Abstract
We have recently reported that Sox2-expressing pituitary stem/progenitor cells contact each other via a tight-junction protein CAR to form stem/progenitor cell niches in the marginal cell layer facing the lumen and in the clusters scattered in the parenchyma of the anterior lobe. However, the microenvironment of the niche for the maintenance of stem cell function in the pituitary remains obscure. In this study of pituitary stem/progenitor cell niches, we have attempted to identify the expression of juxtacrine factor ephrin and its receptor. We have found that ephrin-B2 is expressed in the pituitary throughout development but changes its localization pattern. Notably, in the adult pituitary, ephrin-B2 immuno-signals occur in SOX2-, E-cadherin-, and CAR-triple-positive stem/progenitor cells in the niches. Our data suggest that ephrin-B2 signaling has an important role in the formation of pituitary stem/progenitor cell niches and in pituitary organogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pituitary gland is constituted of two anatomically different entities, the adenohypophysis (anterior pituitary) composed of the anterior and intermediate lobes, and the neurohypophysis of the posterior lobe. In particular, the anterior lobe has five endocrine cell types, and a number of transcription factors and growth factors regulate differentiation into each cell type during embryonic organogenesis (Davis et al. 2010; Vankelecom and Chen 2014; Zhu et al. 2007). In addition, non-endocrine cells, of which a major fraction is known to be folliculostellate cells as defined by the presence of S100β, are also present (Vila-Porcile 1972). Mechanisms of regeneration and of the cell supply system from stem/progenitor cells in the adult pituitary are important issues. Recent studies have demonstrated that sex-determining region Y-box 2 (Sox2)-expressing cells play an important role in the regeneration of endocrine cells in vivo and in vitro (Andoniadou et al. 2013; Chen et al. 2009; Fauquier et al. 2008; Fu et al. 2012; Fu and Vankelecom 2012; Rizzoti et al. 2013). In addition, we have reported that SOX2-positive stem/progenitor cells have some populations defined by a pituitary-specific transcription factor PROP1 (prophet of PIT1) and a paired-related homeobox transcription factor, namely PRRX1 and 2, in the adult pituitary (Higuchi et al. 2014; Yoshida et al. 2009, 2011).
Stem/progenitor cells are known to maintain their stemness in the microenvironment, the so-called niche, which has been identified in some tissues such as the brain, intestine, and skin (Chen and Chuong 2012). Recently, Chen et al. (2013) have reported that CAR (coxsackievirus and adenovirus receptor encoded by the Cxadr gene), which is able to form the homophilic tight junction, exists in the SOX2/E-cadherin-double-positive cells in the marginal cell layer (MCL) and in the clusters scattered in the parenchyma of the anterior lobe, forming the pituitary stem/progenitor niches. These data show that cell adhesion has an important role in the regulation of pituitary stem cell function, as similarly observed in niches such as the crypts in the intestine (Pitulescu and Adams 2010; Sato and Clevers 2013).
Recent studies have revealed that ephrin signaling exists in various niches such as the subventricular zone in the brain (Nomura et al. 2010), the crypts of the intestine (Batlle et al. 2002), and the hair follicles of the skin (Genander et al. 2010), and that it regulates stem cell function. Ephrin and its receptor are signal molecules localized in cell membranes and have a unique juxtacrine signaling mechanism that is activated by cell adhesion. Their interaction raises bidirectional signal transduction into both the ephrin receptor-expressing cell (this process is termed forward signaling) and the ephrin-expressing cell (reverse signaling), and plays a role in cell locomotion and boundary formation via the regulation of cell repulsion and migration in many tissues (Batlle et al. 2002; Daar 2012; Holmberg et al. 2006). Ephrin receptors are a subfamily of receptor tyrosine kinases and are composed of two subclasses: receptor A with nine members and receptor B with five members (Murai and Pasquale 2003; Pasquale 2005). Their ligands, the ephrins (Eph receptor interacting proteins), are cell-surface associated proteins and are divided into two subclasses: ephrin-A with five members and ephrin-B with three members based on their structure and function. Whereas ephrin-As typically bind to receptor-A, and ephrin-Bs bind to receptor-B, a few exceptions, such as ephrin-B2 and -B3, bind to receptor-A4 (Jensen 2000). In particular, ephrin-B signaling has been reported to construct stem/progenitor cell niches in various tissues, such as the crypt (Batlle et al. 2002), subgranular zone (Ashton et al. 2012), and subventricular zone (Nomura et al. 2010). In the crypt, which is known as a stem/progenitor cell niche in the intestine, ephrin signaling plays a role in holding ephrin-receptor-B2-positive stem cells (crypt base columnar cell) and their niche cells, i.e., ephrin-receptor-B3-positive Paneth cells, in the bottom of the crypt via repulsion against ephrin-B1 and -B2-positive differentiating progenitor cells and terminally differentiated cells (Batlle et al. 2002). During neurogenesis, ephrin-B2 is expressed in astrocytes and promotes neuronal differentiation in the adult subgranular zone (Ashton et al. 2012). Moreover, Nomura et al. (2010) have reported that the inhibition of ephrin-B1 and -B2 or ephrin receptor-B1 and -B2 in the subventricular zone causes the transition of astrocytes into ependymal cells in the adult neural stem cell niche. Therefore, ephrin-B signaling plays a role in the formation and regulation of niches. Although Vankelecom (2010) has reported that some ephrin-Bs and their receptor-Bs are enriched in the pituitary stem cell-side population fraction, no follow-up studies have been reported on ephrin and its receptor in the pituitary.
