Abstract
Phenotypic changes in culture hamper the identification and characterization of cultured podocytes and parietal epithelial cells of the Bowman’s capsule (PECs). We have recently established culture conditions that restore podocytes to their differentiated phenotypes. We compared podocytes and PECs cultured under the same conditions to determine the unique characteristics of the two cell types. Performing this comparison under the same conditions accentuated these differences. Podocytes behaved like non-epithelial cells by extending cell processes even at confluence. By contrast, PECs behaved like typical epithelial cells by maintaining a polygonal appearance. Other differences were identified using immunostaining and RT-PCR; podocytes expressed high levels of podocyte-specific markers while PECs expressed high levels of PEC-specific markers. However, while podocytes expressed low levels of PEC markers, PECs expressed low levels of podocyte markers. Therefore, the identification of podocytes and PECs in culture requires the evaluation of respective cell markers and the expression of markers for other cell types.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Visceral and parietal epithelial cells of the Bowman’s capsule in the kidney have been studied extensively because the visceral cells play a critical role in maintaining the glomerular filtration barrier and causing glomerulosclerosis (Pavenstädt et al. 2003). By contrast, parietal epithelial cells (PECs) are involved in the formation of crescents and adhesions in glomerular diseases (Nagata 2016). In addition, it has been shown recently that PECs are also necessary and required in focal segmental glomerulosclerosis (Kuppe et al. 2015, 2019; Lazareth et al. 2019). Visceral epithelial cells which are referred to as podocytes, exhibit a very elaborate morphology. Podocytes have several primary processes radiating from the cell body that ramify into thinner processes. The resultant fine processes, or foot processes, create interdigitations between adjacent cells (Ichimura et al. 2015). The paracellular spaces between foot processes are bridged by slit diaphragms, which are intercellular junctions that are unique to podocytes (Rodewald and Karnovsky 1974). On the other hand, PECs exhibit a simple morphology that resembles that of squamous epithelial cells with one or two primary cilia (Arakawa and Tokunaga 1977). PECs are connected by typical epithelial intercellular junctions including tight junctions and adherens junctions (Webber and Blackbourn 1971). In accordance with these structural differences, these cell types express specific marker proteins such as nephrin, podocin and WT1 for podocytes and Pax8, Pax2 and claudin-1 for PECs, respectively (Ohse et al. 2009; Ohtaka et al. 2002; Pavenstädt et al. 2003). Thus, podocytes and PECs have distinct in vivo differences in terms of morphology, gene expression and protein expression.
The culture system is very valuable for clarifying responses or identifying features of a specific cell type. Unfortunately, the distinction between podocytes and PECs becomes obscured in culture. While in culture, podocytes lose their specific phenotypes. Specifically, podocytes lose their foot processes and slit diaphragms and experience a decrease in the expression of podocyte-specific genes over time (Andrews and Stauver 1979; Yaoita et al. 1995, 2014). Podocyte cell lines also express low levels of podocyte-specific genes and show a polygonal morphology at confluence (Chittiprol et al. 2011). On the other hand, PECs also maintain a polygonal morphology in vivo and express all podocyte-specific genes in primary culture (Guhr et al. 2013; Yaoita et al. 1991). To overcome the uncertain identification entailed in phenotypic changes, Kabgani et al. established for the first time primary podocyte and PEC cultures with proven origin by using genetically tagged mouse lines (Kabgani et al. 2012). One of their conclusions was that PECs and podocytes cannot be unambiguously distinguished in culture using conventional markers, although the two cell types retain a distinct expression profile depending on their origin. Thus, identification and characterization of podocytes and PECs in culture remain a major problem.
