Abstract
Ethion, an organophosphorus pesticide, is used worldwide and has potential for toxicity and inflammation. There are very limited data on the pulmonary and genotoxic effects of ethion especially when the exposure is combined with lipopolysaccharide. Therefore, we used a mouse model to test the hypothesis that prolonged exposure to ethion alone or in conjunction with lipopolysaccharide (LPS) will cause lung inflammation and genotoxicity in a mouse model. Swiss albino (n = 30) were divided into a control (n = 10) and two treatment groups (n = 10; each group). The treatment groups were orally administered ethion (4 or 2 mg/kg/animal/day; n = 10 each) dissolved in corn oil for 90 days. After 90 days of exposure, five animals from each of the groups were challenged with 80 μg Escherichia coli lipopolysaccharide (LPS) intranasally and the remaining five animals with normal saline solution via the same route. Ethion at both dosages induced lung inflammation as indicated by increased (p < 0.05) perivascular and peribronchial accumulation of inflammatory cells along with thickening of the alveolar septal wall. Ethion at 4 mg/kg altered (p < 0.05) the mRNA and protein expression of TLR-9 and IL-1β in the lungs and induced genotoxicity in blood cells as determined by single cell gel electrophoresis (Comet assay). Further, both dosages of ethion in combination with E. coli LPS caused genotoxicity and increased (p < 0.05) pulmonary expression of TLR-4, TLR-9 and IL-1β. The data taken together suggest ethion induces lung inflammation and interaction between ethion and LPS increases genotoxicity in blood cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pesticides are used to protect agricultural and industrial commodities from pests. The rise in overall food demand has led to intensive agricultural practices that require substantial use of pesticides in India and other countries. India has emerged as the second biggest producer of pesticides in Asia and occupies the 12th position globally (Mathur 2010). Organophosphorus (OP) pesticides were introduced in India in the 1960s and are the most widely used pesticides at the industrial as well as domestic level (Aktar et al. 2009).
Ethion (O,O,O′,O′-Tetraethyl S,S′-methylene bis (phosphorodithioate)), an OP pesticide, is extensively used globally on a variety of crops including vegetables, fruits, cotton and ornamental crops. Other uses include topical applications on livestock to control skin parasites or insects (EPA 1989). Workers in the manufacturing plants and those in the agriculture sector may become exposed to ethion directly or indirectly. Ethion has a maximum permissible residue level (MRL) up to 5.0 mg kg−1 with oral LD50 of 40 mg kg−1 in mice (Kidd and James 1991). Levels of ethion have been found above the permissible limits in drinking water, vegetables and human blood in India (Thakur et al. 2008). The detection of ethion in 23.1% human colostrum samples indicates its entry into the food chain and an early exposure of the developing immune system of the infant to ethion (Srivastava et al. 2011).
Oral exposure to ethion is genotoxic in chicks (Bhunya and Jena 1994) and Anopheles culicifacies (Marwaha 2015). Ethion is known to be highly toxic to fish and freshwater invertebrates (EPA 1989). It causes acetylcholinesterase inhibition in erythrocytes (Bhatti et al. 2010, 2011), loss of body weight (Bhatti et al. 2010), lung and respiratory disorders (Dewan et al. 2008) and hematological alterations (Bhatti et al. 2011). Chronic exposure to OP pesticides has also been related to reduced pulmonary function in farmers/workers (Fareed et al. 2013). However, the pulmonary effects of ethion have not been studied in a systematic manner.
Lipopolysaccharides (LPS) are a primary pro-inflammatory constituent of the Gram-negative bacterial cell wall and often prevalent in agricultural and occupational environments such as food storage and processing facilities, grain and textile mills and animal confinements (Liebers et al. 2006; Thorn 2001). There remains a strong possibility that farm workers may become co-exposed to pesticides and LPS (Thorn 2001). LPS elicit macrophage activation, fever, septic shock and acute lung injury (Kitamura et al. 2001; Tschernig et al. 2008). Our previous studies suggest that the interaction of LPS with various other pesticides alters pulmonary inflammation (Merkowsky et al. 2016; Pandit et al. 2016, 2017; Sethi et al. 2017; Tewari et al. 2017) and genotoxic potential (Kaur et al. 2016b).
Long-term exposure to low levels of the pesticides such as acephate enhances LPS-induced production of pro-inflammatory cytokines such as interleukin (IL)-1β, tumor necrosis factor (TNF)-α and interferon (IFN)-γ in rats (Singh and Jiang 2002), which may be attributable to upregulation of the Toll-like receptor (TLR)-4 signaling pathway (Duramad et al. 2006). TLR4 engagement activates macrophages, neutrophils and other immune cells leading to the production of IL-1β and TNF-α (Andonegui et al. 2003). Further, IL-1β (Dinarello 1996; Becker et al. 1999) and TNF-α (Baer et al. 1998) are expressed in lungs during inflammation, infection, or injury. TLR-9 is also highly expressed in chronic obstructive pulmonary disease (Schneberger et al. 2013), lung cancer (Caixia et al. 2015) and following exposure to indoxacarb (Kaur et al. 2016a).
Though ethion is banned in many countries including the USA, New Zealand and those in the European Union and Africa it is still extensively used in northern India (Paranjape et al. 2014). Currently, there are no data on the combined effects of ethion and LPS on lung inflammation, the pulmonary expression of inflammatory molecules and genotoxicity in blood cells. Ethion is used in farming and simultaneous LPS exposure is common. Therefore, we tested the hypothesis that prolonged exposure to ethion alone or in conjunction with LPS will cause lung inflammation and genotoxicity in a mouse model.
Material and methods
Experimental animals
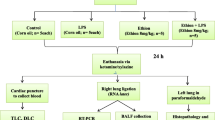
Healthy albino male mice (n = 30), aged 6–7 weeks, were maintained under the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), India. The experiment protocols were permitted by the Institutional Animal Ethics Committee, Guru Angad Dev Veterinary and Animal Sciences University (GADVASU), Ludhiana. The animals (n = 5/cage) were housed in cages at the small animal house facilities of the university. Commercial pellets (Ashirwad Industries, Chandigarh) and drinking water were provided ad libitum. The animals were acclimatized for 2 weeks before the start of the experiment. All animals were found healthy before the start of the experiment.