In this study, our aim has been to identify the expression of juxtacrine factor ephrin-B and its receptors in the pituitary stem/progenitor cell niches by immunohistochemistry. We have been able to demonstrate that a juxtacrine factor ephrin-B2 exists in the SOX2-, E-cadherin-, and CAR-triple-positive stem/progenitor cells in the MCL and in the niches scattered in the parenchyma of the anterior lobe. These data suggest that ephrin-B2 signaling plays an important role in the formation of the pituitary stem/progenitor cell niches.
Materials and methods
Animals
Intact male Wistar-Imamichi rats and Wistar-crlj S100β-GFP (green fluorescent protein) transgenic strain rats (Itakura et al. 2007) were housed individually in a temperature-controlled room under a 12-h light/12-h dark cycle. Determination of pregnancy was made by the observation of a vaginal plug on day 0.5 of gestation. The present study was approved by the Institutional Animal Care and Use Committee, Meiji University, based on NIH Guidelines for the Care and Use of Laboratory Animals.
Quantitative real-time polymerase chain reaction
Total RNA was prepared from the whole pituitaries of embryonic day 13.5 (E13.5; n = 15), E14.5 (n = 10), E16.5 (n = 14), E18.5 (n = 11), E20.5 (n = 10), and postnatal day 0 (P0; n = 9) rats and from the anterior lobes and intermediate/posterior lobes of P5 (n = 9), P15 (n = 8), and P60 (n = 3) rats by using ISOGEN (Nippon Gene, Tokyo, Japan). Reverse transcripts were synthesized with PrimeScript Reverse Transcriptase (Takara Bio, Otsu, Japan) by using 1 μg total RNA after DNase I treatment and were subjected to quantitative real-time polymerase chain reaction (PCR) on an ABI Prism 7500 Real-Time PCR System (Applied Biosystems, Foster City, Calif., USA). Reactions were performed in SYBR Green-Real Time PCR Master Mix Plus (Toyobo, Osaka, Japan), including 0.6 μM of the specific primer set for each gene. Nucleotide sequences of the primers used were: rat ephrin-B2, 5′-CCAACAAGACGTCCAGAGC-3′ and 5′-CCTGCGAATAAGGCCACTT-3′; rat TATA-box-binding protein (Tbp), 5′-GATCAAACCCAGAATTGTTCTCC-3′ and 5′-ATGTGGTCTTCCTGAATCCC-3′. Each sample was measured in duplicate in two independent experiments, and data were calculated by the comparative CT method (DDCT method) to estimate the mRNA copy number relative to Tbp as an internal standard. The DNA sequence of the PCR product of each sample was confirmed by nucleotide sequencing (data not shown).
Immunohistochemistry
Immunostaining was performed after fixation of tissue with 95 % ethyl alcohol or the HOPE (HEPES-glutamic acid buffer-mediated organic solvent protection effect) Fixative System (Polysciences, Warrington, Penn., USA), which preserves antigenic structures without any type of protein cross-linking (Olert et al. 2001). The method of fixation was selected according to the combination of antibodies used for counter-staining (Table 1). Fixation and immunostaining were performed as follows: for ethyl alcohol fixation, freshly prepared pituitaries of E12.5, E13.5, E16.5, E19.5, P0, P3, P15, and P60 rats were embedded in Tissue-Tek O.C.T compound (Sakura Finetek Japan, Tokyo, Japan) and were frozen immediately. Cryosections (7 μm thick) from a sagittal plane for embryonic and P0 pituitaries and from a coronal plane for postnatal pituitaries were mounted on glass slides (Matsunami, Osaka, Japan), followed by fixation in 95 % ethyl alcohol for 30 min at −20 °C.
For HOPE-fixed paraffin sections, the pituitaries of P60 rats were fixed with HOPE solution I for 24 h at 4 °C, followed by immersion in an ice-cold HOPE solution II in acetone for 2 h at 4 °C and three times in acetone for 2 h. After dehydration, tissues were transferred immediately into pre-warmed low-melting-point paraffin and incubated overnight at 55 °C. Paraffin sections (6 μm thick) from a coronal plane were mounted on glass slides. After deparaffinization and hydration, sections were antigen-retrieved by an ImmunoSaver (0.05 % citraconic anhydride solution, pH 7.4; Nisshin EM, Tokyo, Japan) for 1 h at 80 °C.
For HOPE-fixed frozen sections, the pituitaries of P60 rats were fixed as described above, followed by immersion in 30 % trehalose in 20 mM HEPES to cryoprotect the tissues. They were then embedded in Tissue-Tek O.C.T compound and frozen immediately. Frozen sections (7 μm thick) were prepared from the coronal plane.
After being washed with 20 mM HEPES-100 mM NaCl, pH 7.5 (HEPES buffer), these sections were reacted with primary antibodies at appropriate dilutions (Table 1) with 10 % (v/v) fetal bovine serum in HEPES buffer (blocking buffer) overnight at 4 °C. Following the immunoreaction, sections were washed with HEPES buffer and then incubated with secondary antibodies, namely Cy3-, Cy5-, or fluorescein isothiocyanate (FITC)-conjugated AffiniPure donkey anti-mouse, rabbit, goat, or guinea pig IgG and chicken IgY (1:500 dilution; Jackson ImmunoResearch, West Grove, Penn., USA). The sections were washed with HEPES buffer and subsequently mounted in VECTASHIELD Mounting Medium with 4′,6-diamidino-2-phenylindole (DAPI; Vector, Burlingame, Calif., USA). Immunofluorescence was observed under fluorescence microscopy with BZ-8000 (KEYENCE, Osaka, Japan). The proportions of each type of ephrin-B2/SOX2-positive cluster in the parenchyma were measured by counting three to six areas (each 1.1–1.4 mm2) in the sections prepared from three P60 rats. The data are presented as means ± SD for three animals.