One of the main reasons for the lack of distinction between podocytes and PECs is that podocytes lose their specific phenotypes in culture. Recently, we established culture conditions that restore podocytes to their differentiated phenotypes (Yaoita et al. 2018). The medium used in the culture conditions contains all-trans-retinoic acid (ATRA) and highly sulfated polysaccharides, including heparin or dextran sulfate (DS) but lacks fetal bovine serum (FBS), which prevents the induction. While highly sulfated polysaccharides trigger the induction, their underlying mechanism of actions remains unknown. In the present study, we tried to clarify distinct characteristics of cultured podocytes and PECs by comparing them under two different culture conditions: primary cultures using standard culture medium with FBS and secondary cultures using the inducing medium. Consequently, we observed distinct differences between the two cell types. While podocytes behaved like non-epithelial cells, PECs behaved similarly to typical epithelial cells. These observations were compatible with their respective levels of adheren junction proteins.
Materials and methods
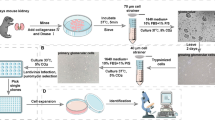
Culture for podocytes and PECs (Fig. 1)
Rat glomeruli were isolated and cultured to obtain outgrowths of podocytes and PECs according to a previously described protocol with minor modifications (Katsuya et al. 2006; Yaoita et al. 1991, 2018). Briefly, rat kidneys were perfused with iron powder to purify the isolated glomeruli. The following procedures were performed differently for the isolation of podocytes and PECs: for podocyte cultures, perfused kidney cortices were digested with collagenase and passed through a 100-μm cell strainer. Glomeruli containing magnetic particles were collected using a magnet and cultured. Because almost all isolated glomeruli were decapsulated and morphologically intact, cellular outgrowths from the glomeruli were used as podocytes (Online Resource 1) (Katsuya et al. 2006; Takemoto et al. 2002; Yaoita et al. 2018). For PEC cultures, glomeruli were mechanically isolated from the perfused kidney cortices using the conventional serial sieving method without collagenase digestion (Yaoita et al. 1991). To remove tubular fragments, isolated glomeruli were washed at least four times with phosphate-buffered saline, collected using a magnet and resuspended. Half of the resultant glomeruli were encapsulated and this portion contained 1% of tubular fragments (Online Resource 1). As reported previously, the conventional sieving method damaged podocytes, which resulted in rare cells growing out from decapsulated glomeruli (Yaoita et al. 1991). Therefore, polygonal cells from encapsulated glomeruli were used as PECs. Glomeruli for podocyte and PEC cultures were cultured on non-coated and collagen type I-coated dishes, respectively. Although total numbers and sizes of cellular outgrowths from the podocytes were not noticeably different between the two types of dishes, the PECs were apparently larger on collagen type I-coated dishes compared to the non-coated dishes. The following culture medium was used for the primary cultures: D-MEM/F-12 (1:1) containing 5% FBS, 0.5% insulin-transferrin-selenium-A liquid media supplement (ITS) and antibiotic solution (100 U/ml penicillin and 100 μg/ml streptomycin).
After 4 days in culture, cellular outgrowths from glomeruli were detached using a trypsin-EDTA solution for PEC culture. A non-enzymatic cell dissociation solution (CDS) was used for podocyte culture, because treatment with trypsin may affect the expression of certain genes in cultured podocytes. Furthermore, treating PECs with CDS resulted in low rates of cell detachment. After passing through a 40-μm cell strainer to remove the remaining glomerular cores, cells were cultured in the same medium on glass slides printed with a highly water-repellent mark with wells 5 mm in diameter (Matsunami Glass Ind., Ltd., Osaka, Japan). Cells were cultured at a density of 1.4 × 104 cells per well and the wells were previously coated with laminin-521 by adding 10 μl of laminin-521 (20 μg/ml) to each well and incubating at 4 °C overnight. After 6 h, at which point most of the cells had attached to the glass slides, the culture medium was changed to D-MEM/F-12 containing 0.5% ITS, 0.5% FBS, 0.2 mg/ml DS, 0.2 μM ATRA and antibiotic solution. After 24 h, the medium was changed to D-MEM/F-12 containing 0.5% ITS, 0.2 μM ATRA and antibiotic solution. After several days in culture, cells were processed for morphological or immunohistochemical analysis or RNA extraction.