Chemicals
Analytical grade Ethion PESTANAL® (45477), Escherichia coli LPS (L3129), corn oil (C8267) and bovine serum albumin (BSA; A3294) were obtained from Sigma-Aldrich, Bangalore, India. Quantifast SYBR Green PCR kit (204054) was procured from Qiagen, India.
Body weight analysis
The change in body weight was calculated by subtracting the initial body weight (body weight at the start of the experiment) from the final body weight (body weight at day 90 of experiment) and expressed as average body weight gain (Kemabonta and Akinhanmi 2013).
Dosages and exposure schedules
Animals were divided randomly into three groups, namely two treatments and one control (n = 10/group). First (EC1) and second (EC2) treatment groups were treated orally daily for 90 days with 4 mg kg−1 (1/10th LD50) and 2 mg kg−1 (1/20th LD50) of ethion dissolved in corn oil, respectively. The lethal dose of ethion given orally in humans is estimated between 50 and 500 mg kg−1 (Gosselin et al. 1984). The doses selected in our experiments are lower than the MRL (5 mg kg−1) of ethion. The control animals were fed orally corn oil daily for 90 days. Mice were observed twice daily during the entire period of study. No animal died during the experiment period.
On completion of 90 days, five animals from each group were administered 80 μl E. coli LPS (1 mg/mL)/animal via the intranasal route while the remaining five animals from each group were given 80 μl of normal saline solution (NSS) via the same route. The animals were euthanized after 9 h of LPS/NSS exposure based on our previous studies (Pandit et al. 2016; Kaur et al. 2016a).
Collection of samples
Whole blood was collected and processed for single cell gel electrophoresis (SCGE), total leukocyte count (TLC) and differential leukocyte count (DLC) analysis on the same day. BAL fluid was collected as described earlier (Pandit et al. 2016) and was subjected to TLC and DLC analysis.
The left lung was inflated with 4% paraformaldehyde solution at 25 cm of water pressure after collection of BAL fluid, followed with immersion in 4% paraformaldehyde solution for 12–16 h at 4 °C for histopathology and immunohistochemistry. The right lung was stored at − 80 °C for detection of TLR-4, TLR-9, IL-1β and TNF-α mRNA expression by real-time (RT) PCR.
Total leukocyte count and differential leukocyte count analysis
Briefly, 20 μl blood/BAL fluid was mixed with 380 μl of white blood cell diluting fluid for TLC analysis. The blood smear was prepared and stained with Leishman stain for differential leukocyte analysis. Neutrophils and lymphocytes were identified and counted on each slide at × 40. About 100 cells per sample were counted and expressed as absolute number of neutrophil and lymphocyte per milliliter of blood. A similar procedure was performed for counting neutrophil in BAL fluid.
Hemotoxylin and eosin staining
The whole lung was embedded in paraffin and processed to obtain 5-μm-thick sections. The sections were stained with hematoxylin and eosin to assess the histopathological changes in the lungs by using × 10 and × 40 objectives. Morphological changes in lungs of five animals per group were examined and were graded semi-quantitatively for perivascular infiltration, peribronchial infiltration, increase in perivascular space, thickening of alveolar septa and sloughing of the epithelium as described earlier (Pandit et al. 2016). Each of these parameters was graded by an evaluator who was blinded to the identity of the samples (0, normal/absent; 1, mild; 2, moderate; 3, severe) and followed by statistical analysis.
Quantitative real-time PCR
The right lung from each animal was subjected to RT-PCR to detect expression of TLR-4, TLR-9, IL-1β and TNF-α mRNA. Total RNA was reverse transcribed to cDNA using oligo-dT primers by first-strand cDNA synthesis kit (Thermo Scientific, USA). The reaction mixture was prepared according to standardized real-time PCR reaction with Quantifast SYBR® Green PCR kit Components. The reaction was carried out in duplicate in RT-PCR (BioRad, USA) with β-actin as an endogenous control. The primer sequences used for the TLR-4 gene were 5’-TGCTGAGTTTCTGATCCATGC-3′ and 5’-TGGCTAGGACTCTGATCATGG-3′ (Zuo et al. 2012), TLR-9 were 5’-TCACAGGGTAGGAAGGCA-3′ and 5’-GAATCCTCCATCTCCCAACA-3′ (Nagajyothi et al. 2012), IL-1β were 5’-TGTAATGAAAGACGGCACACC-3′ and 5’-TCTTCTTTGGGTATTGCTTGG-3′ (Yuan et al. 2013) and TNF-α were 5’-TGTCTACTGAACTTCGGGGT-3′ and 5’-TCCACTTGGTGGTTTGCTAC-3′ (Villar et al. 2013). Data analysis was done by the ΔCT method for relative quantification.
Immunohistochemistry
The paraffin sections of the left lung were subjected to immunohistochemistry as per Sethi et al. (2011, 2013). The sections were stained with primary antibodies against TLR-4 (goat polyclonal TLR4; M-16; sc12511; dilution 1:400), TLR-9 (goat polyclonal TLR-9; C-20; dilution 1:800), IL-1β (goat polyclonal IL-1β; R-20; dilution 1:200) and TNF-α (goat polyclonal TNF-α; N-19; sc1350; dilution 1:2000) for 1 h followed by a suitable secondary antibody (polyclonal rabbit anti-goat immunoglobulins/HRP (Dako P0449); dilution 1:800) for 30 min. The color was developed with a commercial kit (SK4100; Vector Laboratories, USA). Finally, hematoxylin was used as a counterstain. Controls included replacing the primary antibody with bovine serum albumin and labeling of sections with an antibody specific for factor VIII-related antigen to identify endothelial cells.
Quantification of immunopositive cells
Immunopositive TLR4, TLR-9, IL-1 β and TNF-α cells were counted in the lungs of five animals per group. Briefly, the cells were counted manually in an area of 0.2 mm2 in 10 fields/section under × 40 objective. The evaluator was blinded to the identity of the treatment groups.