Absorption test of anti-human ephrin-B2 antibodies
The cDNA of rat ephrin-B2 corresponding to a full-length coding sequence was cloned in frame into the pET32a vector (Novagen, Darmstadt, Germany) and generated the TrxA-His-tag fused ephrin-B2 protein in Escherichia coli BL21 (DE3) Codon Plus RIPL (Stratagene, La Jolla, Calif., USA). The protein was isolated by using His-tag Mag beads (Toyobo). Immunohistochemistry was performed by using mouse monoclonal IgG (clone EFR-163 M) or rabbit IgG against human ephrin-B2 pre-absorbed with TrxA-His-tag peptide or TrxA-His-tag fused rat ephrin-B2 protein at a 1 : 5 molar ratio against IgG.
Results
Ontogeny of ephrin-B2 gene expression and localization of its protein in adult pituitary
The expression of the ephrin-B2 gene during rat pituitary development was examined by quantitative real-time PCR analysis (Fig. 1a). The result demonstrated that the ephrin-B2 gene was expressed throughout all stages tested, and that its expression level gradually decreased, at least in the anterior lobe. The expression levels in the intermediate and posterior lobes were higher than those in the anterior lobe during postnatal periods.
Ontogeny of ephrin-B2 expression and the localization of its protein in adult pituitary. a Quantitative real-time polymerase chain reaction (PCR) was performed to estimate the mRNA level of the rat ephrin-B2 by using total RNAs extracted from the whole pituitaries on embryonic day 13.5 (E13.5) to postnatal day 0 (P0) and from the anterior (AL) and intermediate/posterior lobes (IL+PL) on P5 to P60. Data were calculated by the comparative CT method to estimate the relative copy number to that of the TATA-box-binding protein gene (Tbp) used as an internal standard. Data are presented as the means ± SD of duplicate PCR in two independent experiments. b–e Immunohistochemistry of adult male rat pituitary on P60 was performed with mouse monoclonal IgG against human ephrin-B2 (clone EFR-163 M), pre-absorbed with TrxA-His-tag peptide (b, d) or TrxA-His-tag fused rat ephrin-B2 protein (c, e). Ephrin-B2 is visualized with Cy3 (red), and merged images with nuclear staining by 4,6-diamidino-2-phenylindole (DAPI, blue) are shown in d, e (AL anterior lobe, IL intermediate lobe, PL posterior lobe). Bars 50 μm
Next, we confirmed the existence of ephrin-B2-positive cells in the rat adult pituitary (P60) and the specificity of the antibody by immunohistochemistry with mouse monoclonal IgG against human ephrin-B2 (clone EFR-163 M) pre-absorbed with TrxA-His-tag peptide (Fig. 1b, d) or TrxA-His-tag fused rat ephrin-B2 protein (Fig. 1c, e). The result demonstrated that ephrin-B2 protein existed in the adult pituitary mainly in the MCL, a pituitary stem/progenitor cell niche (Allaerts and Vankelecom 2005; Chen et al. 2005, 2013; Garcia-Lavandeira et al. 2009; Gremeaux et al. 2012; Yoshida et al. 2011). Immunoreactive signals were not found in the posterior lobe (Fig. 1b, d). Pre-absorption tests confirmed that immunoreacted signals of ephrin-B2 in the pituitary were bona fide. Moreover, the same result was obtained with another rabbit polyclonal IgG against human ephrin-B2 (data not shown).
Ephrin-B2-positive cells are localized in embryonic and postnatal pituitaries changing their localization pattern
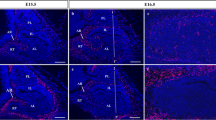
Immunohistochemistry of ephrin-B2 was performed for rat pituitaries from E12.5 to P60 (Fig. 2). On E12.5 during the early stage of pituitary development, two distinct localizations of ephrin-B2-positive signals in the cell membrane were detected in the invaginating oral epithelium (Fig. 2a–c). Immunoreacted signals polarized in the apical cell membranes were localized in the dorsal region of the invaginating oral epithelium. Another type of signal non-polarized in the basolateral cell membranes was mainly localized in the ventral region. On E13.5, when the invaginating oral epithelium detached from the oral cavity and formed the pituitary primordium of Rathke’s pouch, two distinct localizations of ephrin-B2-positive signals in the cell membranes became clearer (Fig. 2d–f). Immunoreacted signals polarized in the apical cell membranes were localized in the MCL and the border of Rathke’s pouch. Signals in the basolateral cell membranes were localized in the rostral tip, which originated from cells in the bilateral area bordering on the oral ectoderm on E12.5 (Fig. 2a, asterisk). On E16.5, at the middle stage of pituitary development when ephrin-B2-positive signals in the rostral tip decreased, positive signals in the basolateral cell membranes were detected in the parenchyma of the intermediate and anterior lobes, whereas the signals polarized in the apical cell membranes were still located in the MCL (Fig. 2g–i). On E19.5, at the late stage of pituitary development (Fig. 2j–l), the number of signals in the basolateral cell membranes increased in the MCL and parenchyma of the intermediate lobe. This phenomenon was enhanced on P0, and the signal intensity in the anterior lobe side (anterior side) of the MCL and in the parenchyma of the anterior lobe became weaker than that in the intermediate lobe (Fig. 2m–o).
