Abstract
Regenerative medicine is a branch of translational research that aims to reestablish irreparably damaged tissues and organs by stimulating the body’s own repair mechanisms via the implantation of stem cells differentiated into specialized cell types. A rich source of adult stem cells is located inside the tooth and is represented by human dental pulp stem cells, or hDPSCs. These cells are characterized by a high proliferative rate, have self-renewal and multi-lineage differentiation properties and are often used for tissue engineering and regenerative medicine. The present review will provide an overview of hDPSCs and related features with a special focus on their potential applications in regenerative medicine of the nervous system, such as, for example, after spinal cord injury. Recent advances in the identification and characterization of dental stem cells and in dental tissue engineering strategies suggest that bioengineering approaches may successfully be used to regenerate districts of the central nervous system, previously considered irreparable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Regenerative medicine combines biomedical science and engineering approaches with the aim of restoring the biological function of damaged tissues and relies on the search of tissue sources to obtain cells useful in therapy approaches (Rodríguez-Lozano et al. 2011; Sedgley and Botero 2012).
A useful source of stem cells is characterized by mesenchymal stem cells (MSCs), since these are capable of self-renewal for a limited time in vitro. Within the vast class of MSCs, hematopoietic stem cells have been the best-studied and widely applied for over 40 years in clinical practice creating the background for bone marrow (BM) transplantation success (Wright et al. 2001). It has been demonstrated that, after bone marrow and adipose tissue, the oral cavity represents an excellent source of MSCs mainly localized in the periodontal ligament, dental follicle and in the dental pulp (Xiao and Nasu 2014).
In this review, we provide an overview of oral MSCs focusing on the use of hDPSCs for future applications in neural regeneration such as spinal cord injury repair.
Mesenchymal stem cells (MSCs): a brief description
MSCs, also known as mesenchymal stromal cells, are classified as multipotent stem cells, due to their capability to differentiate into various cellular lineages (Caplan 1991; Karaoz et al. 2013; Kopen et al. 1999).
Although the most studied source of MSCs derive from BM, MSCs can be extracted from a variety of tissues, including adipose tissue, umbilical cord, blood (Koch et al. 2007; Schuh et al. 2009), Wharton’s jelly (Wang et al. 2004), amniotic fluid (Roubelakis et al. 2007), skeletal muscle tissue and periosteum (Kisiel et al. 2012), liver tissue (Najimi et al. 2017), lung tissue (Shi 2015), menstrual blood (Ren et al. 2016; Ulrich et al. 2013), gingiva and periodontal tissue (Mrozik et al. 2010; Otabe et al. 2012).
Different subtypes of cells have been isolated from dental tissue (Fig. 1). Dental mesenchymal stem cells (DMSCs) can be obtained from erupting primary teeth or extracted teeth, making their isolation simpler and less invasive than aspiration of BM-MSCs. Interestingly, they are considered pluripotent, showing peculiar characteristics related to their markers expression (such as MYC and SOX2) and morphological aspects depending on the area of extraction (Yalvac et al. 2010).
Anatomical niches of dental mesenchymal stem cells mostly used in neuro-regenerative approaches. Classification and neurogenic induction properties are based on the expression on their cell surface of neuronal markers (this figure was realized with elements from Servier Medical Art: www.servier.fr/servier-medical-art)
Dental pulp stem cells (DPSCs)
Human dental pulp stem cells (hDPSCs) originate from ectodermal cells that migrate from the neural tube into the oral region and differentiate into mesenchymal cells (Nuti et al. 2016). The staminal feature of hDPSCs in the adult tissue is preserved by the lack of environmental differentiation stimuli in the dental pulp, a niche sealed by mineralized dentin (d’Aquino et al. 2008). hDPSCs are able to maintain and repair periodontal tissue, are characterized by a high proliferation rate (Nuti et al. 2016) and show plasticity in multi-lineage differentiation. Indeed, a number of in vitro studies have described the possibility to obtain osteoblasts-, chondrocytes-, adipocytes-, odontoblasts-, neural- and myocytes-like cells from hDPSCs (Bonaventura et al. 2018; Nuti et al. 2016; Sonoda et al. 2015).
hDPSCs are known to express mesenchymal- (CD13, CD29, CD44 and CD146) (Martens et al. 2012), staminal- (OCT3/4, NANOG and SSEA4) (Kerkis et al. 2006) and specific neuronal markers (βIII-tubulin, S100, Nestin, Synaptophysin) (Li et al. 2019; Martens et al. 2012). Recently, Niehage et al. (Niehage et al. 2016) identified new hDPSC surface proteins, such as tumor necrosis factor receptor superfamily proteins (CD40, CD120a, CD261, CD262, CD264 and CD266), some integrins (alpha-4, alpha-6 and alpha-10) and IL receptors (CD121a, CD130, CD213a1, CD217 and CDw210b).