Confocal immunofluorescence microscopy and electron microscopy
Indirect immunofluorescence microscopy using antibodies specific for podocyte and PEC markers and scanning electron microscopy were performed as previously described (Yaoita et al. 1995, 2001). The following antibodies were used for immunohistochemical analysis: mouse monoclonal anti-α-catenin (clone: αCAT-7A4, Invitrogen Corporation, Camarillo, CA, USA), rabbit monoclonal anti-vimentin (clone: EPR3776, Abcam plc, Cambridge, UK), rabbit polyclonal anti-active caspase 3 (Abcam plc), mouse monoclonal anti-cadherin 6 (clone: 2, Transduction Laboratories, Lexington, KY, USA), rabbit polyclonal anti-podocin antibody (Immuno-Biological Laboratories Co., Ltd., Gunma, Japan), rabbit polyclonal anti-PAX8 antibody (Proteintech Group, Inc., Rosemont, IL, USA), guinea pig polyclonal anti-nephrin antibody (Progen Biotechnik GmbH, Heidelberg, Germany), mouse monoclonal anti-synaptopodin antibody (Progen Biotechnik GmbH), rabbit polyclonal anti-WT1 antibody (Santa Cruz Biotechnology, Dallas, TX, USA). Normal rabbit serum and mouse monoclonal anti-keyhole limpet hemocyanin antibody (clone: #11711, R&D Systems, Inc., Minneapolis, MN, USA) were used as negative controls.
Western blotting and quantitative RT-PCR analysis
Protein extraction, Western blotting, RNA extraction, cDNA synthesis and RT-PCR were performed as previously described (Yaoita et al., 2001, 2014). To measure the amount of specific mRNA in each sample, a standard curve was generated for each run using serial dilutions of cDNA from isolated glomeruli for PEC cultures that contain both podocytes and PECs. Gene expression was normalized to levels of Gapdh mRNA. To quantify mRNA for each gene evaluated by RT-PCR, it was expressed relative to that in the isolated glomeruli, which was assigned a value of 1.0. The sequences of the primers used are shown in Table 1.
RNA sequencing
RNA-Seq was performed using a SOLiD 5500 System (Applied Biosystems, Carlsbad, CA) according to the manufacturer’s instructions. Briefly, the quantity and quality of RNA were assessed using a NanoDrop 2000 (Thermo Fisher Scientific, Waltham, MA) and an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). The depletion of ribosomal RNA from the samples and the construction of the library were performed using a SOLiD Total RNA-Seq Kit (Applied Biosystems). The quality of the library was assessed using an Agilent 2100 Bioanalyzer and sequenced to produce 75-bp paired-end reads. The reads obtained during this analysis were deposited into the Avadis NGS (Agilent Technologies) to determine reads per kilobase of exons per million mapped reads.
Statistical analysis
All data were expressed as mean values ± the standard deviation. The assessment of statistically significant differences between podocytes and PECs was made using Mann-Whitney U test. Differences were considered statistically significant if P < 0.05.
Results
Phase-contrast microscopic differences
In primary cultures, podocytes frequently formed elongated, branched cell processes while PECs at the periphery of polygonal cell colonies occasionally extended simple processes (Fig. 2, Online Resources 2 and 3). The processes of podocytes often overlapped with other cell processes and the bodies of adjacent cells. Such overlapping was not observed among the cultured PECs. Podocytes separated easily from each other to form large intercellular spaces, whereas PECs remained in close proximity to other cells without intercellular spaces and maintaining their polygonal appearance. The behavior was not different between cells cultured on collagen type I-coated dishes and non-coated dishes except for the number of cellular outgrowths found on PECs as described in Materials and methods (data not shown).