Localization of ephrin-B2 during rat pituitary development. Ethyl-alcohol-fixed cryosections were prepared in a sagittal plane from pituitaries of E12.5 (a–c), E13.5 (d–f), E16.5 (g–i), E19.5 (j–l), and P0 (m–o) and in a coronal plane from pituitaries of P3 (p–r), P15 (s–u), and P60 (v–x). Ephrin-B2 is visualized with Cy3 (red), and the merged images with nuclear staining by DAPI (blue) are shown. Boxed areas in a, d, g, j, m, p, s, v are shown at higher magnification in b, c, e, f, h, i, k, l, n, o, q, r, t, u, w, x (dotted lines outline of anterior and intermediate lobes on E12.5 to P0, asterisk in a region forming the rostral tip, AL anterior lobe, IL intermediate lobe, PL posterior lobe). Bars 50 μm (a, d, g, j, m, p, s, v), 10 μm (w, x)
On P3, before the initiation of the postnatal pituitary growth wave (Chen et al. 2006, 2009, 2013; Taniguchi et al. 2001; Yoshida et al. 2011), ephrin-B2-positive cells formed multiple cell layers beneath both sides of the MCL (Fig. 2p–r). The signal intensity was stronger in the intermediate side than in the anterior side of the MCL. On P15, when the postnatal growth wave was almost completed, multiple cell layers formed by ephrin-B2-positive cells disappeared, and ephrin-B2-positive cells remained in the single cell layer of the MCL in addition to being scattered in the parenchyma of the anterior lobe (Fig. 2s–u). In the adult pituitary on P60 (Fig. 2v, w), ephrin-B2-positive signals both in the apical and in the basolateral cell membranes were observed in the MCL, and the signal intensity was stronger in the intermediate side than in the anterior side. In the parenchyma of the anterior lobe, ephrin-B2-positive cells formed cell clusters (Fig. 2x). These results demonstrated that ephrin-B2 proteins were localized in both the apical and the basolateral membranes of the cells in the pituitary throughout life and mainly settled in the MCL (a pituitary stem/progenitor cell niche) and in the cell clusters in the parenchyma of the anterior lobe.
Ephrin-B2 co-exists with stem/progenitor cell markers, SOX2 and E-cadherin, in adult pituitary
To confirm that ephrin-B2 was present in the pituitary stem/progenitor cells, we examined the co-localization of ephrin-B2 and the pituitary stem/progenitor cell markers, SOX2 (Fig. 3a–h) and E-cadherin (Fig. 3i–p) in the adult pituitary (P60). Double-immunostaining for ephrin-B2 and SOX2 demonstrated that most of the ephrin-B2-positive cells, which were localized in the MCL (Fig. 3a–d) and the parenchyma of the anterior lobe (Fig. 3e–h), were positive for SOX2. However, a small population of ephrin-B2-positive/SOX2-negative cells (Fig. 3a–d, arrows) and ephrin-B2-negative/SOX2-positive cells (Fig. 3e–h, closed arrowheads) were also found. In particular, SOX2-positive cells forming clusters in the parenchyma tended to be positive for ephrin-B2 at a high frequency (Fig. 3e–h, open arrowheads). Immunohistochemistry for another stem/progenitor cell marker, E-cadherin, demonstrated a similar pattern (Fig. 3i–p).
Immunostaining for ephrin-B2 and stem/progenitor cell markers, SOX2 and E-cadherin, in the adult pituitary. a–h Immunostaining for ephrin-B2 (clone EFR-163 M visualized with Cy3, red; a, e) and SOX2 (Cy5, green; b, f) in ethyl-alcohol-fixed cryosections prepared from the pituitary on P60. Merged images without (c, g) and with DAPI (blue; d, h) are shown. i–p Immunostaining for ephrin-B2 (rabbit IgG visualized with Cy3, red; i, m) and E-cadherin (Cy5, green; j, n) in HOPE-fixed paraffin sections. Merged images without (k, o) and with nuclear staining by DAPI (blue; l, p) are shown (dotted lines border of marginal cell layer, arrows ephrin-B2-single-positive cells, closed arrowheads SOX2-single-positive cells, open arrowheads ephrin-B2/SOX2-double-positive clusters, AL anterior lobe, IL intermediate lobe, MCL marginal cell layer). Bars 20 μm
Ephrin-B2 exists in CAR-positive adult pituitary stem/progenitor cell niches
We have recently reported that a cell-surface receptor protein, CAR, which is able to form a homophilic tight junction, is co-localized with SOX2 and E-cadherin and composes putative stem/progenitor cell niches in the MCL and the parenchyma of the anterior lobe (Chen et al. 2013). To examine whether ephrin-B2 exists in the CAR-positive stem/progenitor cell niches, we performed double-immunostaining for ephrin-B2 and CAR in the adult pituitary (Fig. 4). The results demonstrated that ephrin-B2 existed in the CAR-positive pituitary stem/progenitor cell niches in the MCL and the parenchyma of the anterior pituitary on P60.
