Site description
Konza Prairie Biological Station (KPBS) is a 3,487-ha tallgrass prairie preserve in the Flint Hills of northeast Kansas, USA (39°05′N, 96°35′W). KPBS experiences a continental climate, with warm, wet summers and cool, dry winters. Mean monthly air temperatures range from −2.7 °C in January to 26.6 °C in July. Mean monthly air temperatures from May to August in 2012 (21.4, 25.1, 30.0, and 24.2 °C, respectively) were above monthly means of the 30 previous years for 3 of the 4 months (18.4, 23.5, 26.6, and 25.7, respectively). Total annual precipitation averages 880 mm (1983–2012; NOAA National Climate Data Center 2013), with 79 % falling during the growing season (April–October). However, precipitation patterns in this region are characterized by high variability among years, ranging from 513 to 1,435 mm annually in the period 1983–2012. Annual precipitation was below average for 2012 (570 mm), with 74 % falling during the growing season (Konza HQ1MET weather station). A matrix of C4 grasses dominates the vegetation, including big bluestem (Andropogon gerardii Vit.), Indian grass (Sorghastrum nutans [L.] Nash), and little bluestem (Schizachyrium scoparium), with a wide variety of subdominant C4 grasses, C3 grasses, sedges, and forbs interspersed (Towne 2002). KPBS has steep topography, with shallow upland cherty silt loam soil and deeper lowland silt clay loam soil. Native (never plowed) upland tallgrass prairie covers the unit of KPBS in which this study was conducted. Between 1994 and 2007, this unit was grazed at 1.7 ha/au−1 (hectares per animal unit) from May to early October by yearling Angus × Hereford steers, but has not been grazed since 2007. From 1994 until 2009, the unit was burned annually in March or April. The unit was not burned in 2010 and 2011, but was burned the year of the study, 2012.
Precipitation and grazing treatments
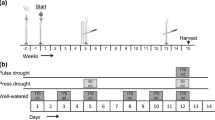
We randomly assigned precipitation treatments (ambient vs. drought) to 48 2 × 2 m plots on relatively level upland tallgrass prairie. At least 3 m separated plots from each other. This experimental manipulation of precipitation, combined with the severe natural drought of 2012, presented the unique opportunity to test vegetation responses under extreme drought conditions for the Flint Hills region. We used passive rainfall interception structures to exclude 80 % of the incident precipitation on drought plots from mid-March to October 2012 using a modification of the shelters tested by Yahdjian and Sala (2002). Shelters measured 2.4 × 2.4 m, with 2.4 × 0.2 m clear Lexan shingles (SABIC Innovative Plastics, Pittsfield, MA, USA) sloping to the south at 15° to account for prevailing southerly winds in the summer. Shingles were bent lengthwise at 120° and spaced to cover 80 % of the total area of the shelter (Fig. S1). Using an Analytical Spectral Devices (ASD) FieldSpec Pro portable spectrometer (Boulder, CO, USA), we determined that at least 90 % of visible light was transmitted by the shingles. All water collected by the shingles was drained at least 2 m from the shelter on the downhill side and away from other plots. While all plots experienced drought conditions for this region due to low precipitation, in the following text we refer to plots with rainfall interception shelters as “drought” plots, and plots without as “ambient” plots.
We randomly assigned herbivory treatments (simulated ungulate grazing vs. no simulated ungulate grazing) to plots within precipitation treatments. To simulate moderate to heavy grazing of bison or cattle in fenced pastures, we selectively clipped graminoids (grasses, sedges, and rushes) to 5 cm above the soil surface using scissors. We made no attempt to exclude other herbivores, including small mammals and insects. We originally planned to clip every 3 weeks, starting in May, but we were only able to clip on 17 May, 12 June, and 3 July because hot, dry weather limited new grass for the remainder of the growing season. Clipping removed 42.7 ± 1.6 % of aboveground biomass in ambient plots, and 54.0 ± 2.6 % of aboveground biomass in drought plots (mean ± SE). Due to an error assigning treatments to plots, ambient/grazed and drought/ungrazed treatment combinations were assigned to 13 plots each, while ambient/ungrazed and drought/grazed treatments were assigned to 11 plots each.