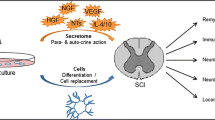
Several soluble factors and cytokines secreted by hDPSCs, such as transforming growth factor beta (TGF-β), prostaglandin E2, interleukin-6 (IL-6) and IL-10, could be immunomodulator candidates for regulation of T lymphocyte function with a profound effect on clinical cell therapy (Demircan et al. 2011). For all these reasons, hDPSCs represent a rising candidate for tissue repair therapies (Sakai et al. 2012) and, in particular, for neuro-regeneration (Fig. 2).
Neuro-regeneration through DPSCs. DPSCs can be easily isolated from adult deciduous teeth. Given their neurogenic induction properties, DPSCs can be in vitro differentiated into mature neurons or different glial cells, such as Schwann cells or oligodendrocytes. These cells can be implanted into CNS to revert neurodegeneration by replacing dead neurons, promoting myelination, or secreting anti-inflammatory chemokines and neurotrophic factors (this figure was realized with elements from Servier Medical Art: www.servier.fr/servier-medical-art)
Stem cells from human exfoliated deciduous teeth (SHED)
Miura et al. (Miura et al. 2003) were the first to isolate and characterize multipotent stem cells from exfoliated deciduous teeth (SHED) within dental pulp tissue. These particular MSCs express Stro-1 and CD146; two early mesenchymal markers also present in cell surfaces of BM-MSCs and DPSCs (Fig. 1). In vitro studies showed that SHEDs are able to differentiate into cells of osteogenic, adipogenic, myogenic and chondrogenic lineages (Bakopoulou et al. 2011a; Kerkis et al. 2006; Miura et al. 2003; Wang et al. 2010).
SHED are able to express some neural progenitor markers, such as nestin and the glial marker glial fibrillary acidic protein (GFAP) at both the mRNA and protein levels (Miura et al. 2003). In vitro neural differentiation studies have also demonstrated that this cell population differentiates into neural cells that are able to survive for more than 10 days when transplanted into an adult rodent brain, leading to an overexpression of neural markers, such as neurofilament (Miura et al. 2003). Wang et al. (Wang et al. 2010) described that SHED are able to form in vitro neural-like spheres in a medium optimized for neural stem cells and to further differentiate into dopaminergic neurons. When differentiated into dopaminergic neurons and transplantated in a rat animal model of Parkinson’s disease, these cells partially improve motor dysfunctions (Gnanasegaran et al. 2016). A recent study reported that SHED therapy reduces neuronal loss over time (do Couto Nicola et al. 2017). In addition, recent reports demonstrated that SHED-conditioned media may afford significant therapeutic improvements for treating autoimmune diseases, such as multiple sclerosis (Shimojima et al. 2016).
Periodontal ligament stem cells (PDLSCs) and stem cells from apical papilla (SCAP)
Two other cell types included in the oral cavity are the periodontal ligament stem cells (PDLSCs) and the apical papilla stem cells (SCAP), a specialized soft connective tissue that physically sustain teeth structure by linking roots and alveolar bone (Beertsen et al. 1997) (Fig. 1). PDLSCs, deriving from the neural crest (Fortino et al. 2014), exhibit immunosuppressive properties that are mediated by soluble factor release (Wada et al. 2009) and are able to maintain their MSC characteristics after in vivo transplantation, which highlights their possible use in cell therapy and neurogenesis (Bueno et al. 2019; Lei et al. 2014). SCAPs have been described by Sonoyama et al. (Sonoyama et al. 2006; Sonoyama et al. 2008) as a lineage with a higher rate of proliferation and a propensity to osteo/odontogenic differentiation (as demonstrated by the expression of CD24 on the cell surface) (Bakopoulou et al. 2011b; Liu et al. 2015). However, several studies have reported the adipogenic and neurogenic differentiation capacity of SCAP (Abe et al. 2007; De Almeida et al. 2014; Sonoyama et al. 2006; Sonoyama et al. 2008). In fact, under standard culture conditions, SCAP physiologically express neural markers (nestin, βIII-tubulin and GFAP) and, after stimulation, produce additional neural markers such as NeuN, medium chain neurofilaments, neuron-specific enolase and glial markers CNPase (Sonoyama et al. 2008). Furthermore, it has been demonstrated that SCAP, seeded onto a synthetic scaffold, when transplanted into immunocompromised mice, produce regeneration of vascularized pulp-like tissue and the formation of dentin-like mineral structures (Huang et al. 2009).