Immunostaining for ephrin-B2 and CAR in the adult pituitary. Immunostaining for ephrin-B2 (clone EFR-163 M visualized with Cy3, red; a, e) and CAR (Cy5, green; b, f) in ethyl-alcohol-fixed cryosections. Merged images without (c, g) and with nuclear staining by DAPI (blue; d, h) are shown (dotted lines border of the marginal cell layer, AL anterior lobe, IL intermediate lobe, MCL marginal cell layer). Bars 20 μm
Some ephrin-B2-positive cells express genes for Prop1 or S100β but not for hormones
We have previously reported that (1) a pituitary-specific transcription factor Prop1 is definitely expressed by SOX2-positive pituitary stem/progenitor cells throughout the stages of pituitary organogenesis, (2) the number of PROP1-positive cells in the MCL greatly decreases after the postnatal growth wave, and (3) afterward, the majority of PROP1-positive cells remain in the parenchyma of the anterior lobe and about 78 % of them express S100β, a marker of folliculostellate cells (Yoshida et al. 2011). To characterize ephrin-B2-positive cells further, we performed co-immunostaining for SOX2 and PROP1 (Fig. 5), and S100β or pituitary hormones (Fig. 6). Triple-immunostaining together with SOX2 and PROP1 revealed that the majority of ephrin-B2-positive clusters in the parenchyma of the anterior lobe were composed of SOX2-single-positive cells and a small amount of SOX2/PROP1-double-positive cells (66.6 ± 3.3 % of all ephrin-B2/SOX2-positive clusters; Fig. 5a–d). Clusters composed of SOX2/PROP1-double-positive cells were also observed (24.2 ± 5.2 %; Fig. 5e–h), and those composed of only SOX2-single-positive cells were found at a low frequency (9.2 ± 2.0 %; Fig. 5i–l). Double-immunostaining together with S100β in the S100β-GFP transgenic rat (Itakura et al. 2007) revealed that a large number of the cells in ephrin-B2-positive clusters were positive for S100β (Fig. 6a). As shown in Fig. 6b–f, none of the cells positive for ephrin-B2, which were in clusters or scattered in the parenchyma of the anterior lobe, produced any pituitary hormone. These data indicated that the ephrin-B2 gene was expressed by pituitary stem/progenitor cells but not by terminally differentiated cells. Furthermore, ephrin-B2-positive clusters in the parenchyma of the anterior lobe were the SOX2/E-cadherin/CAR-triple-positive stem/progenitor cell niches but were inhomogeneous regarding the expression of Prop1.
Triple-immunostaining for ephrin-B2, SOX2, and a pituitary specific progenitor marker, PROP1. Immunostaining for ephrin-B2 (clone EFR-163 M visualized with Cy3, red; a, e, i), SOX2 (Cy5, green; b, f, j), and PROP1 (FITC, white; c, g, k) in ethyl-alcohol-fixed cryosections prepared from the pituitary on P60. Merged images with nuclear staining by DAPI (blue) are shown right (d, h, l). Clusters composed of SOX2-positive and PROP1/SOX2-positive cells (a–d), SOX2/PROP1-positive cells (e–h), and SOX2-positive/PROP1-negative cells (i–l) are shown. Bars 20 μm
Localization of ephrin-B2 in S100β-GFP-positive cells and hormone-producing cells. Immunostaining was performed for ephrin-B2 (rabbit IgG) and green fluorescent protein (GFP) by using HOPE-fixed paraffin sections prepared from adult S100β-GFP transgenic rat on P60 (a). HOPE-fixed frozen sections of adult pituitary (P60) were used for immunostaining for ephrin-B2 (clone EFR-163 M) and the following hormones: growth hormone (GH, b), prolactin (PRL, c), thyroid-stimulating hormone β subunit (TSHβ, d), adrenocorticotrophic hormone (ACTH, e), and luteinizing hormone β subunit (LHβ, f). Merged images with ephrin-B2 (visualized with Cy3, red) and GFP or hormones (Cy5, green) are shown. Bars 20 μm
Discussion
We have previously reported that CAR exists in SOX2-positive cells that are located in the MCL and scattered in the parenchyma of the anterior lobe, and that these cells compose the pituitary stem/progenitor cell niches via tight junctions (Chen et al. 2013). Therefore, we have hypothesized that cell adhesion is an important factor for the construction of the niches and the regulation of stem cell function. We have checked the expression of all B class ephrins (data not shown) and here focus on ephrin-B2 because of its known role in the neural stem cell (Nomura et al. 2010). Our results confirm that ephrin-B2-positive cells are involved in pituitary organogenesis and settle within the SOX2/E-cadherin/CAR-triple positive pituitary stem/progenitor cell niches.
Although the localization pattern of the ephrin-B2-positive cells is similar to that of CAR in the adult pituitary, that of ephrin-B2 in the embryonic pituitary is different and drastically changes at every stage of pituitary organogenesis. These data suggest that the ephrin-B2 signal is involved not only in the construction of the pituitary stem/progenitor cell niches, but also in pituitary organogenesis. Indeed, ephrin signaling is known to play a role in boundary formation during the development of many tissues, such as the crypts of the intestine (Batlle et al. 2002) and the rhombomere (Mellitzer et al. 1999). In pituitary organogenesis, only ephrin receptor-A5 is reported to be expressed in the neurohypophysis (Zarbalis and Wurst 2000). Our data demonstrate that ephrin-B2-positive cells are mainly localized in the rostral tip and MCL on E13.5. Growth factor signaling such as that of bone morphogenetic protein 2, which is initially expressed in the most ventral region of Rathke’s pouch, and of sonic hedgehog, which is expressed in the oral ectoderm, has been reported to be involved in the formation of the ventral boundary between Rathke’s pouch and the oral ectoderm via the induction of many transcription factors (Dasen and Rosenfeld 2001; Treier et al. 1998). Regardless of the many studies that have addressed growth factor signaling, little is known about the mechanism of boundary formation between the rostral tip and Rathke’s pouch. Our present data are the first to indicate that ephrin-B2 plays a role in the boundary formation of the rostral tip during pituitary development.