Suitability of treatment levels
We set up precipitation interception shelters before severe drought conditions developed in 2012, giving us the opportunity to test bud bank response to grazing under severe drought conditions. April–October precipitation for 2012 was 408 mm, 60 % of the average precipitation during these months from 1894 to 2011 (NOAA National Climate Data Center 2013). With 80 % reduction of growing season rainfall, drought treatments received 82 mm of rain during the growing season, and about 150 mm total for the year. Point measurements of soil moisture at the end of June indicated that soil moisture in the top 10 cm was approximately 12 % in ambient plots, but 6–7 % in drought plots. While this is quite dry, during the drought of 1934, Weaver and Albertson (1936) reported less than 2 % soil moisture at similar depths for most of the 1934 growing season in a prairie near Lincoln, Nebraska. Similarly, clipping treatments removed 42.7 ± 1.6 % and 54.0 ± 2.6 % (mean ± SE) of aboveground biomass in ambient and drought plots respectively, similar to grazing intensity under moderate stocking rates for this region (Towne et al. 2005). A broader range of drought and grazing treatments may reveal further nuances of vegetation responses to these disturbances, but the levels we selected are reasonable and yield interesting insights into differing responses of grasses, sedges, and forbs under these climate and grazing management scenarios.
Vegetation sampling
All plant community sampling occurred in the central 1 × 1 m area of each plot to minimize edge effects. Clipped aboveground biomass of graminoids within grazed plots was collected, dried at 60 °C for at least 48 h and weighed to the nearest 0.01 g. Total end-of-season aboveground biomass of both graminoids and forbs in grazed and ungrazed plots was harvested on 17 September 2012 by clipping at the soil surface. We used end-of-season graminoid and forb aboveground biomass from ungrazed plots to estimate graminoid and forb aboveground net primary productivity. For grazed plots we estimated graminoid aboveground net primary productivity by pooling end-of-season graminoid biomass with all previously clipped graminoid biomass. We estimated forb aboveground net primary productivity in grazed plots using end-of-season aboveground forb biomass.
End-of-season aboveground shoot density was estimated in September 2012 by counting and identifying shoots to species in four 10 × 10 cm quadrats randomly located within plots. Estimates of shoot density for each species were used to test for shifts in aboveground taxonomic species richness and community structure. To estimate bud bank density, we harvested four 0.01-m3 soil cores (10 × 10 × 10 cm) directly below the quadrats used for shoot counts. We harvested all soil cores between 27 and 29 November 2012, immediately placing them in sealable plastic bags and storing them at 4 °C until processing. To process, we rinsed soil from the belowground samples (no more than 3 weeks prior to examination) and examined belowground plant organs using a dissecting microscope, trimming roots to allow thorough examination of the belowground structures. Belowground buds were counted to estimate bud bank density and buds were assigned to species using bud morphology, phyllotaxy, morphology of the attached root system, and morphology of any remaining aboveground parts, as in Carter et al. (2012). Live rhizome biomass for each species was estimated by drying live rhizomes of each species within the 0.01-m3 soil cores at 60 °C for at least 48 h and weighing to the nearest 0.01 g.
We used species richness to describe community diversity. Belowground, we estimated species richness by counting the number of species present in the four 0.01-m3 soil cores per plot. Aboveground, we estimated species richness by counting the number of species in the four 0.01-m2 quadrats directly above each soil core. We calculated the number of buds per shoot by dividing the mean bud density of a plot by the mean shoot density of that plot. Buds per shoot is a measure of bud production standardized by the density of aboveground shoots, and has been used as an index of meristem limitation in grasslands (Dalgleish and Hartnett 2006).
Statistical analyses
We performed all analyses with R 3.0 (R Core Team 2013). We used analysis of variance to test for changes in aboveground net primary productivity of graminoids and forbs; to test for changes in shoot density, bud density, buds per shoot, and live rhizome biomass of grasses, sedges, and forbs; and to test for changes in taxonomic species richness of shoots and buds. Species-level data for grasses, sedges, and forbs were summed when analyzing shoot, bud, and rhizome abundance. Clipping and drought treatments and their interaction were included as fixed effects in the model. We tested the assumption of normality with visual examination of histograms and normal-quantile plots and with the Shapiro–Wilk test. When not normal, we performed generalized linear modeling using the gamma distribution (inverse link) for continuous variables and the negative binomial distribution (log link) for counts, using the glm function in R. We used Type III sums of squares to account for slightly unbalanced sample sizes.
