Abstract
Genetic studies on hereditary kidney diseases and in vivo experimental model studies have revealed a critical role for the podocyte in glomerular function and disease. Primary podocyte cultures as well as immortalized podocyte cell lines have been used extensively to study podocyte function. Although, primary cells often more closely resemble the in vivo cells, they may have only a finite replicative life span before they reach senescence. Therefore, the success of studies using primary cell cultures depends on standardized isolation and culture protocols that allow reproducible generation of stable primary cultures.
This chapter describes the isolation of primary podocytes with a proven origin using the novel technology of cell-specific genetic tagging. Podocytes are isolated from glomeruli from a podocyte-specific transgenic reporter mouse. The podocyte-specific reporter gene beta-galactosidase is used to identify and specifically isolate the labeled podocytes from other glomerular cells by FACS.
Access provided by CONRICYT – Journals CONACYT. Download protocol PDF
Similar content being viewed by others
Key words
1 Introduction
Within the glomerulus , the filtration unit of the kidney, podocytes form the visceral epithelial layer on the glomerular capillaries. Podocytes are essential for the structure and function of the glomerular filtration barrier. In the last two decades, it has become apparent that podocyte injury is an early and critical event in many forms of glomerular disease [1]. Cultured podocytes are an essential and indispensable tool to study biological processes in podocytes in a defined environment, without confounding influences of renal hemodynamics and paracrine signaling by different neighboring cell types [2]. The earliest reports of primary podocyte cultures date from the late 1970s [3, 4]. Strikingly, the methods used and the challenges encountered then are still relevant today.
The main challenge is to identify and separate podocytes from other kidney cell types in culture. Generally, podocytes are isolated from cellular outgrowths from isolated glomeruli. The use of isolated glomeruli is necessary to enrich the fraction of podocytes, as podocytes contribute little of the total kidney cells. The glomerulus still contains four different cell types, i.e., the glomerular endothelial cells, mesangial cells, podocytes, and parietal epithelial cells, all of which may be present in the cellular glomerular outgrowth. Since podocytes have a lower cell turnover, they are easily overgrown by the other glomerular cell types. Identification of podocytes is mainly done on the basis of cell morphology of cell-specific marker expression. However, it is well known that virtually all cells undergo significant phenotypic changes as soon as they are removed from their physiological environment and placed into a culture flask. Even in the relative short culture period to grow glomerular outgrowths, rapid phenotypic changes of the outgrowing podocytes can be observed [5]. These phenotypic changes make clear-cut identification of podocytes in culture problematic.
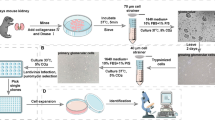
To address these problems, at least in part, irreversible genetic tagging of podocytes in vivo is used to unequivocally determine the origin of cellular outgrowths from isolated glomeruli in primary culture (see Fig. 1 for details of the transgenic mouse model). This cell-specific genetic label is used to isolate the primary podocytes from glomerular outgrowths using fluorescence-activated cell sorting (FACS) (Fig. 2) [5]. This approach also avoids several cloning steps by limited dilution including multiple cell passages and population doublings, which could affect the phenotype of the cells.
Genetic map of the transgenic Pod-rtTA/LC1/R26R mice. Pod-rtTA: The podocyte promoter (derived from the human podocin gene, NPHS2) drives expression of the tetracycline-inducible reverse transactivator (rtTA2-M2) in podocytes . LC1: Expression of Cre recombinase and a reporter gene (luciferase, not relevant in this context) are expressed under the control of a “tetracycline-inducible” promoter containing Tet-responsive elements (TRE). Expression is activated reversibly after administration of doxycycline (Dox) in podocytes. R26R: A stop signal (a neocassette) is irreversibly excised by Cre recombination, so that expression of beta-galactosidase (LacZ) is activated irreversibly under the control of the ubiquitously active R26R locus
Flow scheme for the isolation of genetically tagged podocytes . Podocytes are specifically and irreversibly labeled by administration of doxycycline in transgenic Pod-rtTA/LC1/R26R mice. Inset in mouse illustrates X-Gal staining of labeled podocytes (blue staining). The mice are perfused with magnetic iron oxide which accumulates in the capillaries of the glomeruli (white arrow). Glomeruli are isolated and cultured. Single-cell suspensions of the glomerular outgrowths are treated with FDG, which is hydrolyzed by β-gal into an insoluble fluorescent product, and are subjected to FACS. After FACS, X-Gal staining can be performed to assess the purity of the podocyte culture (blue staining, black arrowhead). The primary podocytes form large arborized cell bodies with several intracytoplasmic extensions, i.e., thickenings
In short, podocytes are irreversibly genetically tagged in vivo in the inducible podocyte reporter mouse (Pod-rtTa/LC1/R26R) [6–8]. The glomeruli containing the labeled podocytes, i.e., expressing beta-galactosidase (β-gal), are isolated and cultured. The β-gal-expressing podocytes are directly isolated from the cellular glomerular outgrowths by FACS. The obtained cultures are >97 % pure, based on the β-gal expression.
2 Materials
-
1.