After 4 days in culture, podocytes and PECs were subcultured at the same cell density to produce confluent subcultures (Fig. 3). Podocytes exhibited an irregular morphology by displaying long, thin cell processes and formed interdigitation of processes by after 6 days of subculturing. By contrast, PECs maintained their polygonal morphology, thus giving them a cobblestone-like appearance. Scanning electron microscopy showed differences in the appearance of podocytes and PECs after only 6 h of subcultutre. Ruffles and single cilia were observed on podocytes and PECs, respectively (Online Resource 4). Four days after subculture, spaces lacking in cells appeared in PEC cultures. Consistently, some cells stained positive for active caspase-3, an apoptosis marker, in PEC cultures but not in the podocyte cultures (Online Resource 5).
Phase-contrast microscopic images of cultured podocytes (a, c, e, g, i) and PECs (b, d, f, h, j) at 6 h (a, b), 24 h (c, d), 2 days (e, f), 4 days (g, h) and 6 days (i, j) after subculture. Podocytes extended cell processes upon confluence, while PECs maintained a polygonal appearance. Spaces lacking in cells were seen in PEC cultures after 4 and 6 days of subculture (asterisks in j). Scale bar = 50 μm
Immunofluorescence microscopic differences
Generally, epithelial cells are typified by a polygonal morphology and high levels of adherens junction (AJ) proteins, including members of cadherin and catenin families. Because PECs had a typical epithelial morphology, which differentiated them from the podocytes, we examined whether the two cell types had different expression levels for α-catenin, an AJ component (Fig. 4). All sites of cell-cell contact for PECs showed positive immunoreactivity for α-catenin throughout the primary and secondary cultures. Unexpectedly, in podocyte cultures, some cells also stained significantly positive for α-catenin at sites of cell-cell contact in primary cultures and during the early phase of secondary culture. The staining was lost in podocyte cultures after the fourth day of subculture. Because the podocyte cultures showed significant immunoreactivity for α-catenin, RNA seq analysis was performed on podocytes after 2 days of subculturing to examine which types of cadherins were expressed. Consequently, it was suggested that cadherin 6 was the most abundantly expressed cadherin in podocyte cultures: the reads per kilobase of exons per million mapped reads (RPKM) for Chd6, Cdh11 and Chd2 were 144.8, 35.4 and 29.7, respectively. Western blot analysis revealed a single band corresponding to the reported molecular mass of cadherin 6 (Online Resource 6) (Paul et al. 1997). The signal was weak but statistically significant in primary podocyte cultures compared to primary PEC cultures. Microscopic images showing cadherin 6 immunoreactivity were identical to images for α-catenin immunoreactivity in PEC and podocyte cultures (Fig. 5). Double-label immunofluorescence microscopy was performed to examine the relationship between cadherin 6 immunoreactivity and the expression of podocin, a podocyte-specific intercellular junction protein. No podocin staining was detected in cultured PECs. On the other hand, podocyte cultures showed variations in immunoreactivity ranging from negative to positive in primary and during the early phase of secondary cultures, where some podocin-positive cells showed significant immunoreactivity for cadherin 6. Almost all podocytes showed intense podocin immunoreactivity at sites of cell-cell contact 4 days after subculture. Coincidentally, cadherin 6 staining disappeared in podocyte cultures at this time. Double-labeling for α-catenin and podocin showed the results that were identical to images obtained after simultaneously detecting cadherin 6 and podocin (data not shown). These findings were similar to the changes observed in the development (Online Resource 7). PECs always showed positive immunoreactivity for α-catenin and cadherin 6 during before and after differentiation. Podocytes only showed immunoreactivity for these proteins during their S-shaped body stage; after the capillary loop stage, they subsequently lost their immunoreactivity.