Plots initially appeared homogeneous when installed while plants were dormant, but detailed vegetation characterization as the plant canopy developed suggested that plots should be blocked by location for analysis. We performed non-metric multi-dimensional scaling (NMDS) using the metaMDS routine in the vegan package (Oksanen et al. 2013) to determine if blocking by location was justified. Species scores were square root-transformed and Wisconsin double standardized prior to analysis (Bray and Curtis 1957). Pairwise dissimilarity between plant communities (plots) was computed using the Bray–Curtis index, using species aboveground shoot and belowground bud counts for each plot. NMDS ordinations confirmed that plots fell into two groups, both above and below ground (Fig. S2), corresponding to plot location within the study area. We included these two location blocks in a mixed model for aboveground net primary productivity, shoot density, bud density, buds per shoot, live rhizome biomass, and species richness, but we found that, for responses that did not include species-specific information (aboveground net primary productivity, shoot density, bud density, buds per shoot, and live rhizome biomass), mixed models accounting for blocks did not perform better than models without blocking. We used Akaike’s information criterion (AIC) to assess model performance. Blocking was therefore only retained for analysis of species richness. When significant interactions between drought and grazing treatments were detected, post hoc pairwise comparisons were used to test for differences among treatment means, adjusting P values for multiple comparisons using the Bonferroni correction. All differences were declared significant at P < 0.05 unless otherwise noted.
We evaluated shifts in below- and aboveground community structure in response to drought and grazing treatments using mean bud density of each species from the four 0.01-m2 soil cores per plot and mean shoot density of each species from the four 0.01-m2 quadrats directly above soil cores. We used partial distance-based redundancy analysis (partial db-RDA), a constrained ordination technique on the community dissimilarity matrix that is able to remove the effect of a random variable before performing the RDA (Legendre and Gallagher 2001). We used the capscale function in the vegan package (Oksanen et al. 2013). Dissimilarity between plant communities (plots) was calculated using the quantitative (abundance) form of the Bray–Curtis index. We modeled the response of community structure as a function of drought and grazing and their interaction, blocking by location as with the analysis of species richness. We assessed the effects of drought and grazing on community structure using a permutation test with 10,000 permutations.
We fit standardized major axis regression models to assess the relationship between bud density and rhizome biomass for the eight species encountered most frequently. Standardized major axis regression was used because both variables had an associated error component. For each species, we first fit a standardized major axis regression model using the data pooled across all treatments. If significant, we then fit models for all drought and grazing treatment combinations and compared slopes of the different treatments using the smatr package in R (Warton et al. 2012). If slopes were different, we performed pairwise comparisons among treatments, adjusting P values using the Sidak correction.
Results
Aboveground net primary productivity
Graminoid aboveground net primary productivity was reduced by 30–40 % in drought plots relative to ambient plots, with a slight increase in aboveground net primary productivity in both drought and ambient plots due to grazing (Fig. 1a). Forb aboveground net primary productivity in ambient/grazed plots was two times greater than all other treatments (110.78 ± 11.06 vs. approximately 50 g m−2 year−1), with no differences in forb aboveground net primary productivity across all other treatment combinations (Fig. 1b).
Effects of drought and grazing treatments on a graminoid and b forb aboveground net primary productivity (ANPP) in grazed (dark gray) and ungrazed (light gray) treatments. Forb analysis excludes three outliers (ambient, not grazed = 269.63 g m−2 year−1; ambient/grazed = 196.16 g m−2 year−1; drought/grazed = 192.26 g m−2 year−1), caused by the presence of large clonal forbs or woody shrubs on the plots. When included in the analysis, drought and clipping effects remain significant (P < 0.01), while the interaction is not (P = 0.194). Bars mean ± SE. Lowercase letters indicate significant differences among all treatment combinations. Sample size numbers are shown inside bars
Community structure and diversity
Analysis of above- and belowground species abundance data suggests that plant community structure marginally shifted within one growing season in response to drought and grazing treatments. The effect of drought on aboveground plant community structure was marginally significant (P < 0.1), with no effect of grazing and no interaction between drought and grazing (Fig. 2a). The effect of grazing on belowground community structure was marginally significant (P < 0.1), with no effect of drought and no interaction between drought and grazing (Fig. 2b). Species richness declined 15 to 20 % both above and below ground in response to drought, across grazing treatments (Fig. 3a, b), although the effect of drought on species richness was only marginally significant aboveground (P < 0.1).