Pod-rtTA/LC1/R26R mice.
-
2.
Doxycycline solution: 5 % sucrose (w/v) and 1 mg/mL doxycycline hydrochloride (Fargon GmbH&Co, Barsbüttel, Germany), in normal tap water.
-
3.
Anesthetic: mixture of 2 % xylazine (20 mg/mL) and 10 % ketamine (100 mg/mL).
-
4.
Iron oxide solution: normal saline (0.9 % NaCl) containing 0.9 % w/v iron (II,III) oxide (Fe3O4, 98 % purity, 20–30 nm particle powder; Alfa Aesar GmbH, Karlsruhe, Germany).
-
5.
Collagenase Type 4 (Worthington, Lakewood, NJ, USA).
-
6.
EGM medium: Endothelial Growth Media (EGM™) Bullet Kit (Lonza, Walkersville, MD USA). To formulate EGM medium, basal medium EBM™ is supplemented with the Bullet Kit components (i.e., human epidermal growth factor [hEGF], hydrocortisone, bovine brain extract [BBE], ascorbic acid, fetal bovine serum [FBS], and gentamicin/amphotericin-B [GA]), according to manufacturer’s protocol. Final concentration of FBS is 20 % v/v.
-
7.
RPMI medium: RPMI 1640 supplemented with 10 % v/v FBS.
-
8.
HBSS-Tween: 0.05 % v/v Tween®20 in Hank’s balanced salt solution (HBSS).
-
9.
Fluorescein di-β-galactopyranoside (FDG) (Molecular Probes, Leiden, the Netherlands).
-
10.
Cell culture antibiotics: penicillin‐streptomycin solution (10,000 U/mL).
-
11.
Trypsin‐EDTA cell culture formulation to remove cells from cell culture vessel.
-
12.
50 mL centrifuge tubes.
-
13.
Six-well cell culture plates.
-
14.
75 cm2 cell culture flask.
-
15.
Automatic FACS cell sorter (e.g., BD FACSAria cell sorter, BD Biosciences, San Jose, CA, USA).
-
16.
Centrifuge (e.g., Multifuge 3L‐R, Thermo Fischer Scientific Inc., Waltham, MA USA).
-
17.
Cell strainer, 70 μm (Becton Dickinson, Bradford, MA, USA).
-
18.
Glutaraldehyde fixative: Prepare a 2 % glutaraldehyde solution in PBS (v/v) from a 25 % glutaraldehyde commercial stock.
-
19.
Triton™ X-100.
-
20.
X-Gal staining solution: 1 mg/mL X-Gal, 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, and 2 mM MgCl2 in PBS [pH 7.8].
-
21.
Aqueous mounting media.
3 Methods
3.1 Isolation of Podocytes
All transgenic animals used to establish these methods were housed under SPF-free conditions, and the procedures were approved by the local state government authorities of LANUV Cologne. All procedures are performed using aseptic techniques.
-
1.
To induce the genetic labeling of the podocytes , the podocyte reporter mice (Pod-rtTA/LC1/R26R) receive the doxycycline solution via drinking water for a total of 10 days. The solution needs to be protected from light and exchanged every 2 days.
-
2.
Mice are anesthetized with ketamine (80 mg/kg bodyweight) and xylazine (4 mg/kg bodyweight) and perfused via the left heart ventricle with 20–40 mL iron oxide solution (see Note 1 ).
-
3.
Kidneys are transferred into 50 mL RPMI medium containing 1 % (v/v) penicillin-streptomycin solution and cut into small fragments.
-
4.
The kidney fragments are incubated for 30 min at 37 °C with 1 mg/mL collagenase Type 4.
-
5.
The small kidney fragments are gently pressed and sieved through a 70 μm nylon mesh cell strainer/sieve. During this process the strainer is repeatedly rinsed with cold HBSS-Tween. The filtrate is collected in 50 mL centrifuge tubes.
-
6.
HBSS-Tween is added to the filtrate to a final volume of 40 mL per 50 mL falcon tube and incubated on ice for 20 min. During this period the relatively large glomeruli form a sediment, whereas cell debris and smaller structures will remain largely in the supernatant. The supernatant is discarded and the sediment is resuspended in 40 mL of HBSS-Tween and incubated on ice for another 20 min.
-
7.
The sediment is resuspended in 1 mL of HBSS-Tween and placed in a magnetic particle concentrator. The “magnetic” glomeruli containing the iron oxide particles will be attracted to the magnet. Other cells/structures will stay in the solution and can be discarded carefully.
-
8.
The glomeruli still bound to the magnet are washed twice with 1 mL HBSS-Tween.
-
9.
The washed glomeruli are resuspended in EGM medium with 1 % penicillin‐streptomycin solution and incubated for 7 to 14 days at 37 °C in a humidified atmosphere containing 95 % air 5 % CO2. During this culture period, the glomeruli will adhere to the culture dish and glomerular cell outgrowths are formed (see Note 2 ).
-
10.
The cellular outgrowths are trypsinized, and the cells are resuspended in RPMI medium containing 1 % penicillin-streptomycin solution and cultured in a 75 cm2 flask in a humidified atmosphere containing 95 % air 5 % CO2.