Single cell gel electrophoresis (Comet assay)
Alkaline single cell gel electrophoresis was performed as per Singh et al. (1988) with few variations (Devi et al. 2000). Briefly, 5 μL of blood and 95 μL of 0.5% low melting point agarose (LMPA) were mixed and applied to 0.75% normal melting agarose coated microscopic slides. The slides were viewed under a fluorescence microscope (Nikon Eclipse 90i) having an excitation filter of 420–490 nm and a barrier filter of 520 nm. One hundred cells per sample were analyzed by Open Comet 1.3 (Gyori et al. 2014). The number of cells evaluated per animal was fixed to 100. Therefore, 500 cells per group were evaluated.
Statistical analysis
Data from TLC, DLC, ∆CT values, histopathology and immunohistochemistry were presented as mean ± standard error (SE) and analyzed statistically by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test to find the significant reduction (p < 0.05) between the levels of effect as well as compared with two-way ANOVA. GraphPad Prism 6 software was used for data analysis and graphical representation of data. One-way and two-way ANOVA analysis did not vary so results with one-way ANOVA are presented. Correlation among different comet parameters was determined by Pearson correlation coefficient and represented using heat maps generated by Statgraphics Centurion XVII software. All the groups were compared with each other and results were considered statistically significant at p < 0.05.
Results
Average body weight gain
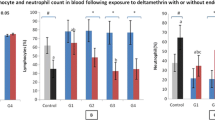
There was no significant difference in initial body weight of different groups and no mortality was observed throughout the experiment. However, treatment groups (EC1 7.36 ± 0.27 g and EC2 8.08 ± 0.44 g) showed less average body weight gain compared to the control (Fig. 1a).
a Average body weight gain (g) among different groups, b TLC (×103), c neutrophil count (×103), d lymphocyte count (×103) per mL blood, e TLC (×103) and f neutrophil count (×103) per milliliter BAL fluid after long-term (90 days) exposure of ethion (4 mg kg−1 and 2 mg kg−1) alone or in combination with LPS. a,b,c,d: no common superscript (a, b, c, d) between two levels of an effect indicates significant difference (p < 0.05) among groups
Total leukocyte count and differential leukocyte count analysis
Blood
LPS treatment resulted in increase in TLC in peripheral blood along with neutrophilia compared to the control animals (Fig. 1b, c). However, animals exposed to ethion at 4 mg kg−1 (EC1) showed a significant decrease in TLC along with lymphocytopenia compared to the control and the LPS groups (Fig. 1b, d). Furthermore, administration of 4 mg kg−1 ethion with LPS (EC1 + LPS) resulted in significant (p < 0.05) lymphocytopenia compared to the control and the LPS only groups (Fig. 1d). There was no difference in TLC in peripheral blood in the EC1 + LPS group compared to the control and the EC1 groups (Fig. 1b). However, a significant decrease in peripheral blood TLC was found in the EC1 + LPS group compared to the animals treated with LPS only (Fig. 1b).
Ethion at 2 mg kg−1 (EC2) did not alter TLC, lymphocyte and neutrophil counts compared to the control animals but significantly reduced TLC, neutrophil and lymphocyte count as compared to the LPS group (Fig. 1b–d). The EC2 + LPS group showed a significant increase in neutrophil count as compared to EC2.
Comparison between EC1 and EC2 revealed no difference in TLC, neutrophil and lymphocyte count (Fig. 1b–d). However, when combined with LPS, EC2 + LPS showed a significant increase in lymphocyte count compared to EC1 + LPS (Fig. 1d).
Bronchoalveolar lavage fluid
Lungs of mice challenged with LPS showed increase in TLC and neutrophil count of bronchoalveolar lavage (BAL) fluid (Fig. 1e, f) compared to the control. However, EC1 did not alter TLC and neutrophil count of BAL fluid compared to the control and the LPS alone. Furthermore, EC1 + LPS induced an increase in TLC and neutrophil count of BAL fluid compared to the control and the EC1 groups (Fig. 1e, f).
EC2 mice showed a significant decrease in neutrophil numbers compared to the LPS group (Fig. 1f) but not compared to the control group. Ethion at 2 mg kg−1 along with LPS (EC2 + LPS) increased TLC and neutrophil count of BAL fluid compared to the EC2 and the control groups. There were no differences in the TLC and neutrophils numbers between the EC1 and EC2, with or without LPS, groups (Fig. 1e, f).
Histopathological examinations
The mice from the control group showed normal histoarchitecture of the lung characterized by the normal alveolar septa and airways epithelium (Table 1; Fig. 2a, b). Semi-quantitative histology revealed that treatment with LPS or both doses of ethion increased (p < 0.05) perivascular and peribronchial accumulation of inflammatory cells along with expansion of perivascular space compared to the control (Table 1; Fig. 2c–f, i, j). The LPS treatment of both EC1 and EC2 mice caused thickening of the alveolar septal wall, peribronchial and perivascular infiltration with increase in size of the perivascular space compared to the control (Fig. 2g–l; Table 1). The data taken together suggested ethion induced lung inflammation with or without LPS.
H&E staining of lung sections showing normal architecture of the airways epithelium (arrow) and alveolar septa in control group (2a, b). Also seen is peribronchial (arrow) and perivascular infiltration (double arrow), infiltration of inflammatory cells around alveolar septa (arrow head), perivascular space (X) and thickening of septal wall (star). Representative histopathological changes in the LPS group (2c, d), EC1 group (2e, f), EC1+ LPS (2 g, h), EC2 (2i, j) and EC2 + LPS (2 k, l) are shown. B bronchiole, BV blood vessel; original magnification 2a,2c,2e,2 g,2i,2k: × 100; 2b,2d,2f,2 h,2j,2l: × 400; inset: × 1000; scale bar = 50 μm
mRNA and protein expression
The pulmonary expression of TLR-4, TLR-9, IL-1β and TNF-α were examined at mRNA (Fig. 3) and protein levels by using RT-PCR and immunohistochemical methods, respectively.
Toll-like receptor 4
There was a 4.68-fold increase in expression of TLR-4 mRNA in the lungs of mice treated with only LPS compared to the control (Fig. 3a). Further, EC1and EC2 mice did not show changes in the expression of TLR-4 mRNA but in combination with LPS an increase in TLR-4 expression compared to the control was noticed.