Distance-based redundancy analysis (db-RDA) of the effect of drought and grazing on a aboveground shoot and b belowground bud community structure. AU Ambient/ungrazed (closed circles); AG ambient/grazed (closed squares); DU drought/ungrazed (open circles); and DG drought/grazed (open squares). Lines connect plots to the treatment centroid. For shoots, drought: F 1,43 = 1.6, P = 0.065; grazing: F 1,43 = 1.3, P = 0.182; drought × grazing: F 1,43 = 1.5, P = 0.117. For buds, drought: F 1,43 = 1.3, P = 0.243; grazing: F 1,43 = 1.6, P = 0.081; drought × grazing: F 1,43 = 0.6, P = 0.87
Effect of treatments on shoot and bud densities
Grass, sedge, and forb shoot densities responded differently to drought and grazing. Neither drought nor grazing reduced grass shoot density (Fig. 4a). Sedge shoot density was reduced approximately 20 % by drought, with no effect of grazing (Fig. 4c). Neither drought nor grazing affected forb shoot density (Fig. 4e).
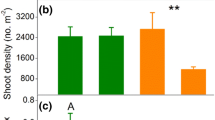
The effect of drought and grazing treatments on aboveground stem density in 0.01-m2 vegetation quadrats (left column) and belowground bud density in 0.01-m3 soil cores (right column) for a, b grasses, c, d sedges, and e, f forbs. All χ 2 tests have 1° of freedom. Bars mean ± SE in grazed (dark gray) and ungrazed (light gray) treatments. Sample size numbers are shown inside bars. Lowercase letters indicate significant differences among treatments (P < 0.05)
As with shoot density, grass, sedge, and forb bud banks responded differently to drought and grazing. Neither drought nor grazing affected grass bud densities (Fig. 4b). Drought reduced sedge bud bank density 50–75 % under ungrazed conditions relative to all other treatment combinations (Fig. 4d). Forb bud density declined 50–75 % under drought, with no effect of grazing (Fig. 4f).
Grasses, sedges, and forbs differed not only in the number of buds per shoot but also in the response of buds per shoot to drought and grazing. Grazing reduced grass buds per shoot from approximately 7.5 to 5 buds per shoot, but drought had no effect on grass buds per shoot (Fig. 5a). In contrast, while grazing had no effect on sedge and forb buds per shoot, drought reduced sedge buds per shoot from approximately 4 to 3 buds per shoot and reduced forb buds per shoot from approximately 27 to 10 buds per shoot (Fig. 5b, c).
The effect of drought and grazing treatments on the number of buds per shoot for a grasses, b sedges, and c forbs. One drought/grazed outlier (30.4 buds per shoot) was excluded in (a). In (b), one drought/grazed plot had no sedge shoots, so buds per shoot was not estimable. In (c), five plots had either no forbs buds or shoots, so buds per shoot was not estimable. Bars mean ± SE in grazed (dark gray) and ungrazed (light gray) treatments. Sample size numbers are shown inside bars
Relationship between bud density and rhizome biomass
Live rhizome biomass reflected the bud bank response to drought and grazing (Fig. 6). We found that grass live rhizome biomass remained constant at 1–1.5 g 0.01 m−2 across grazing and precipitation treatments (Fig. 6a). Sedge live rhizome biomass was reduced by drought only under ungrazed, drought conditions relative to all other treatment combinations (Fig. 6b). Neither drought nor grazing affected forb live rhizome biomass (Fig. 6c).