-
11.
When the cells have grown to 80 % confluence, the cells are trypsinized and resuspended in RPMI medium, centrifuged at 600 × g, and resuspended in 1 mL RPMI medium containing 1 % penicillin-streptomycin solution.
-
12.
The cell suspension is placed in a magnetic particle concentrator. Free iron oxide particles and remaining glomerular structures containing iron oxide will be attracted to the magnet. The oxide-free cell suspension can be transferred to a clean 1.5 mL test tube.
-
13.
Fluorescein di-β-galactopyranoside (FDG) is added to the cell suspension to a final concentration of 2 mM and incubated for 1 min at 37 °C (see Note 3 ).
-
14.
Subsequently, the samples are diluted tenfold with RPMI medium and incubated on ice for 60 min. The fluorescent-labeled podocytes are sorted from non-labeled cells at 514 nm using FACS (BD FACSAria II cell sorter).
-
15.
Primary podocytes are cultured in RPMI medium containing 1 % penicillin-streptomycin solution at 37 °C in a humidified atmosphere containing 95 % air 5 % CO2 (see Note 4 ).
3.2 Characterization of Isolated Podocytes
Primary podocytes have a distinct phenotype. When the culture is below 80 % confluence, the cells show a large cell body with several arborized intracytoplasmic extensions (Fig. 2) (see Note 4 ).
The podocyte origin and purity of the culture can be verified by enzymatic X-Gal staining. The enzymatic X-Gal staining is used to detect the constitutive beta-galactosidase expression in the labeled podocytes . For the enzymatic X-Gal staining:
-
1.
Cells are washed with HBSS and fixed with 2 % glutaraldehyde for 5 min.
-
2.
Cells are washed with HBSS and treated with 1 % Triton® X-100 for 5 min.
-
3.
The cells are washed again with HBSS and incubated overnight at 37 °C in a humidified atmosphere in X-Gal staining solution.
-
4.
On the next day, samples are counterstained with eosin, washed in tap water, and mounted using aqueous mounting media.
-
5.
Examine microscopically.
4 Notes
-
1.
A minimum of 20 mL Iron oxide solution is used for the perfusion . Successful perfusion of the kidney, with the black-colored iron oxide solution, will turn the color of the perfused kidney gray. When necessary, larger volumes of the iron oxide solution, up to 40 mL, can be used.
-
2.
The isolated glomeruli in culture should not be disturbed for at least a week; any movement of the culture dish will prevent adherence of the glomeruli to the culture plate and the formation of glomerular cell outgrowths.
-
3.
FDG is a substrate for beta-galactosidase. Nonfluorescent FDG is sequentially hydrolyzed by β-galactosidase, first to fluorescein monogalactoside (FMG) and then to highly fluorescent fluorescein.
-
4.
Primary podocyte should be passaged when the monolayer is about 90 % confluent. The cells show restricted growth if the cell density is too low. Therefore, primary podocytes should be seeded to a confluence of 40 % or higher.
References
Wiggins RC (2007) The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int 71:1205–1214
Shankland SJ, Pippin JW, Reiser J, Mundel P (2007) Podocytes in culture: past, present, and future. Kidney Int 72:26–36
Kreisberg JI, Hoover RL, Karnovsky MJ (1978) Isolation and characterization of rat glomerular epithelial cells in vitro. Kidney Int 14:21–30
Striker GE, Killen PD, Farin FM (1980) Human glomerular cells in vitro: isolation and characterization. Transplant Proc 12:88–99
Kabgani N, Grigoleit T, Schulte K et al (2012) Primary cultures of glomerular parietal epithelial cells or podocytes with proven origin. PLoS One 7:e34907
Shigehara T, Zaragoza C, Kitiyakara C et al (2003) Inducible podocyte-specific gene expression in transgenic mice. J Am Soc Nephrol 14:1998–2003
Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB (2003) Podocyte-specific expression of cre recombinase in transgenic mice. Genesis 35:39–42
Soriano P (1999) Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21:70–71
Acknowledgments
This work was supported by the German Research Foundation (Grant BO 3755/1-1 to BS), by the Genzyme Renal Innovation Program (GRIP, to BS). Additional support came from the eRARE consortium “Rare-G” (01 GM 1208A to MJM) and TP17 of SFB/Transregio 57 of the German Research Foundation (to MJM). MJM is a member of the SFB/Transregio 57 DFG consortium “mechanisms of organ fibrosis .” We thank Regina Lanzmich for excellent technical support and helpful discussions, and we thank the Q1 platform of the SFB/Transregio 57 for implementing the above-described protocol into routine practice.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this protocol
Cite this protocol
Smeets, B., Kabgani, N., Moeller, M.J. (2016). Isolation and Primary Culture of Murine Podocytes with Proven Origin. In: Hewitson, T., Smith, E., Holt, S. (eds) Kidney Research. Methods in Molecular Biology, vol 1397. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-3353-2_1
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3353-2_1
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-3351-8
Online ISBN: 978-1-4939-3353-2
eBook Packages: Springer Protocols