In relation to the formation of niches scattered in the parenchyma of the anterior lobe, Gremeaux et al. (2012) suggest that two processes take place: cluster outgrowth from the MCL and stem/progenitor cell migration from MCL. We have previously proposed the latter process by demonstrating that epithelial-mesenchymal transition (EMT) provokes the migration of CAR-positive stem/progenitor cells from the MCL by the indirect verification of the expression of Vimentin, an EMT-associated marker (Chen et al. 2013). During the postnatal period, ephrin-B2 occurs in CAR-positive cells localized in the MCL and in the clusters scattered in the parenchyma of the anterior lobe. Notably, at the initiation of the postnatal growth wave (P3), ephrin-B2-positive cells form multiple cell layers beneath the MCL, changing their membrane polarization to basolateral, followed by migration to compose the postnatal pituitary stem/progenitor niches in the parenchyma of the anterior lobe after P15, similar to CAR-positive cells (Chen et al. 2013). Ephrin-Bs signaling is known to have a role in regulating cell attachment and migration, such as in ovary-, glioma-, melanoma-, and intestinal-epithelia-derived cell lines (Hafner et al. 2005; Meyer et al. 2005; Nakada et al. 2010). Cowan and Henkemeyer (2001) have reported that the phosphorylated cytoplasmic domain of ephrin-B recruits the adaptor protein GRB4, and following its binding, proteins including PAXILLIN and FAK promote cell spreading and movement. Therefore, CAR- and ephrin-B2-positive cells probably migrate from the MCL, with ephrin-B2 signaling playing a role in pituitary cell migration. This can be achieved by cells expressing a partner ephrin receptor that should exist around the MCL to activate ephrin-B2 signaling. Hence, studies of ephrin-B2 and its receptor might become a good tool for demonstrating (1) whether CAR- and ephrin-B2-positive stem/progenitor cells migrate from the MCL and (2) the presence of the pituitary niche cells. From another point of view, the intensities of both ephrin-B2- and CAR-positive signals are commonly stronger in the intermediate side than the anterior side of the MCL, and both proteins contain a PSD-95, Dlg, ZO-1 (PDZ)-binding domain in the intracellular C-terminal region (Arvanitis and Davy 2008; Bewley et al. 1999; van Raaij et al. 2000; Xi et al. 2012). These data suggest that ephrin-B2 and CAR interact via PDZ-domain-containing proteins and cooperate in the signaling for cell attachment, migration, proliferation, and maintenance of stemness.
Our previous quantitative and qualitative analyses of immunohistochemistry demonstrated that, during the early postnatal pituitary growth wave, the number of PROP1/SOX2-positive cells significantly decreased by a change in their properties in the MCL, whereas they were maintained in the niches in the parenchyma (Higuchi et al. 2014; Yoshida et al. 2011). At that time, it was not clear whether SOX2/PROP1-double positive cells and SOX2-positive/PROP1-negative cells had different roles in the cell supply system in the adult pituitary niches. In this study, we have confirmed that ephrin-B2 exists in the adult stem/progenitor cell niches in the parenchyma of the anterior lobe, and that these niches are classified into three groups with regard to the expression of Prop1. Most of the niches are composed of SOX2-single-positive cells and a small number of SOX2/PROP1-double-positive cells. The second type is composed of SOX2/PROP1-double-positive cells. The third type of niche, composed only of SOX2-single-positive cells, is also found at a low frequency. Our data demonstrate that about 90 % of the pituitary stem/progenitor cell niches in the parenchyma of the adult anterior lobe include PROP1-positive cells. Andoniadou et al. (2013) have reported that most of the SOX2-positive cells are in a quiescent state in the adult pituitary under normal physiological conditions by using Sox2-lineage tracing. These findings indicate that most of the pituitary stem/progenitor cell niches in the parenchyma are in a waiting state for differentiation on demand.
In summary, we have demonstrated that a juxtacrine factor ephrin-B2 exists in the SOX2/E-cadherin/CAR-triple-positive pituitary stem/progenitor cell niches in the adult pituitary. Thus, the present data suggest that ephrin-B2 signaling plays important roles in the formation of pituitary stem/progenitor cell niches and in pituitary organogenesis.