Effects of drought and grazing treatments on belowground live rhizome biomass for a grasses, b sedges, and c forbs, estimated in the same 0.01-m3 soil cores used for bud density estimates. Bars mean ± SE in grazed (dark gray) and ungrazed (light gray) treatments. Sample size numbers are shown inside bars. Lowercase letters indicate significant differences among treatments (P < 0.05)
Live rhizome biomass predicted total bud bank density fairly well (r 2 = 0.47; Fig. 7a). However, predictions improved when analyses were performed separately for grasses (r 2 = 0.671; Fig. 7b) and sedges (r 2 = 0.724; Fig. 7c), but not for forbs (r 2 = 0.21; Fig. 7d). Analyzing the most common plant species individually improved predictions even more (Fig. 8). The number of buds per gram of rhizome biomass varied widely among species, ranging from 34.3 buds per gram for Carex meadii to 123.2 buds per gram for Symphotrichum ericoides. Within species, however, buds per rhizome biomass did not vary by drought or grazing treatment (Fig. 8; Table 1).
Relationship between bud density and rhizome biomass for a the entire plant community, b grasses, c sedges, and d forbs. Because both variables have an error component, standardized major axis regression was used to model the relationship. Regression statistics correspond to the analysis with all treatments pooled, as indicated by the dashed line
Relationship between bud density and live rhizome biomass for the eight species encountered most frequently in plots. Regression statistics correspond to the analysis with all treatments pooled, as indicated by the dashed line. Sample sizes and tests of slopes are given in Table 1
Discussion
Shifts in plant community structure and diversity
Taking advantage of a severe, natural drought in addition to our precipitation interception treatment, we investigated the effects of grazing under severe drought conditions. Contrary to our first hypothesis, we observed similar changes in both above- and belowground community structure within one growing season in response to drought and grazing treatments. While both drought and grazing appeared to affect community structure, only drought reduced species richness both above and below ground. Many earlier studies have documented differential drought and grazing tolerance among plant species (Tucker et al. 2011; Mullahey et al. 1990; Albertson and Weaver 1942). When graminoids are abundant, large ungulate grazers generally avoid feeding on forbs in tallgrass prairies, resulting in increased relative abundance and diversity of forbs in grazed prairies (Vinton et al. 1993; Hartnett et al. 1996; Biondini et al. 1998). Our results suggest that short-term forb responses to grazing depend on the relative level of water stress. Forb aboveground net primary productivity and bud density only increase in response to selective graminoid herbivory in ambient plots not exposed to precipitation reductions. This corroborates previous work which found that climate mediates the response of vegetation to grazing (Biondini et al. 1998).
We found few responses where grazing exacerbated the short-term effects of drought, but several examples where grazing had no effect or ameliorated short-term effects of drought. The only example of grazing causing additional damage was the reduction in C4 grass flowering shoot density (data not shown), where C4 flowering shoot density was zero in plots subjected to both drought and grazing. In contrast, total aboveground net primary productivity from grazed plots was greater than aboveground net primary productivity from ungrazed plots, regardless of precipitation treatment. Other studies have also documented overcompensation of aboveground productivity and increased relative growth rates in the first year of clipping tallgrass prairie grasses, but decreased aboveground productivity and relative growth rates with multiple years of defoliation (Weaver and Hougen 1939; Vinton and Hartnett 1992). Tuomi et al. (1994) demonstrated that bud sensitivity to herbivory may facilitate short-term overcompensation, but that repeated grazing could cause long-term declines in plant growth.
Responses of graminoid and forb bud banks
Contrary to our second hypothesis, we did not find that the synergistic effects of drought and grazing degraded the bud bank to a greater extent than when either disturbance is applied alone. Our study clearly indicates that the grass bud bank is stable when disturbed separately and concurrently by drought and grazing, facilitating vegetation stability in tallgrass prairie. Large ungulate grazers preferentially feed on grass in tallgrass prairie (Augustine and McNaughton 1998), and grasses tolerate grazing by maintaining their meristems at or below the soil surface, out of the reach of large ungulate herbivores (Nilsson et al. 1996). Even after imposing drought and grazing treatments in a year of low ambient precipitation and record heat, we found that grass bud bank density remained constant, ready to recruit to shoots when favorable conditions returned. As a corollary, greater relative abundance of species that respond strongly to drought and grazing, such as sedges and forbs in tallgrass prairie, may lead to greater variation in community composition when disturbed.