The omission of primary antibody did not show any color development in the lung sections (Fig. 4e). The sections incubated with vWF stained only the endothelium (Fig. 4f). The airway epithelial and alveolar septal cells in the lungs of control animals showed weak TLR-4 reaction (data not shown). LPS alone or in combination with ethion (EC1 + LPS, EC2 + LPS) resulted in a significant increase in the number of TLR-4 positive cells compared to the control (Fig. 4a). However, neither EC1 nor EC2 treatments altered the number of TLR-4 expressing cells (Fig. 4a).
The number of a TLR-4, b TLR-9, c IL-1β and d TNF-α positive cells after long-term ethion exposure in combination with LPS. Lung section stained without primary antibody (4e) does not show any color development in the airways epithelium and staining with vWF (4f) showed immunopositive reaction in endothelial cells (arrow). Representative images of TLR4 (4g), TLR9 (4h), IL-1β (4i) and TNF-α (4j) immunopositive cells. Original magnification 4e,4f,4g,4h,4i,4j,4k: × 400. a,b,c,d: no common superscript between two levels of an effect indicates significant difference (p < 0.05) among groups
Toll-like receptor 9
The TLR9 mRNA expression did not vary in the LPS and EC2 groups compared to the control; however, EC1, EC1 + LPS and EC2 + LPS increased the expression compared to the control (Fig. 3b).
The normal lung showed minimal reaction for TLR-9 mainly localized in alveolar septal cells, vascular and bronchial endothelium (data not shown). Treatment with LPS increased TLR-9 immunopositive cells compared to the control (Fig. 4b). EC1 or EC2 did not alter the number of TLR-9 reactive cells (Fig. 4b). However, EC1 + LPS and EC2 + LPS significantly increased the numbers of cells stained for TLR-9 compared to the control (Fig. 4b).
Interleukin 1β
LPS or EC1 alone increased the IL-1β mRNA expression by 4.56- and 2.2-folds as compared to the control, respectively (Fig. 3c). However, EC2 did not modify the IL-1β mRNA expression compared to the control. Further, EC1 + LPS and EC2 + LPS increased the IL-1β mRNA expression compared to the control, EC1 and EC2 but did not differ from the LPS group.
The bronchial epithelial and septal cells showed weak IL-1β immunoreactivity in the control and EC1 group (data not shown). LPS and EC1 + LPS significantly increased immunopositive IL-1β cells in the alveolar epithelium and alveolar septa (Fig. 4c). However, EC2 and EC2 + LPS did not differ in terms of number of IL-1β immunopositive cells from each other and from other groups (Fig. 4c).
Tumor necrosis factor-α
There was no significant change in TNF-α mRNA expression following treatment with LPS and/or both doses of ethion (Fig. 3d). However, TNF-α staining was localized in the airway epithelial cells, septal cells and cells adhering vascular endothelium. The expression was more intense towards the luminal side of the bronchial epithelium (data not shown). LPS showed a significant increase in the number of TNF-α immunopositive cells compared to all other groups (Fig. 4d). Further, an increase in TNF-α expression was observed in EC1 + LPS compared to the control (Fig. 4d).
Single cell gel electrophoresis
The damaged cells showed the appearance of comet while undamaged cells were intact without a tail. LPS or EC1 resulted in increase in tail length, tail DNA percent and olive moment and a decrease in head DNA percent compared to the control (Fig. 5). The tail and head parameters did not show any significant differences between the LPS and EC1 groups. However, EC1 + LPS showed a significant increase in tail length and olive moment compared to EC1 alone and a significant increase in tail DNA percent and a significant decrease in head DNA percent compared to LPS alone. EC2 did not induce a change in the tail parameters (Fig. 5) compared to the control; however, in combination with LPS (EC2 + LPS), there was a significant (p < 0.05) increase in tail parameters and a significant (p < 0.05) decrease in head DNA percent compared to the control. The comparison between both the treatments revealed that EC1 resulted in a significant increase in tail DNA percent and olive moment and a significant decrease in head DNA percent compared to EC2, suggesting long-term ethion exposure at 4 mg kg−1 has genotoxic potential.
There was a significant positive correlation of tail length with tail DNA percent (r = 0.92, p < 0.05), tail moment (r = 0.97, p < 0.05) and olive moment (r = 0.98, p < 0.05) between the control and LPS (Fig. 6). Ethion at 4 mg kg−1 with/without LPS showed a significant positive correlation of tail length with tail moment and olive moment (r = 0.84, p < 0.05). Similarly, ethion at 2 mg kg−1 alone/with LPS showed a significant correlation of tail length with tail moment (r = 0.93, p < 0.05) and olive moment (r = 0.91, p < 0.05).
Discussion
Ethion has been and is being extensively used in agriculture. There is however scarcity of data on the pulmonary effects of ethion especially in combination with LPS. Therefore, we investigated the pulmonary effects along with the genotoxic potential of ethion alone or in combination with LPS in a mouse model. The data show induction of pulmonary inflammation and genotoxicity in mice exposed to ethion.
In the present study, both ethion exposures (EC1 and EC2) significantly reduced the average body weight gain compared to the control group as reported earlier following exposure to OP pesticide chlorpyriphos (Ambali et al. 2011) and oral administration of 2.7 mg kg−1day−1ethion for 7–28 days in rats (Bhatti et al. 2010).
We found that LPS exposure alone significantly increased TLC of blood along with neutrophilia compared to the control. LPS increased peripheral blood leukocytes and neutrophils (Copeland et al. 2005). Interestingly, exposure to 4 mg kg−1 ethion decreased TLC and was accompanied by lymphocytopenia compared to the control, which has also been observed after exposure to indoxacarb in mice (Kaur et al. 2016b), organophosphate in rats (Ambali et al. 2011; Jiv and Roy 2013), lambda-cyalothrin in female rabbits (Basir et al. 2011), fenvalerate, monocrotophos and endosulfan in broiler chickens (Garg et al. 2004). Reduced blood TLC may be directly related to either decreased production of blood cells from the lymphoid organs or augmented cell lysis (Sinha and Thaker 2014). Infiltration of lymphocytes into organs where the pesticide is acting may also contribute to lymphocytopenia in peripheral blood. However, 2 mg kg−1 ethion alone or in combination with LPS did not alter TLC, neutrophil and lymphocyte percentages of blood compared to the control. The data taken together suggest that ethion at 4 mg kg−1 with or without LPS resulted in significant lymphocytopenia.