Abbreviations
- CAR:
-
Coxsackievirus and adenovirus receptor
- DAPI:
-
4,6-Diamidino-2-phenylindole
- FITC:
-
Fluorescein isothiocyanate
- GFP:
-
Green fluorescent protein
- HOPE:
-
HEPES-glutamic acid buffer-mediated organic solvent protection effect
- MCL:
-
Marginal cell layer
- PCR:
-
Polymerase chain reaction
- PDZ:
-
PSD-95, Dlg, ZO-1
- PROP1:
-
Prophet of PIT1
- SOX2:
-
Sex-determining region Y-box 2
References
Allaerts W, Vankelecom H (2005) History and perspectives of pituitary folliculo-stellate cell research. Eur J Endocrinol 153:1–12
Andoniadou CL, Matsushima D, Mousavy Gharavy SN, Signore M, Mackintosh AI, Schaeffer M, Gaston-Massuet C, Mollard P, Jacques TS, Le Tissier P, Dattani MT, Pevny LH, Martinez-Barbera JP (2013) Sox2(+) stem/progenitor cells in the adult mouse pituitary support organ homeostasis and have tumor-inducing potential. Cell Stem Cell 13:433–445
Arvanitis D, Davy A (2008) Eph/ephrin signaling: networks. Genes Dev 22:416–429
Ashton RS, Conway A, Pangarkar C, Bergen J, Lim KI, Shah P, Bissell M, Schaffer DV (2012) Astrocytes regulate adult hippocampal neurogenesis through ephrin-B signaling. Nat Neurosci 15:1399–1406
Batlle E, Henderson JT, Beghtel H, van den Born MM, Sancho E, Huls G, Meeldijk J, Robertson J, van de Wetering M, Pawson T, Clevers H (2002) Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell 111:251–263
Bewley MC, Springer K, Zhang YB, Freimuth P, Flanagan JM (1999) Structural analysis of the mechanism of adenovirus binding to its human cellular receptor, CAR. Science 286:1579–1583
Chen CC, Chuong CM (2012) Multi-layered environmental regulation on the homeostasis of stem cells: the saga of hair growth and alopecia. J Dermatol Sci 66:3–11
Chen J, Hersmus N, Van Duppen V, Caesens P, Denef C, Vankelecom H (2005) The adult pituitary contains a cell population displaying stem/progenitor cell and early embryonic characteristics. Endocrinology 146:3985–3998
Chen J, Crabbe A, Van Duppen V, Vankelecom H (2006) The notch signaling system is present in the postnatal pituitary: marked expression and regulatory activity in the newly discovered side population. Mol Endocrinol 20:3293–3307
Chen J, Gremeaux L, Fu Q, Liekens D, Van Laere S, Vankelecom H (2009) Pituitary progenitor cells tracked down by side population dissection. Stem Cells 27:1182–1195
Chen M, Kato T, Higuchi M, Yoshida S, Yako H, Kanno N, Kato Y (2013) Coxsackievirus and adenovirus receptor-positive cells compose the putative stem/progenitor cell niches in the marginal cell layer and parenchyma of the rat anterior pituitary. Cell Tissue Res 354:823–836
Cowan CA, Henkemeyer M (2001) The SH2/SH3 adaptor Grb4 transduces B-ephrin reverse signals. Nature 413:174–179
Daar IO (2012) Non-SH2/PDZ reverse signaling by ephrins. Semin Cell Dev Biol 23:65–74
Dasen JS, Rosenfeld MG (2001) Signaling and transcriptional mechanisms in pituitary development. Annu Rev Neurosci 24:327–355
Davis SW, Castinetti F, Carvalho LR, Ellsworth BS, Potok MA, Lyons RH, Brinkmeier ML, Raetzman LT, Carninci P, Mortensen AH, Hayashizaki Y, Arnhold IJ, Mendonca BB, Brue T, Camper SA (2010) Molecular mechanisms of pituitary organogenesis: in search of novel regulatory genes. Mol Cell Endocrinol 323:4–19
Fauquier T, Rizzoti K, Dattani M, Lovell-Badge R, Robinson IC (2008) SOX2-expressing progenitor cells generate all of the major cell types in the adult mouse pituitary gland. Proc Natl Acad Sci U S A 105:2907–2912
Fu Q, Vankelecom H (2012) Regenerative capacity of the adult pituitary: multiple mechanisms of lactotrope restoration after transgenic ablation. Stem Cells Dev 21:3245–3257
Fu Q, Gremeaux L, Luque RM, Liekens D, Chen J, Buch T, Waisman A, Kineman R, Vankelecom H (2012) The adult pituitary shows stem/progenitor cell activation in response to injury and is capable of regeneration. Endocrinology 153:3224–3235
Garcia-Lavandeira M, Quereda V, Flores I, Saez C, Diaz-Rodriguez E, Japon MA, Ryan AK, Blasco MA, Dieguez C, Malumbres M, Alvarez CV (2009) A GRFa2/Prop1/stem (GPS) cell niche in the pituitary. PLoS One 4:e4815
Genander M, Holmberg J, Frisen J (2010) Ephrins negatively regulate cell proliferation in the epidermis and hair follicle. Stem Cells 28:1196–1205
Gremeaux L, Fu Q, Chen J, Vankelecom H (2012) Activated phenotype of the pituitary stem/progenitor cell compartment during the early-postnatal maturation phase of the gland. Stem Cells Dev 21:801–813
Hafner C, Meyer S, Hagen I, Becker B, Roesch A, Landthaler M, Vogt T (2005) Ephrin-B reverse signaling induces expression of wound healing associated genes in IEC-6 intestinal epithelial cells. World J Gastroenterol 11:4511–4518
Higuchi M, Yoshida S, Ueharu H, Chen M, Kato T, Kato Y (2014) PRRX1 and PRRX2 distinctively participate in pituitary organogenesis and cell supply system. Cell Tissue Res 357:323–335