Mechanism of changes in bud density
We found that changes in rhizome biomass by drought and grazing treatments reflected changes in bud bank density, suggesting that bud mortality is linked to the senescence of the parent rhizome. Furthermore, the number of buds per unit rhizome biomass did not vary among grazing or drought treatments for any species or group. This suggests that in tallgrass prairie, buds and their parent rhizomes tend to be lost and gained at similar rates across varying drought and grazing regimes. Loss of biomass to herbivores, and senescence of aboveground plant organs during severe drought and may have changed bud density in forbs and sedges in our study by limiting the growth of new rhizomes or increasing mortality of existing rhizomes.
The ratio of buds per shoot we observed in response to drought and grazing treatments agreed with previous reports. In previous work, we found that the number of buds per shoot did not change with drought or irrigation relative to ambient conditions (VanderWeide et al. 2014). In a separate study, we found that grazing reduced the number of buds per shoot for grasses by 15–25 % in 3 of the 4 years observed (VanderWeide 2013). Mullahey et al. (1991) also found that defoliation reduced bud production of Calamovilfa longifolia (Hook.) Scribn. and Andropogon hallii Hack. Like this previous work, our current study demonstrated that drought does not change the number of buds per shoots, but that grazing reduces grass buds per shoot up to 25 %. Thus, we think that grass shoots that emerge in response to grazing do not produce buds at similar rates to ungrazed shoots. Since grass bud production per unit rhizome biomass is stable when disturbed, bud production per shoot may be depressed because fewer phytomers, and thus fewer buds, are produced on rhizomes. When fewer buds are produced per shoot under grazed conditions (N’Guessan and Hartnett 2011; Mullahey et al. 1990, 1991), grasses maintain bud bank density by increasing shoot density.
The excellent correlation between bud density and live rhizome biomass may be a useful clonal plant trait that could be used to compare clonal strategies and responses to environmental variation within and among species. Further research should explore the implications of buds per unit rhizome biomass for growth, survival, and responses to disturbance. Live rhizome biomass may also provide an index of bud bank density that allows better comparisons among observers, studies, and species.
Conclusions
Contrary to our hypothesis, community structure and diversity shifted both above and below ground, demonstrating that even though buds and rhizomes are protected below ground, drought and grazing affect their growth and survival. Since bud density and rhizome biomass reflected each other, with no change in bud density per unit rhizome biomass across treatments, we can deduce that buds and rhizomes are lost and gained at similar rates. The tight relationship between bud density and rhizome biomass suggests that rhizome biomass deserves further testing as an easily measured index of bud bank density.
References
Albertson FW, Weaver JE (1942) History of the native vegetation of western Kansas during seven years of continuous drought. Ecol Monogr 12:25–51. doi:10.2307%2F1948421
Anderson RC (2006) Evolution and origin of the Central Grassland of North America: climate, fire, and mammalian grazers. J Torrey Bot Soc 133:626–647. doi:10.3159/1095-5674(2006)133[626:EAOOTC]2.0.CO;2
Augustine DJ, McNaughton SJ (1998) Ungulate effects on the functional species structure of plant communities: herbivore selectivity and plant tolerance. J Wildl Manag 62:1165–1183
Benson EJ, Hartnett DC (2006) The role of seed and vegetative reproduction in plant recruitment and demography in tallgrass prairie. Plant Ecol 187:163–177. doi:10.1007/s11258-005-0975-y
Beschta RL, Donahue DL, DellaSala DA, Rhodes JJ, Karr JR, O’Brien MH, Fleischner TL, Deacon Williams C (2013) Adapting to climate change on western public lands: addressing the ecological effects of domestic, wild, and feral ungulates. Environ Manag 51:474–491. doi:10.1007/s00267-012-9964-9