Double-label immunofluorescence microscopic images showing α-catenin and vimentin at day 4 of primary culture (a, b) and after 6 h (c, d), 2 days (e, f) and 4 days (g, h) of subculture. Vimentin (red) was localized in cell bodies and cell processes while α-catenin (green) was localized at sites of cell-cell contact. Nuclei are colored blue with DAPI. Podocytes lost α-catenin staining after an increased length of time in culture (a, c, e, g), while PECs still showed high levels of α-catenin immunoreactivity during culture (b, d, f, h). gl: glomerulus. Scale bar = 50 μm in a, b, 25 μm in c–h
Double-label immunofluorescence microscopic images for cadherin 6 and podocin at day 4 of primary culture (a, b) and after 6 h (c, d), 2 days (e, f), 4 days (g, h) and 8 days (i, j) of subculture. Cadherin 6 (green) and podocin (red) were localized at cell-cell contact sites. Nuclei are colored blue with DAPI. Some podocin-positive cells also stained positive for cadherin 6 (a, c, e). Podocytes lost cadherin 6 immunoreactivity and increased their levels of podocin after an increased length of time in culture (a, c, e, g, i). By contrast, PECs showed intense staining for cadherin 6 but not podocin during culture (b, d, f, h, j). Podocyte formed sites of cell-cell contact, which showed extremely finely inflections after 8 days of subculture (inset in i). gl: glomerulus. Scale bar = 25 μm in a–j, 5 μm in inset i
Due to the expression of AJ proteins in cultured podocytes, the expression of PAX8, another PEC marker, was examined along with nephrin, another podocyte-specific intercellular junction protein. The results also coincided with those regarding the relationship between AJ protein and podocin levels. Cultured PECs always stained positive for PAX8 and negative for nephrin. In podocyte cultures, cells showed varying levels of nephrin. Some nephrin-positive podocytes stained positive for PAX8 in the primary cultures and during the early phase of secondary cultures. During the later phase of secondary culture, PAX8 staining disappeared while most cells showed increased levels of nephrin.
In this study, podocyte markers in PECs were also detected using immunofluorescence microscopy. Most PECs showed significantly immunoraeactivity for WT1 and synaptopodin. Nevertheless, their immunoreactivity was much less pronounced compared to that of podocytes (Online Resource 8).
As shown by podocin and nephrin staining, the morphology of cell-cell contact sites of podocytes was conspicuously different from that of PECs (Figs. 5i and 6c). While images of stained AJ proteins showed linear sites of cell-cell contact for PECs, sites of cell-cell contact for podocytes were elaborately inflective.
Double-label immunofluorescence microscopic images showing nephrin and PAX8 after 2 days (a, b) and 8 days (c, d) of subculture. Nephrin (green) was localized at sites of cell-cell contact, while PAX8 (red) was localized in nuclei. Some nephrin-positive cells also stained positive for PAX8 (a). Podocytes showed decreased PAX8 staining and increased nephrin staining after an increased time in culture (a, c). Meanwhile, PECs showed intense staining for PAX8 but not nephrin during culture (b, d). The morphology of nephrin staining was identical to that of podocin (inset in c) in podocytes after 8 days of subculture. Scale bar = 25 μm in a–d, 5 μm in inset in c
Differences in RT-PCR
Time-dependent changes in the gene expression for podocyte and PEC markers were quantified using RT-PCR. The expression levels of most podocyte markers were highest after 2 days of subculture, which preceded the changes in protein expression as observed in immunofluorescence images (Fig. 7, Online Resource 9). RT-PCR was used to successfully detect all podocyte and PEC markers in both the podocyte and PEC cultures. However, most of the expression levels for these markers differed greatly between the podocyte and PEC cultures.