Holmberg J, Genander M, Halford MM, Anneren C, Sondell M, Chumley MJ, Silvany RE, Henkemeyer M, Frisen J (2006) EphB receptors coordinate migration and proliferation in the intestinal stem cell niche. Cell 125:1151–1163
Itakura E, Odaira K, Yokoyama K, Osuna M, Hara T, Inoue K (2007) Generation of transgenic rats expressing green fluorescent protein in S-100beta-producing pituitary folliculo-stellate cells and brain astrocytes. Endocrinology 148:1518–1523
Jensen PL (2000) Eph receptors and ephrins. Stem Cells 18:63–64
Mellitzer G, Xu Q, Wilkinson DG (1999) Eph receptors and ephrins restrict cell intermingling and communication. Nature 400:77–81
Meyer S, Hafner C, Guba M, Flegel S, Geissler EK, Becker B, Koehl GE, Orso E, Landthaler M, Vogt T (2005) Ephrin-B2 overexpression enhances integrin-mediated ECM-attachment and migration of B16 melanoma cells. Int J Oncol 27:1197–1206
Murai KK, Pasquale EB (2003) “Eph”ective signaling: forward, reverse and crosstalk. J Cell Sci 116:2823–2832
Nakada M, Anderson EM, Demuth T, Nakada S, Reavie LB, Drake KL, Hoelzinger DB, Berens ME (2010) The phosphorylation of ephrin-B2 ligand promotes glioma cell migration and invasion. Int J Cancer 126:1155–1165
Nomura T, Goritz C, Catchpole T, Henkemeyer M, Frisen J (2010) EphB signaling controls lineage plasticity of adult neural stem cell niche cells. Cell Stem Cell 7:730–743
Olert J, Wiedorn KH, Goldmann T, Kuhl H, Mehraein Y, Scherthan H, Niketeghad F, Vollmer E, Muller AM, Muller-Navia J (2001) HOPE fixation: a novel fixing method and paraffin-embedding technique for human soft tissues. Pathol Res Pract 197:823–826
Pasquale EB (2005) Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol 6:462–475
Pitulescu ME, Adams RH (2010) Eph/ephrin molecules—a hub for signaling and endocytosis. Genes Dev 24:2480–2492
Rizzoti K, Akiyama H, Lovell-Badge R (2013) Mobilized adult pituitary stem cells contribute to endocrine regeneration in response to physiological demand. Cell Stem Cell 13:419–432
Sato T, Clevers H (2013) Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science 340:1190–1194
Taniguchi Y, Kominami R, Yasutaka S, Shinohara H (2001) Mitoses of existing corticotrophs contribute to their proliferation in the rat pituitary during the late fetal period. Anat Embryol (Berl) 203:89–93
Treier M, Gleiberman AS, O’Connell SM, Szeton DP, McMahon JA, McMahon AP, Rosenfeld MG (1998) Multistep signaling reqirements for pituitary organogenesis in vivo. Genes Dev 12:1691–1704
van Raaij MJ, Chouin E, van der Zandt H, Bergelson JM, Cusack S (2000) Dimeric structure of the coxsackievirus and adenovirus receptor D1 domain at 1.7 Å resolution. Structure 8:1147–1155
Vankelecom H (2010) Pituitary stem/progenitor cells: embryonic players in the adult gland? Eur J Neurosci 32:2063–2081
Vankelecom H, Chen J (2014) Pituitary stem cells: where do we stand? Mol Cell Endocrinol 385:2–17
Vila-Porcile E (1972) The network of the folliculo-stellate cells and the follicles of the adenohypophysis in the rat (pars distalis). Z Zellforsch Mikrosk Anat 129:328–369
Xi HQ, Wu XS, Wei B, Chen L (2012) Eph receptors and ephrins as targets for cancer therapy. J Cell Mol Med 16:2894–2909
Yoshida S, Kato T, Susa T, Cai L-Y, Nakayama M, Kato Y (2009) PROP1 coexists with SOX2 and induces PIT1-commitment cells. Biochem Biophys Res Commun 385:11–15
Yoshida S, Kato T, Yako H, Susa T, Cai L-Y, Osuna M, Inoue K, Kato Y (2011) Significant quantitative and qualitative transition in pituitary stem/progenitor cells occurs during the postnatal development of the rat anterior pituitary. J Neuroendocrinol 23:933–943
Zarbalis K, Wurst W (2000) Expression domains of murine ephrin-A5 in the pituitary and hypothalamus. Mech Dev 93:165–168
Zhu X, Gleiberman AS, Rosenfeld MG (2007) Molecular physiology of pituitary development: signaling and transcriptional networks. Physiol Rev 87:933–963
Acknowledgments
The authors thank Dr. A.F. Parlow and the NIDDK for antibodies against pituitary hormones, Dr. S. Tanaka at Shizuoka University for the antibody against human ACTH and GH, and Mr. K. Kawai for his excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Saishu Yoshida and Takako Kato contributed equally to this work.
This work was partially supported by JSPS KAKENHI grant nos. 21380184 to Y.K. and 24580435 to T.K. and by a research grant (A) to Y.K. from the Institute of Science and Technology, Meiji University. The study was also supported by the Meiji University International Institute for BioResource Research (MUIIR).
Rights and permissions
About this article
Cite this article
Yoshida, S., Kato, T., Higuchi, M. et al. Localization of juxtacrine factor ephrin-B2 in pituitary stem/progenitor cell niches throughout life. Cell Tissue Res 359, 755–766 (2015). https://doi.org/10.1007/s00441-014-2054-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-014-2054-y