Biondini ME, Patton BD, Nyren PE (1998) Grazing intensity and ecosystem processes in a northern mixed-grass prairie, USA. Ecol Appl 8:469–479. doi:10.1890/1051-0761(1998)008[0469:GIAEPI]2.0.CO;2
Bray JR, Curtis JT (1957) An ordination of the upland forest communities of southern Wisconsin. Ecol Monogr 27:325–349. doi:10.2307/1942268
Busso CA, Mueller RJ, Richards JH (1989) Effects of drought and defoliation on bud viability of two caespitose grasses. Ann Bot 63:477–485
Carter DL, VanderWeide BL, Blair JM (2012) Drought-mediated shoot and below-ground bud dynamics in restored grasslands. Appl Veg Sci 15:470–478. doi:10.1111/j.1654-109X.(2012)01200.x
Curtin C, Western D (2008) Grasslands, people, and conservation: over-the-horizon learning exchanges between African and American pastoralists. Conserv Biol 22:870–877. doi:10.1111/j.1523-1739.2008.00945.x
Dalgleish HJ, Hartnett DC (2006) Below-ground bud banks increase along a precipitation gradient of the North American Great Plains: a test of the meristem limitation hypothesis. New Phytol 171:81–89. doi:10.1111/j.1469-8137.2006.01739.x
Dalgleish HJ, Hartnett DC (2009) The effects of fire frequency and grazing on tallgrass prairie productivity and plant structure are mediated through bud bank demography. Plant Ecol 201:411–420. doi:10.1007/978-90-481-2798-6_4
Frank DA, Groffman PM (1998) Ungulate vs. landscape control of soil C and N processes in grasslands of Yellowstone National Park. Ecology 79:2229–2241. doi:10.1890/0012-9658(1998)079[2229:UVLCOS]2.0.CO;2
Hartnett DC, Hickman KR, Walter LEF (1996) Effects of bison grazing, fire, and topography on floristic diversity in tallgrass prairie. J Range Manag 49:413–420
Hayes DC, Seastedt TR (1987) Root dynamics of tallgrass prairie in wet and dry years. Can J Bot 65:787–791
Hendrickson JR, Briske DD (1997) Axillary bud banks of two semiarid perennial grasses: occurrence, longevity, and contribution to population persistence. Oecologia 110:584–591. doi:10.1007/s004420050199
Illius AW, O’Connor TG (1999) On the relevance of nonequilibrium concepts to arid and semiarid grazing systems. Ecol Appl 9:798–813. doi:10.1890/1051-0761(1999)009[0798:OTRONC]2.0.CO;2
Koerner SE, Collins SL, Blair JM, Knapp AK, Smith MD (2013) Rainfall variability has minimal effects on grassland recovery from repeated grazing. J Veg Sci. doi:10.1111/jvs.12065
Legendre P, Gallagher ED (2001) Ecologically meaningful transformations for ordination of species data. Oecologia 129:271–280. doi:10.1007/s004420100716
McNaughton SJ (1993) Grasses and grazers, science and management. Ecol Appl 3:17–20
McSherry ME, Ritchie ME (2013) Effects of grazing on grassland soil carbon: a global review. Glob Change Biol 19:1347–1357. doi:10.1111/gcb.12144
Min SK, Zhang X, Zwiers FW, Hegerl GC (2011) Human contribution to more-intense precipitation extremes. Nature 470:378–381. doi:10.1038/nature09763
Mullahey JJ, Waller SS, Moser LE (1990) Defoliation effects on production and morphological development of little bluestem. J Range Manag 43:497–500
Mullahey JJ, Waller SS, Moser LE (1991) Defoliation effects on yield and bud and tiller numbers of two Sandhills grasses. J Range Manag 44:241–245
N’Guessan M, Hartnett DC (2011) Differential responses to defoliation frequency in little bluestem (Schizachyrium scoparium) in tallgrass prairie: implications for herbivory tolerance and avoidance. Plant Ecol 212:1275–1285. doi:10.1007/s11258-011-9904-4
Nilsson P, Tuomi J, Astrom M (1996) Bud dormancy as a bet-hedging strategy. Am Nat 147:269–281
NOAA National Climate Data Center (2013) www.ncdc.noaa.gov. Accessed June 2013
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H (2013) vegan: community ecology Package. http://CRAN.R-project.org/package=vegan. R package version 2.0.7
Ott JP, Hartnett DC (2012) Contrasting bud bank dynamics of two co-occurring grasses in tallgrass prairie: implications for grassland dynamics. Plant Ecol 213:1437–1448. doi:10.1007/s11258-012-0102-9
Patricola CM, Cook KH (2013) Mid-twenty-first century warm season climate change in the central United States. part we: regional and global model predictions. Clim Dyn 40:551–568. doi:10.1007/s00382-012-1605-8