Lung inflammation was determined through analyses of BAL, histopathology and immunohistochemistry. BAL fluid cytology is used for the diagnosis and evaluation of lung inflammation (Meyer 2007). In the present study, LPS challenge increased TLC and neutrophils in BAL fluid compared to the control. Increase in BAL cell number is considered a hallmark of lung inflammation (Lucas et al. 2002; Mei et al. 2007). Although pesticides such as chlorpyriphos (Chougule et al. 2013) and carbaryl (Dong et al. 1998) have been shown to increase neutrophil counts in BAL fluid, the exposure to ethion did not alter the TLC and neutrophil count in BAL fluid compared to the control. The combination of ethion and LPS however increased TLC and neutrophil numbers in BAL fluid to suggest that LPS may be the primary agonist in this process. The semi-quantitative data from the present study showed that both dosages of ethion induced lung inflammation, which was illustrated by infiltration of inflammatory cells around the perivascular and peribronchial regions. Pesticides such as imidacloprid (Pandit et al. 2016) and fipronil (Merkowsky et al. 2016) in mice, diazinon in the guinea pig (Rady 2009) and OP pesticides in various experimental animals (Alavanja et al. 2004; Hemmati et al. 2005; Yavuz et al. 2008) induced lung inflammation. Taken together, the data showed that ethion alone or in combination with LPS induced lung inflammation.
Toll-like receptors are central to host responses to microbial molecules in various organs including the lung. While bacterial LPS binds to and signals via TLR-4, bacterial CpG molecules interact with TLR-9 to induce their cellular effects including release of various inflammatory cytokines (Lu et al. 2008; An et al. 2002). To understand the mechanisms of lung inflammation observed in our studies, we examined the expression of TLR-4 and TLR-9 mRNA and protein in the lungs. E. coli LPS challenge significantly increased the mRNA expression of TLR-4 and produced an intense TLR-4 reaction in septal and airway epithelial cells compared to the control. LPS stimulation upregulates gene expression of TLR-4 (Lu et al. 2008) and TLR-9 (An et al. 2002) via the NF-kB and p38 MAPK signal pathways, respectively. TLR-4 further triggers signaling events to activate host defense in human and mice (Flo et al. 2001; Takeda and Shizuo 2004). Ethion at 4 mg kg−1 increased TLR-9 mRNA expression but in combination with LPS it significantly increased TLR-4 and TLR-9 mRNA expression along with immunopositive activity in airway epithelial cells compared to the control. TLR-9 expression increases in physiological stress in the lungs and is associated with the severity of lung injury (Droemann et al. 2005). Pesticides such as paraquat induce acute lung injury leading to elevated TLR-9 mRNA expression in the lung (Qian et al. 2015). The data taken together suggested that ethion at a higher dose (4 mg kg−1) alone or in combination with LPS at both the doses increased TLR-9 gene transcription while TLR-4 expression was mainly elevated due to LPS. Further studies using TLR-4- and TLR-9-deficient mice are needed to elucidate the specific roles of these molecules in lung inflammation induced by ethion alone or in combination with LPS.
The stimulation of TLR-4 induces the expression of critical pro-inflammatory cytokines (IL-1β and TNF-α) that are crucial to trigger strong immune responses. IL-1β (Dinarello 1996; Becker et al. 1999) and TNF-α (Baer et al. 1998) are expressed during lung inflammation or injury. IL-1β is one of the most important cytokines implicated in the commencement and prevalence of lung inflammation (Dinarello 1996) and is produced by alveolar macrophages (Becker et al. 1999). Ethion at 4 mg kg−1 alone significantly increased the expression of IL-1β mRNA. The data suggested that ethion-induced IL-1β increase is independent of TLR-4. Our experiments did not explain the mechanism (s) but it may be attributed to activation of other pathways viz. P2X7 receptor activation, IL-converting enzyme protease-activating factor and NLRP1 that activate caspase 1 (Grishman et al. 2012). Further, ethion at 4 mg kg−1 in combination with LPS also significantly increased the expression of IL-1β mRNA, IL-1β immunopositive reactivity and number of immunopositive IL-1β cells, suggesting LPS-induced TLR-4 activation. The data suggested that histological signs of lung inflammation may be due to induction of IL-1β through activation of TLR-4 following challenge with ethion alone or when combined with LPS.
Interestingly, no significant changes were found in TNF-α gene expression among different groups as reported earlier with imidacloprid (Pandit et al. 2016) and parathion (Proskocil et al. 2013). However, the immunopositive TNF-α was localized in airway epithelial, septal cells and vascular endothelium. On the contrary, dichlorodiphenyltrichloroethane (DDT) induced an increase in the expression of TNF-α along with apoptosis through a p38 mitogen activated protein kinases (MAPK)-dependent mechanism in endometrial Ishikawa and human embryonic kidney (HEK) 293 cells (Frigo et al. 2004). Our experiments did not explain the reasons for the lack of changes in TNF-α mRNA expression. But it may be possible that lung inflammation induced by ethion alone or along with LPS might be employing inflammatory mediators other than TNF-α or we might have missed the time point when TNF-α mRNA was altered. The data taken together suggested mRNA expression of TLR-4, TLR-9, IL-1β and TNF-α did not vary following co-exposure to ethion and LPS compared to LPS alone, suggesting no synergistic effect between ethion and LPS.