Dental follicle stem cells (DFSCs)
DFSCs were isolated for the first time in 2005 from dental follicles, an ecto-mesenchymal-derived connective tissue (Morsczeck et al. 2005; Zeichner-David et al. 2003). Several studies have demonstrated that hDFSCs have the capacity to differentiate into multiple cell lineages such as osteoblastic, adipogenic and neurogenic lineages (Yao et al. 2008). In addition, since they can be easily obtained during various surgical procedures, hDFSCs represent a good alternative source of MSC suitable for regenerative purposes in cell therapy.
Regenerative effects of DPSCs in neurodegenerative conditions
Despite the fact that BMSCs are the most used MSCs for treating a large range of diseases, they possess several disadvantages linked to bone marrow isolation and lower proliferation rates (Huang et al. 2008; Stenderup et al. 2003). Dental stem cells show MSC-like properties and possess neural characteristics thanks to their origin from the neural crest, their neural marker expression and their ability to secrete neurotrophic factors (Király et al. 2011; Nosrat et al. 2001, 2004). In particular, different studies have confirmed the enormous potential of DPSCs as sources of neuro-regenerative factors. For example, the use of new technologies made possible the differentiation of DPSCs into retinal ganglion-like cells in a three-dimensional network resembling the natural environment of retinal cells. These studies proposed DPSCs as candidates for glaucoma treatment and retinal degenerative diseases (Bray et al. 2014; Roozafzoon et al. 2015).
A specific interest regards the capacity of DPSC to differentiate into oligodendrocytes using a medium enriched in Olig2 factor. Surprisingly, DPSC-derived oligodendrocytes significantly increased the in vivo myelination of peripheral nerves, suggesting the potential use of DPSCs in the cure of myelin-related diseases (Askari et al. 2014). Furthermore, recent discoveries on new factors that induce oligodendrocytes proliferation (Alvarez-Saavedra et al. 2016) extend the possibilities of using DPSCs as a source of myelin. Finally, a recent study reported that DPSC transplantation exerts a neurotrophic effect onto Schwann cells, contributing to peripheral nerve regeneration (Yamamoto et al. 2016). DPSCs can also be used as a source of neurotrophic factors, Aβ-degrading enzyme (such as neprisilyn–NEP) and antiapoptotic factors, rendering DPSCs as promising candidates for secretome-based therapy in neurodegenerative diseases (Gnanasegaran et al. 2017; Mita et al. 2015; Wang et al. 2017).
All together, these results indicate that hDPSCs may promote regeneration of damaged neuron cells in disease models and serve as a useful cell source for the treatment of neurodegenerative diseases (Ullah et al. 2017; Yang et al. 2017) (Fig. 2).
Clinical development of DPSCs in neuro-regeneration
The most promising clinical applications of DPSCs involve the correction of metabolic diseases and treatment of liver diseases with high mortality rates, such as cirrhosis and hepatocellular carcinoma (Ishkitiev et al. 2010; Ohkoshi et al. 2017). Furthermore, DPSCs have become the preferred alternative to harvesting stem cells during hepatic transplantation (Lei et al. 2014). When DPSCs are cultured on hydrogels, they can spontaneously differentiate into both odontogenic and osteogenic phenotypes (Ishkitiev et al. 2010).
Immortalization and neural differentiation of DPSCs are now a reality. Recent studies (Urraca et al. 2015) have demonstrated that DPSCs transplanted in vivo present even more stable characteristics than the cells differentiated in vitro (Lei et al. 2014). These findings make dental tissue-derived stem cells an excellent pre-clinical model in cell therapy and tissue engineering studies.