R Core Team. R: A Language and Environment for Statistical Computing (2013) R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org
Rogers WE, Hartnett DC (2001) Temporal vegetation dynamics and recolonization mechanisms on different-sized soil disturbances in tallgrass prairie. Am J Bot 88:1634–1642
Samson F, Knopf F (1994) Prairie conservation in North America. Bioscience 44:418–421
Seastedt TR (1985) Maximization of primary and secondary productivity by grazers. Am Nat 126:559–564
Towne EG (2002) Vascular plants of Konza Prairie Biological Station: an annotated checklist of species in a Kansas tallgrass prairie. SIDA Contr Bot 20:269–294
Towne EG, Hartnett DC, Cochran RC (2005) Vegetation trends in tallgrass prairie from bison and cattle grazing. Ecol Appl 15:1550–1559. doi:10.1890/04-1958
Tucker SS, Craine JM, Nippert JB (2011) Physiological drought tolerance and the structuring of tallgrass prairie assemblages. Ecosphere 2:art48 doi: 10.1890/ES11-00023.1
Tuomi J, Nilsson P, Astrom M (1994) Plant compensatory responses: bud dormancy as an adaptation to herbivory. Ecology 75:1429–1436
VanderWeide BV (2013) Grazing and drought in tallgrass prairie: the role of belowground bud banks in vegetation dynamics. PhD dissertation, Kansas State University, Manhattan
VanderWeide BV, Hartnett DC, Carter DL (2014) Belowground bud banks of tallgrass prairie are insensitive to multi-year, growing season drought. Ecosphere 5:art103. doi: 10.1890/ES14-00058.1
Vinton MA, Hartnett DC (1992) Effects of bison grazing on Andropogon gerardii and Panicum virgatum in burned and unburned tallgrass prairie. Oecologia 90:374–382. doi:10.1007/BF00317694
Vinton MA, Hartnett DC, Finck EJ, Briggs JM (1993) Interactive effects of fire, bison (Bison bison) grazing and plant community structure in tallgrass prairie. Am Midl Nat 129:10–18
Volaire F, Seddaiu G, Ledda L, Lelievre F (2009) Water deficit and induction of summer dormancy in perennial Mediterranean grasses. Ann Bot 103:1337–1346. doi:10.1093/aob/mcp080
Warton DI, Duursma RA, Falster DS, Taskinen S (2012) Smatr 3–an R package for estimation and inference about allometric lines. Methods Ecol Evol 3:257–259. doi:10.1111/j.2041-210X.2011.00153.x
Weaver JE, Albertson FW (1936) Effects of the great drought on the prairies of Iowa, Nebraska, and Kansas. Ecology 17:567–639
Weaver JE, Hougen V (1939) Effect of frequent clipping on plant production in prairie and pasture. Am Midl Nat 21:396–414
Yahdjian L, Sala OE (2002) A rainout shelter design for intercepting different amounts of rainfall. Oecologia 133:95–101. doi:10.1007/s00442-002-1024-3
Zwicke M, Alessio GA, Thiery L, Falcimagne R, Baumont R, Rossignol N, Soussana JF, Picon-Cochard C (2013) Lasting effects of climate disturbance on perennial grassland above-ground biomass production under two cutting frequencies. Glob Change Biol 19:3435–3448. doi:10.1111/gcb.12317
Author contribution statement
BV and DH designed the study. BV executed the experiments, performed analyses, and wrote the manuscript.
Acknowledgments
We thank Anita Dille, Carolyn Ferguson, and Tony Joern for comments on an earlier version of the manuscript. We also thank three anonymous reviewers for critical comments that improved the manuscript. We thank Kathryn Sebes for assistance with field work, Leigh Murray for statistical advice, and John Blair for access to additional rain interception shelters. During this project, B.L.V. was supported by Konza Prairie Long Term Ecological Research Station, Kansas State University Division of Biology, US National Science Foundation GK-12 Fellowship DGE-0841414. The experiments comply with the current laws of the United States where they were performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Bryan Foster.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
VanderWeide, B.L., Hartnett, D.C. Belowground bud bank response to grazing under severe, short-term drought. Oecologia 178, 795–806 (2015). https://doi.org/10.1007/s00442-015-3249-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-015-3249-y