Finally, we evaluated the genotoxic effects of ethion because pesticides can induce cell death, genomic instability and cancer (Luzhna et al. 2013). The comet assay is a reliable tool in the domain of genetic toxicology (Betti et al. 1993) due to its potential to identify damaged DNA based on differential migration when subjected to electrophoresis (Lee and Steinert 2003). The whole blood usage for single cell gel electrophoresis circumvents the tedious job of separating different cells, which may in turn lead to oxidative DNA damage (Speit et al. 2003). Long-term ethion exposure at 4 mg kg−1 revealed a significant increase in tail parameters and a significant decrease in head DNA percent, suggesting the genotoxic potential of ethion at 4 mg kg−1. The ethion-induced DNA damage has been reported earlier using chromosomal aberrations in chicks (Bhunya and Jena 1994) and Anopheles culicifacies (Marwaha 2015). Malathion also caused DNA damage in a freshwater teleost (Kumar et al. 2010) and rats (Réus et al. 2008). We found that LPS exposure also resulted in a significant increase in tail length, tail DNA percent, olive moment and a significant decrease in head DNA percent, suggesting DNA damage as observed earlier in mice (Kaur et al. 2016b). Further, ethion at 4 mg kg−1 combined with LPS resulted in a significant increase in olive moment compared to LPS alone. Whereas ethion at 2 mg/kg alone did not show any genotoxic potential, its combination with LPS caused a significant increase in tail length, tail DNA percent, olive moment and a decrease in head DNA percent compared to the control. The genotoxin effects could be due to the oxidative stress generated by chronic exposure to pesticides (Abdollahi et al. 2004). The data indicate ethion at 4 mg/kg induced genotoxicity and the synergistic genotoxic effect of co-exposure of ethion with LPS.
Conclusions
We conclude that long-term exposure of ethion at 2 and 4 mg/kg induces lung inflammation and ethion at 4 mg/kg alone or in combination with LPS results in genotoxicity in blood cells. The data are significant because the dosages of ethion used in our study are within MRL and may have implications for human health including genotoxicity. However, further studies are warranted for better understanding of molecular mechanisms of effects of ethion on the pulmonary system, mechanisms underlying DNA damage and cell death and its potential impact on other organs such as the liver.
References
Abdollahi M, Mostafalou S, Pournourmohammadi S, Shadnia S (2004) Oxidative stress and cholinesterase inhibition in saliva and plasma of rats following subchronic exposure to malathion. Comp Biochem Physiol C Toxicol Pharmacol 137:29–34
Aktar MW, Sengupta D, Chowdhury A (2009) Impact of pesticides use in agriculture: their benefits and hazards. Interdiscip Toxicol 2:1–12
Alavanja M, Hoppin J, Kamel F (2004) Health effects of chronic pesticide exposure: cancer and neurotoxicity. Annu Rev Public Health 25:155–197
Ambali SF, ShuaibK ER, Orieji BC, Shittu M, Akande M (2011) Hyperglycemia induced by subchronic co-administration of chlorpyrifos and lead in Wistar rats: role of pancreatic lipoperoxidation and alleviating effect of vitamin C. Biol Med 3:6–14
An H, Xu H, Y Y, Zhang M, Qi R, Yan X, Liu S, Wang W, Guo Z, Qin Z, Cao X (2002) Up-regulation of TLR9 gene expression by LPS in mouse macrophages via activation of NF-κB, ERK and p38 MAPK signal pathways. Immunol Lett 81:165–169
Andonegui G, Bonder CS, Green F, Mullaly SC, Zbytnuik L, Raharjo E, Kubes P (2003) Endothelium-derived Toll-like receptor-4 is the key molecule in LPS-induced neutrophil sequestration into lungs. J Clin Invest 111:1011–1020
Baer M, Dillner A, Schwartz RC, Sedon C, Nedospasov S, Johnson PF (1998) Tumor necrosis factor alpha transcription in macrophages is attenuated by an autocrine factor that preferentially induces NF-κB p50. Mol Cell Biol 18:5678–5689
Basir A, Khan A, Mustafa R, Khan MZ, Rizvi F, Mahmood F, Yousaf A (2011) Toxicopathological effects of lambdacyhalothrin in female rabbits (Oryctolagus cuniculus). Human Exp Toxicol 30:591–602
Becker S, Clapp WA, Quay J, Frees KL, Koren HS, Schwartz D (1999) Involvement of microbial components and toll-like receptors 2 and 4 in cytokine responses to air pollution particles. Am J Resp Crit Care Med 160:1309–1318
Betti C, Barale R, Pool-Zobel BVL (1993) Comparative estudies on cytotoxic and genotoxic effects of two organic mercury compounds in lymphocytes and gastric mucosa cells of Spraque–Dawley rats. Environ Mol Mutagen 22:173–180
Bhatti GK, Kiran R, Sandhir R (2010) Modulation of ethion-induced hepatotoxicity and oxidative stress by vitamin E supplementation in male Wistar rats. Pest Biochem Physiol 98:26–32
Bhatti GK, Bhatti JS, Kiran R, Sandhir R (2011) Biochemical and morphological perturbations in rat erythrocytes exposed to ethion: protective effect of vitamin E. Cell Mol Biol 57:70–79
Bhunya SP, Jena GB (1994) Evaluation of genotoxicity of a technical grade organophosphate insecticide, Tafethion (ethion), in chicks. In Vivo 8:1087–1089
Caixia G, Tiankui Q, Bin Z, Sujuan Y (2015) Effect of Toll-like receptor 9 expression in postoperative radiotherapy in lung cancer survivors. Carcinog Teratog Mutagen 27:294–298
Chougule AA, Sethi RS, Schneberger D, Brar RS, Gill JPS, Singh B (2013) Chlorpyriphos induces lung inflammation and alters response to E. coli lipopolysaccharide challenge. FASEB J 27:1166.17