The efficacy of DPSCs in pre-clinical studies is based on two different mechanisms: neuro-regeneration and neuroprotection (Mita et al. 2015). DPSCs showed remarkable tissue regenerative capability after spinal cord injury through their immunomodulatory, differentiation and protection capacity (do Couto Nicola et al. 2017; Yang et al. 2017). Additional progress in their clinical neuro-regeneration application is represented by the ability of DPSCs to repair peripheral nerve injury (Ullah et al. 2017).
Currently, the gold standard treatment for peripheral nerve injury is nerve grafting but this technique has several disadvantages, such as donor site morbidity (Sultan et al. 2019). Independent studies have demonstrated that DPSCs ameliorate peripheral nerve injuries, such as sciatic nerve injury (Kolar et al. 2017; Yamamoto et al. 2016) and multiple sclerosis (Shimojima et al. 2016). More recently, DPSCs significantly ameliorated the motor defects in a cerebellar ataxia animal model (Aliaghaei et al. 2019).
Dental mesenchymal stem cells (DPSCs) and spinal cord injury
Spinal cord injury (SCI) is characterized by the loss of neuronal cells as a consequence of a physical trauma, due to inflammatory responses triggered by the mechanical trauma (Ahmed et al. 2016; Crowe et al. 1997; Schwab and Bartholdi 1996; Thuret et al. 2006). DMSC-based therapies have shown promising results in SCI treatment (Wang et al. 2017). DPSC can be differentiated into Schwann-like glial cells, becoming able to secrete neurotrophic factors (NTF) and promote survival and neurite outgrowth (Choo et al. 2008). However, many difficulties still exist in the use of DMSCs for SCI regeneration, such as the low rate of cell engraftment and survival after transplantation. In order to overcome these issues, engineered 3D scaffolds have been proposed for DMSCs delivery after SCI, which may provide a surrounding environment conferring a mechanical support to promote cell adhesion, migration and in vivo differentiation (Mead et al. 2017). In this regard, different in vivo studies have highlighted the significant impact of DMSCs as a promising strategy for neuronal repair, functional recovery and tissue regeneration after SCI. A preliminary study in animal models of SCI demonstrated the therapeutic potential of DPSCs through a paracrine-mediated mechanism that promotes axon regeneration and survival of endogenous neurons and glia within and around the lesion site (Martens et al. 2014). Transplanted neural induced SHED in a rat SCI site is known to improve locomotion (Taghipour et al. 2011). Some authors administered neural-differentiated DPSCs combined with a chitosan scaffold into a chronic contusive SCI rat model (Zhang et al. 2016). In this DPSC-/chitosan scaffold-treated group, a greater amount of BDNF, GDNF, b-NGF and NT3 was found in the site of lesion and was responsible for hind limb locomotor recovery. Recently, a thermosensitive heparin-poloxamer (HP) hydrogel containing DPSCs and bFGF was used as an optimal combination of scaffold, cell and growth factors for neuronal regeneration as well as functional recovery after SCI (Luo et al. 2018).
Conclusion
Since DPSCs are widely available and easily accessible, they represent an alternative to traditional therapies in the management of neurological disorders, including SCI. DPSCs hold several advantages over BM-MSCs, such as less invasive isolation and superior ex vivo proliferation. However, several aspects of these stem cells still need to be fully investigated, such as their differentiation potential into the cells of interest, their ability to produce and secrete neurotrophic factors, their homing properties and their immune response modulatory abilities.