Discussion
In the present study, the first outgrowths from decapsulated glomeruli isolated using iron powder and collagenase digestion were used as podocytes. Meanwhile, cells from encapsulated glomeruli isolated using the conventional sieving method were used as PECs (Katsuya et al. 2006; Yaoita et al. 1991, 2018). In the secondary cultures, podocytes shared several features with podocytes in vivo, as did the latter cell type with PECs in vivo. The former showed distinct immunoreactivity for nephrin, podocin, synaptopodin and WT1. The high levels of expressions for these genes were comparable with those of isolated glomeruli. In particular, these cells exhibited interdigitations with nephrin and podocin concentrated at sites of cell-cell contact; this is the most distinctive character of podocytes in vivo. On the other hand, PECs did not show interdigitations, but rather a simple, polygonal morphology. They showed intense immunoreactivity for PAX8, a PEC-specific marker but not for podocin or nephrin. The levels of expression for PAX2 and PAX8 were much higher than those of isolated glomeruli. These findings indicate the origin of podocytes and PECs, respectively. Furthermore, these results show that culturing conditions in this study may be used to emphasize their unique features and make them distinguishable from each other. Isolated glomeruli used in cultures for PECs contained numbers of decapsulated and encapsulated glomeruli. However, isolation of glomeruli using the serial sieving method damages podocytes and causes them to undergo apoptosis, possibly due to mechanical stress (Ishikawa and Kitamura 1998). Therefore, cellular outgrowths arise from encapsulated glomeruli but not from decapsulated glomeruli isolated using this method (Yaoita et al. 1991). In the case of the former method, collagenase digestion may help decrease the stiffness of renal tissue to minimize mechanical stress during the isolation process. This may help increase the survival of podocytes during the isolation process.
Differences in morphology between the two cell types were apparent in primary and secondary cultures. In primary cultures, cells grow out from the isolated glomeruli into a vast cell-free space that does not exist under physiological conditions in vivo. In this situation, podocytes often had large intercellular spaces and elongated branched cell processes. By contrast, PECs remained in close contact with their neighbors and maintained a polygonal appearance. The existence of large intercellular spaces between podocytes suggests a weakening of intercellular binding forces. This hypothesis was supported by the results of immunostaining and Western blot analysis. Podocytes had lower levels of α-catenin and cadherin 6 compared to levels observed in PECs. These results are not surprising since catenins and cadherins are major components of AJs, which join the actin cytoskeleton to the plasma membrane and form adhesive contacts between cells. Under confluent conditions in secondary culture, podocytes and PECs also showed differences in morphology. Podocytes tended to extend cell processes to form interdigitations while PECs sustained a polygonal appearance. In parallel, the immunoreactivity of α-catenin and cadherin 6 became weaker in podocytes, while PECs maintained intense staining for the proteins. It has been reported that using transfection to induce the expression of classic cadherins in L cells, which have little cadherin expression at baseline, strengthened cell-cell binding creates a polygonal morphology (Nose et al. 1988). This polygonal appearance may not be maintained if cadherin activity is blocked (Hatta et al. 1985). The polygonal morphology of PECs, therefore, is related to their high levels of expression for AJ proteins. Podocytes developed cell processes extensively after the decrease in AJ protein expressions, which coincides with the morphological differences between podocytes in the S-shaped body to those of the capillary loop stage of development. Extensions of cell processes between podocytes may require the loss of AJ proteins. Thus, AJ proteins may also serve as markers for distinguishing the two cell types. The above observations regarding cellular morphology, such as the large intercellular spaces observed in primary cultures, the formation of cell processes during confluence and the disappearance of AJ proteins represent non-epithelial features of podocytes. These changes contrast sharply with typical epithelial features of PECs.