Copeland S, Warren HS, Lowry SF, Calvano SE, Remick D (2005) Acute inflammatory response to endotoxin in mice and humans. Clin Vaccine Immunol 12:60–67
Devi KD, Banu BS, Grover P, Jamil K (2000) Genotoxic effect of lead on mice using SCGE (comet assay). Toxicol 145:195–201
Dewan A, Patel AB, Pal RR, Jani UJ, Singel VC, Panchal MD (2008) Mass ethion poisoning with high mortality. Clin Toxicol Phila 46:85–88
Dinarello CA (1996) Biologic basis for interleukin-1 in disease. Blood 87:2095–2147
Dong W, Gilmour MI, Lambert AL, Selgrade MK (1998) Enhanced allergic responses to house dust mite by oral exposure to carbaryl in rats. Toxicol Sci 44:63–69
Droemann D, Albrecht D, Gerdes J, Ulmer AJ, Branscheid D, Vollmer E, Dalhoff K, Zabel P, Goldmann T (2005) Human lung cancer cells express functionally active Toll-like receptor 9. Resp Res 6:1
Duramad P, Tager IB, Leikauf J, Eskenazi B, Holland NT (2006) Expression of Th1/Th2 cytokines in human blood after in vitro treatment with chlorpyrifos, and its metabolites, in combination with endotoxin LPS and allergen Der p1. J Appl Toxicol 26:458–465
EPA (U.S. Environmental Protection Agency) (1989) Pesticide factsheet number 209: Ethion. Office of pesticides and toxic substances, Washington, D.C., pp 5–64
Fareed M, Pathak MK, Bihari V, Kamal R, Srivastava AK, Kesavachandran CN (2013) Adverse respiratory health and hematological alterations among agricultural workers occupationally exposed to organophosphate pesticides: a cross-sectional study in North India. PLoS One 8:e69755
Flo TH, Halaas O, Torp S, Ryan L, Lien E, Dybdahl B, Sundan A, Espevik T (2001) Differential expression of Toll-like receptor 2 in human cells. J Leukoc Biol 69:474–481
Frigo DE, Tang Y, Beckman BS, Scandurro AB, Alam J, Burow ME, McLachlan JA (2004) Mechanism of AP-1-mediated gene expression by select organochlorines through the p38 MAPK pathway. Carcinogenesis 25:249–261
Garg UK, Pal AK, Jha GJ, Jadhao SB (2004) Pathophysiological effects of chronic toxicity with synthetic pyrethroid, organophosphate and chlorinated pesticides on bone health of broiler chicks. Toxicol Pathol 32:364–369
Gosselin RE, Smith RP, Hodge HC (1984) Clinical toxicology of commercial products, 5th edn. Williams and Wilkins, Baltimore, pp 5–45
Grishman EK, White PC, Savani RC (2012) Toll-like receptors, the NLRP3 inflammasome, and interleukin-1β in the development and progression of type 1 diabetes. Pediatr Res 71:626–632
Gyori BM, Venkatachalam G, Thiagarajan PS, Hsu D, Clement MV (2014) OpenComet: an automated tool for comet assay image analysis. Redox Biol 2:457–465
Hemmati AA, Nazari Z, Ghafurian N, Ranjbari N, Darabi B (2005) Study of the effects of sodium cromlyn on levels of TNFα, IL1-β and collagen in the paraquat induced pulmonary fibrosis in rat. Toxicol Lett 158:S139–S139
Jiv KS, Roy AK (2013) Role of curcumin and cumin on hematological parameters of Profenofos exposed mice—Mus Musculus. Int J Current Pharmaceutical Rev Res 4:120–127
Kaur S, Mukhopadhyay CSM, Sethi RS (2016a) Chronic exposure to indoxacarb and pulmonary expression of TLR-9 in mice. Veterinary World 9:1282–1286
Kaur S, Mukhopadhyay CSM, Arora JS, Sethi RS (2016b) Indoxacarb interaction alters immunotoxic and genotoxic potential of endotoxin. J Pest Sci 41:65–70
Kemabonta KA, Akinhanmi FO (2013) Toxicological effects of Chlorpyrifos, Dichlorvos and Alpha Cypermethrin on adult albino mice, Mus musculus. PAT. 9:1–17 ISSN: 0794–5213
Kidd H, James DR (1991) The agrochemicals handbook, 3rd edn. Royal Society of Chemistry Information Services, Cambridge
Kitamura Y, Hashimoto S, Mizuta N, Kobayashi A, Kooguchi K, Fujiwara I, Nakajima H (2001) Fas/FasL-dependent apoptosis of alveolar cells after lipopolysaccharide induced lung injury in mice. Am J Resp Crit Care Med 163:762–769
Kumar R, Nagpure NS, Kushwaha B, Srivastava SK, Lakra WS (2010) Investigation of the genotoxicity of Malathion to freshwater teleost fish Channa Punctatus (Bloch) using the micronucleus test and comet assay. Arch Environ Contam Toxicol 58:123–130
Lee RF, Steinert S (2003) Use of the single cell gel electrophoresis/comet assay for detecting DNA damage in aquatic (marine and freshwater) animals. Mutation Res 544:43–64
Liebers V, Brüning T, Raulf-Heimsoth M (2006) Occupational endotoxinexposure and possible health effects on humans. Am J Ind Med 49:474–491
Lu YC, Yeh WC, Ohashi PS (2008) LPS/TLR4 signal transduction pathway. Cytokine 42:145–151
Lucas R, Lijnen HR, Suffredini AF, Pepper MS, Steinberg KP, Martin TR, Pugin J (2002) Increased angiostatin levels in bronchoalveolar lavage fluids from ARDS patients and from human volunteers after lung instillation of endotoxin. Thromb and Haemost 87:966–971
Luzhna L, Kathiria P, Kovalchuk O (2013) Micronuclei in genotoxicity assessment: from genetics to epigenetics and beyond. Front Genet 4:131
Marwaha L (2015) In vivo mutagenicity assessment of ethion pesticide using polytene chromosomes of Anopheles culicifacie. Asian J Pharmacol Toxicol 3:7–13
Mathur SC (2010) Future of Indian pesticides industry in next millennium. Pestic Inf 24:9–23
Mei SH, McCarter SD, Deng Y, Parker CH, Liles WC, Stewart DJ (2007) Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med 4:e269
Merkowsky K, Sethi RS, Gill JPS, Singh B (2016) Fipronil induces lung inflammation in vivo and cell death in vitro. J Occup MedToxicol 11:10. https://doi.org/10.1186/s12995-016-0102-0
Meyer KC (2007) Bronchoalveolar lavage as a diagnostic tool. Semin Respir Crit Care Med 28:5