References
Abe S, Yamaguchi S, Amagasa T (2007) Multilineage cells from apical pulp of human tooth with immature apex. Oral Sci Int 4:45–58
Ahmed NE-MB, Murakami M, Hirose Y, Nakashima M (2016) Therapeutic potential of dental pulp stem cell secretome for Alzheimer’s disease treatment: an in vitro study. Stem Cells Int 2016:8102478
Aliaghaei A, Boroujeni ME, Ahmadi H, Bayat A-H, Tavirani MR, Abdollahifar MA, Pooyafar MH, Mansouri V (2019) Dental pulp stem cell transplantation ameliorates motor function and prevents cerebellar atrophy in rat model of cerebellar ataxia. Cell Tissue Res 376:179-187
Alvarez-Saavedra M, De Repentigny Y, Yang D, O’Meara RW, Yan K, Hashem LE, Racacho L, Ioshikhes I, Bulman DE, Parks RJ (2016) Voluntary running triggers VGF-mediated oligodendrogenesis to prolong the lifespan of Snf2h-null ataxic mice. Cell Rep 17:862–875
Askari N, Yaghoobi MM, Shamsara M, Esmaeili-Mahani S (2014) Human dental pulp stem cells differentiate into oligodendrocyte progenitors using the expression of Olig2 transcription factor. Cells Tissues Organs 200:93–103
Bakopoulou A, Leyhausen G, Volk J, Tsiftsoglou A, Garefis P, Koidis P, Geurtsen W (2011a) Assessment of the impact of two different isolation methods on the osteo/odontogenic differentiation potential of human dental stem cells derived from deciduous teeth. Calcif Tissue Int 88:130–141
Bakopoulou A, Leyhausen G, Volk J, Tsiftsoglou A, Garefis P, Koidis P, Geurtsen W (2011b) Comparative analysis of in vitro osteo/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP). Arch Oral Biol 56:709–721
Beertsen W, McCulloch CA, Sodek J (1997) The periodontal ligament: a unique, multifunctional connective tissue. Periodontology 2000 13:20–40
Bonaventura G, La Cognata V, Iemmolo R, Zimbone M, Contino A, Maccarrone G, Failla B, Barcellona ML, Conforti FL, D’Agata V, Cavallaro S (2018) Ag-NPs induce apoptosis, mitochondrial damages and MT3/OSGIN2 expression changes in an in vitro model of human dental-pulp-stem-cells-derived neurons. Neurotoxicology 67:84–93
Bray A, Cevallos R, Gazarian K, Lamas M (2014) Human dental pulp stem cells respond to cues from the rat retina and differentiate to express the retinal neuronal marker rhodopsin. Neuroscience 280:142–155
Bueno C, Martínez-Morga M, Martínez S (2019) Non-proliferative adult neurogenesis in neural crest-derived stem cells isolated from human periodontal ligament. bioRxiv https://doi.org/10.1101/325613
Caplan AI (1991) Mesenchymal stem cells. J Orthop Res 9:641–650
Choo AM, Liu J, Dvorak M, Tetzlaff W, Oxland TR (2008) Secondary pathology following contusion, dislocation, and distraction spinal cord injuries. Exp Neurol 212:490–506
Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Crowe MS (1997) Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med 3:73–76
d’Aquino R, Papaccio G, Laino G, Graziano A (2008) Dental pulp stem cells: a promising tool for bone regeneration. Stem Cell Rev 4:21–26
De Almeida JFA, Chen P, Henry MA, Diogenes A (2014) Stem cells of the apical papilla regulate trigeminal neurite outgrowth and targeting through a BDNF-dependent mechanism. Tissue Eng A 20:3089–3100
Demircan PC, Sariboyaci AE, Unal ZS, Gacar G, Subasi C, Karaoz E (2011) Immunoregulatory effects of human dental pulp-derived stem cells on T cells: comparison of transwell co-culture and mixed lymphocyte reaction systems. Cytotherapy 13:1205–1220
do Couto Nicola F, Marques MR, Odorcyk F, Arcego DM, Petenuzzo L, Aristimunha D, Vizuete A, Sanches EF, Pereira DP, Maurmann N (2017) Neuroprotector effect of stem cells from human exfoliated deciduous teeth transplanted after traumatic spinal cord injury involves inhibition of early neuronal apoptosis. Brain Res 1663:95–105
Fortino VR, Chen RS, Pelaez D, Cheung HS (2014) Neurogenesis of neural crest-derived periodontal ligament stem cells by EGF and bFGF. J Cell Physiol 229:479–488