Cells that stained positive for AJ proteins and PAX8 were detected during the primary and early secondary phase of secondary phases of cultured podocytes using immunofluorescence microscopy. Since there was a possibility that podocyte cultures stained positive for AJ proteins or PAX8 due to PEC contamination, staining for podocin or nephrin was used to distinguish between cultured PECs and podocytes (Figs. 5 and 6). Neither podocin nor nephrin staining was detected in PEC cultures, indicating that podocin and nephrin were specific markers for podocytes. Double-label immunofluorescence microscopy demonstrated that some of the podocin-positive or nephrin-positive cells stained positive for cadherin 6 or PAX8 during the early phase of podocyte culture. These findings indicate that podocytes may express PEC markers in culture. Therefore, it seems plausible that significant levels of PAX2 detected in podocyte cultures using RT-PCR may originate from podocytes rather than possible contamination with PECs. In addition, weak but significant immunostaining for WT1 and synaptopodin in most of the cultured PECs corroborates the idea that PECs may express most podocyte-specific genes in culture, which coincides with previous studies (Guhr et al. 2013: Yaoita et al. 2001). These findings suggest that most of the markers have relatively different levels of expression between the two cell types. Therefore, expression of podocyte and PEC markers do not necessarily confirm the existence of podocytes and PECs, respectively. Rather, identification of origin also needs to confirm low expression levels for markers of the other cell types.
References
Andrews PM, Stauver M (1979) In vitro incubation and study of kidney glomerular epithelial cells. Kidney Int 15:80–87
Arakawa M, Tokunaga J (1977) A scanning electron microscope study of the human Bowman's epithelium. Contrib Nephrol 6:73–78
Chittiprol S, Chen P, Petrovic-Djergovic D, Eichler T, Ransom RF (2011) Marker expression, behaviors, and responses vary in different lines of conditionally immortalized cultured podocytes. Am J Physiol Renal Physiol 301:F660–F671
Guhr SS, Sachs M, Wegner A, Becker JU, Meyer TN, Kietzmann L, Schlossarek S, Carrier L, Braig M, Jat PS, Stahl RA, Meyer-Schwesinger C (2013) The expression of podocyte-specific proteins in parietal epithelial cells is regulated by protein degradation. Kidney Int 84:532–544
Hatta K, Okada TS, Takeichi M (1985) A monoclonal antibody disrupting calcium-dependent cell-cell adhesion of brain tissues: possible role of its target antigen in animal pattern formation. Proc Natl Acad Sci U S A 82:2789–2793
Ichimura K, Miyazaki N, Sadayama S, Murata K, Koike M, Nakamura K, Ohta K, Sakai T (2015) Three-dimensional architecture of podocytes revealed by block-face scanning electron microscopy. Sci Rep 5:8993. https://doi.org/10.1038/srep08993
Ishikawa Y, Kitamura M (1998) Spontaneous apoptosis of podocytes in explanted glomeruli. Kidney Int 54:2008–2013
Kabgani N, Grigoleit T, Schulte K, Sechi A, Sauer-Lehnen S, Tag C, Boor P, Kuppe C, Warsow G, Schordan S, Mostertz J, Chilukoti RK, Homuth G, Endlich N, Tacke F, Weiskirchen R, Fuellen G, Endlich K, Floege J, Smeets B, Moeller MJ (2012) Primary cultures of glomerular parietal epithelial cells or podocytes with proven origin. PLoS One 7:e34907
Katsuya K, Yaoita E, Yoshida Y, Yamamoto Y, Yamamoto T (2006) An improved method for primary culture of rat podocytes. Kidney Int 69:2101–2106
Kuppe C, Gröne HJ, Ostendorf T, van Kuppevelt TH, Boor P, Floege J, Smeets B, Moeller MJ (2015) Common histological patterns in glomerular epithelial cells in secondary focal segmental glomerulosclerosis. Kidney Int 88:990–998