Nagajyothi F, Desruisseaux MS, Machado FS, Upadhya R, Zhao D, Schwartz GJ, Teixeira MM, Albanese C, Lisanti MP, Chua SC, Weiss LM, Scherer PE, Tanowitz HB (2012) Response of adipose tissue to early infection with Trypanosoma cruzi (Brazil strain). J Infect Dis 205:830–840
Pandit AA, Choudhary S, Ramneek SB, Sethi RS (2016) Imidacloprid induced histomorphological changes and expression of TLR-4 and TNFα in lung. Pest Biochem Physiol. https://doi.org/10.1016/j.pestbp.2016.02.004
Pandit AA, Mukhopadhyay CS, Verma R, Singh B, Sethi RS (2017) Expression of TLR-9 and IL-1β following concomitant exposure to imidacloprid and endotoxin. Pestic Res J 29:243–250
Paranjape K, Gowariker V, Krishnamurthy VN, Gowariker S. (2014). The pesticide encyclopedia. Cabi
Proskocil BJ, Bruun DA, Jacoby DB, van Rooijen N, Lein PJ, Fryer AD (2013) Macrophage TNF-α mediates parathion-inducedairway hyperreactivity in guinea pigs. Am J Physiol Lung Cell Mol Physiol 304:L519–LL29
Qian J, Liu L, Chen L, Lu X, Zhu C (2015) Increased toll-like receptor 9 expression is associated with the severity of paraquat-induced lung injury in mice. Hum Exp Toxicol 34:430–438
Rady MI (2009) Effects of exposure to Diazinon on the lung and small intestine of Guinea pig, histological and some histochemical changes. Braz Arch Biol Technol 52:317–326
Réus GZ, Valvassori SS, Nuernberg H, Comim CM, Stringari RB, Padilha PT, Leffa DD, Tavares P, Dagostim G, Paula MM, Andrade VM, Quevedo J (2008) DNA damage after acute and chronic treatment with malathion in rats. J Agric Food Chem 56:7560–7565
Schneberger D, CaldwellS KR, Singh B (2013) Expression of Toll-like receptor 9 in mouse and human lungs. J Anat 222:495–503
Sethi RS, Brar RS, Singh O, Singh B (2011) Immunolocalization of pulmonary intravascular macrophages, TLR4, TLR9 and IL-8 in normal and Pasteurella multocida-infected lungs of water buffalo (Bubalus bubalis). J Comparative Pathol 144:135–144
Sethi RS, Schneberger D, Singh B (2013) Characterization of the lung epithelium of wild-type and TLR9(-/-) mice after single and repeated exposures to chicken barn air. Exp Toxicol Pathol 65:357–364
Sethi RS, Schneberger D, Charavarymath C, Singh B (2017) Pulmonary innate inflammatory responses to occupational contaminants of farming. Cell Tissue Res 367:627–642
Singh AK, Jiang Y (2002) Immunotoxicity of acute acephate exposure in control or IL-1-challenged rats: correlation between the immune cell composition and corticosteroid concentration in blood. J Appl Toxicol 22:279–291
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low level of DNA damage in individual cells. Exp Cell Res 17:184–187
Sinha S, Thaker AM (2014) Study on the impact of lead acetate pollutant on immunotoxicity produced by thiamethoxam pesticide. Ind J Pharmacol 46:596–600
Speit G, Witton-Davies T, Heepchantree W, Trenz K, Hoffman H (2003) Investigations on the effect of cigarette smoking in the comet assay. Mutat Res 542:33–42
Srivastava S, Narvi SS, Prasad SC (2011) Levels of select organophosphates in human colostrum and mature milk samples in rural region of Faizabad district, Uttar Pradesh, India. Hum Exp Toxicol 30:1458–1463
Takeda K, Shizuo A (2004) TLR signaling pathways. Semin Immunol 16:3–9
Tewari A, Sethi RS, Banga HS, Singh B, Gill JPS (2017) Concomitant effect of low dose of lindane and intranasal lipopolysaccharide on respiratory system of mice. Human Exp Toxicol. https://doi.org/10.1177/0960327116685889
Thakur J, Rao B, Rajwanshi A, Parwana HK, Kumar R (2008) Epidemiological study of high cancer among rural agricultural community of Punjab in Northern India. Int J Env Res Public Health 5:399–407
Thorn J (2001) The inflammatory response in humans after inhalation of bacterial endotoxin: a review. Inflamm Res 50:254–261
Tschernig T, Janardhan KS, Pabst R, Singh B (2008) Lipopolysaccharide induced inflammation in the perivascular space in lungs. J Occup Med Toxicol 3:17. https://doi.org/10.1186/1745-6673-3-17
Villar SR, Ronco MT, Fernandez BR, Roggero E, Lepletier A, Manarin R, Savino W, Perez AR, Bottasso O (2013) Tumor necrosis factor-α regulates glucocorticoid synthesis in the adrenal glands of Trypanosomacruzi acutely-infected mice. PLoS One 8:e63814
Yavuz Y, Yurumez Y, Ciftci IH, Sahin O, Saglam H, Buyukokuroglu M (2008) Effect of diphenhydramine on myocardial injury caused by organophosphate poisoning. Clin Toxicol Phila 46:67–70
Yuan H, Luigi F, Gabriel N (2013) Toll-like receptor agonists stimulate Nlrp3-dependent IL-1β production independently of the purinergic P2X7 receptor in dendritic cells and in vivo. J Immunol 190:334–339
Zuo DC, Choi S, Shahi PK, Kim MY, Park CG, Kim YD, Lee J, Chang IY, Lee HS, Yeom SC, Moon HJ, Seong SY, So I, Jun JY (2012) Action of lipopolysaccharide on interstitial cells of cajal from mouse small intestine. Pharmacology 90:151–159
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The experiment protocols were permitted by the Institutional Animal Ethics Committee, Guru Angad Dev Veterinary and Animal Sciences University (GADVASU), Ludhiana.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the Institutional Animal Ethics Committee, Guru Angad Dev Veterinary and Animal Sciences University (GADVASU), Ludhiana.
Rights and permissions
About this article
Cite this article
Verma, G., Mukhopadhyay, C.S., Verma, R. et al. Long-term exposures to ethion and endotoxin cause lung inflammation and induce genotoxicity in mice. Cell Tissue Res 375, 493–505 (2019). https://doi.org/10.1007/s00441-018-2912-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-018-2912-0