Kuppe C, Leuchtle K, Wagner A, Kabgani N, Saritas T, Puelles VG, Smeets B, Hakroush S, van der Vlag J, Boor P, Schiffer M, Gröne HJ, Fogo A, Floege J, Moeller MJ (2019) Novel parietal epithelial cell subpopulations contribute to focal segmental glomerulosclerosis and glomerular tip lesions. Kidney Int 96:80–93
Lazareth H, Henique C, Lenoir O, Puelles VG, Flamant M, Bollée G, Fligny C, Camus M, Guyonnet L, Millien C, Gaillard F, Chipont A, Robin B, Fabrega S, Dhaun N, Camerer E, Kretz O, Grahammer F, Braun F, Huber TB, Nochy D, Mandet C, Bruneval P, Mesnard L, Thervet E, Karras A, Le Naour F, Rubinstein E, Boucheix C, Alexandrou A, Moeller MJ, Bouzigues C, Tharaux PL (2019) The tetraspanin CD9 controls migration and proliferation of parietal epithelial cells and glomerular disease progression. Nat Commun 10:3303
Nagata M (2016) Podocyte injury and its consequences. Kidney Int 89:1221–1230
Nose A, Nagafuchi A, Takeichi M (1988) Expressed recombinant cadherins mediate cell sorting in model systems. Cell 54:993–1001
Ohse T, Chang AM, Pippin JW, Jarad G, Hudkins KL, Alpers CE, Miner JH, Shankland SJ (2009) A new function for parietal epithelial cells: a second glomerular barrier. Am J Physiol Renal Physiol 297:F1566–F1574
Ohtaka A, Ootaka T, Sato H, Soma J, Sato T, Saito T, Ito S (2002) Significance of early phenotypic change of glomerular podocytes detected by Pax2 in primary focal segmental glomerulosclerosis. Am J Kidney Dis 39:475–485
Paul R, Ewing CM, Robinson JC, Marshall FF, Johnson KR, Wheelock MJ, Isaacs WB (1997) Cadherin-6, a cell adhesion molecule specifically expressed in the proximal renal tubule and renal cell carcinoma. Cancer Res 57:2741–2748
Pavenstädt H, Kriz W, Kretzler M (2003) Cell biology of the glomerular podocyte. Physiol Rev 83:253–307
Rodewald R, Karnovsky MJ (1974) Porous substructure of the glomerular slit diaphragm in the rat and mouse. J Cell Biol 60:423–433
Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, Betsholtz C (2002) A new method for large-scale isolation of kidney glomeruli from mice. Am J Pathol 161:799–805
Webber WA, Blackbourn J (1971) The permeability of the parietal layer of Bowman's capsule. Lab Investig 25:367–373
Yaoita E, Yamamoto T, Saito M, Kawasaki K, Kihara I (1991) Desmin-positive epithelial cells outgrowing from rat encapsulated glomeruli. Eur J Cell Biol 54:140–149
Yaoita E, Yamamoto T, Takashima N, Kawasaki K, Kawachi H, Shimizu F, Kihara I (1995) Visceral epithelial cells in rat glomerular cell culture. Eur J Cell Biol 67:136–144
Yaoita E, Kurihara H, Sakai T, Ohshiro K, Yamamoto T (2001) Phenotypic modulation of parietal epithelial cells of Bowman's capsule in culture. Cell Tissue Res 304:339–349
Yaoita E, Yoshida Y, Nameta M, Zhang Y, Fujinaka H, Magdeldin S, Xu B, Yamamoto T (2014) Heparin increasing podocyte-specific gene expressions. Nephrology (Carlton) 19:195–201
Yaoita E, Yoshida Y, Nameta M, Takimoto H, Fujinaka H (2018) Induction of interdigitating cell processes in podocyte culture. Kidney Int 93:519–524
Funding
This work was supported by research grants from the Japanese Ministry of Education, Culture, Sports, Science and Technology (No. 16K09606 and No. 19K08698 to EY).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
All the animal experiments were conducted in compliance with the protocol, which was reviewed by the Institutional Animal Care and Use Committee of Niigata University and approved by the President of Niigata University (Permit Number: #28 Niigata Univ. Res. 345-2, Niigata Univ. Res.SA-00210).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Oyama, T., Yaoita, E., Yoshida, Y. et al. Distinct differences between cultured podocytes and parietal epithelial cells of the Bowman’s capsule. Cell Tissue Res 380, 581–591 (2020). https://doi.org/10.1007/s00441-020-03170-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-020-03170-4