Gnanasegaran N, Govindasamy V, Abu Kasim N (2016) Differentiation of stem cells derived from carious teeth into dopaminergic-like cells. Int Endod J 49:937–949
Gnanasegaran N, Govindasamy V, Mani V, Kasim NHA (2017) Neuroimmunomodulatory properties of DPSCs in an in vitro model of Parkinson’s disease. IUBMB Life 69:689–699
Huang AHC, Snyder BR, Cheng PH, Chan AW (2008) Putative dental pulp-derived stem/stromal cells promote proliferation and differentiation of endogenous neural cells in the hippocampus of mice. Stem Cells 26:2654–2663
Huang GT-J, Yamaza T, Shea LD, Djouad F, Kuhn NZ, Tuan RS, Shi S (2009) Stem/progenitor cell–mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng A 16:605–615
Ishkitiev N, Yaegaki K, Calenic B, Nakahara T, Ishikawa H, Mitiev V, Haapasalo M (2010) Deciduous and permanent dental pulp mesenchymal cells acquire hepatic morphologic and functional features in vitro. J Endod 36:469–474
Karaoz E, Okcu A, Ünal ZS, Subasi C, Saglam O, Duruksu G (2013) Adipose tissue-derived mesenchymal stromal cells efficiently differentiate into insulin-producing cells in pancreatic islet microenvironment both in vitro and in vivo. Cytotherapy 15:557–570
Kerkis I, Kerkis A, Dozortsev D, Stukart-Parsons GC, Massironi SMG, Pereira LV, Caplan AI, Cerruti HF (2006) Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells Tissues Organs 184:105–116
Király M, Kádár K, Horváthy DB, Nardai P, Rácz GZ, Lacza Z, Varga G, Gerber G (2011) Integration of neuronally predifferentiated human dental pulp stem cells into rat brain in vivo. Neurochem Int 59:371–381
Kisiel AH, McDuffee LA, Masaoud E, Bailey TR, Esparza Gonzalez BP, Nino-Fong R (2012) Isolation, characterization, and in vitro proliferation of canine mesenchymal stem cells derived from bone marrow, adipose tissue, muscle, and periosteum. Am J Vet Res 73:1305–1317
Koch TG, Heerkens T, Thomsen PD, Betts DH (2007) Isolation of mesenchymal stem cells from equine umbilical cord blood. BMC Biotechnol 7:26
Kolar MK, Itte VN, Kingham PJ, Novikov LN, Wiberg M, Kelk P (2017) The neurotrophic effects of different human dental mesenchymal stem cells. Sci Rep 7:12605
Kopen GC, Prockop DJ, Phinney DG (1999) Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci 96:10711–10716
Lei M, Li K, Li B, Gao L-N, Chen F-M, Jin Y (2014) Mesenchymal stem cell characteristics of dental pulp and periodontal ligament stem cells after in vivo transplantation. Biomaterials 35:6332–6343
Li D, Zou X-Y, El-Ayachi I, Romero LO, Yu Z, Iglesias-Linares A, Cordero-Morales JF, Huang GT-J (2019) Human dental pulp stem cells and gingival mesenchymal stem cells display action potential capacity in vitro after neuronogenic differentiation. Stem Cell Rev Rep 15:67–81
Liu J, Yu F, Sun Y, Jiang B, Zhang W, Yang J, Xu GT, Liang A, Liu S (2015) Concise reviews: Characteristics and potential applications of human dental tissue-derived mesenchymal stem cells. Stem Cells 33:627–638
Luo L, Albashari AA, Wang X, Jin L, Zhang Y, Zheng L, Xia J, Xu H, Zhao Y, Xiao J, He Y, Ye Q (2018) Effects of transplanted heparin-poloxamer hydrogel combining dental pulp stem cells and bFGF on spinal cord injury repair. Stem Cells Int 2018:2398521
Martens W, Wolfs E, Struys T, Politis C, Bronckaers A, Lambrichts I (2012) Expression pattern of basal markers in human dental pulp stem cells and tissue. Cells Tissues Organs 196:490–500
Martens W, Sanen K, Georgiou M, Struys T, Bronckaers A, Ameloot M, Phillips J, Lambrichts I (2014) Human dental pulp stem cells can differentiate into Schwann cells and promote and guide neurite outgrowth in an aligned tissue-engineered collagen construct in vitro. FASEB J 28:1634–1643
Mead B, Logan A, Berry M, Leadbeater W, Scheven BA (2017) Concise review: dental pulp stem cells: a novel cell therapy for retinal and central nervous system repair. Stem Cells 35:61–67
Mita T, Furukawa-Hibi Y, Takeuchi H, Hattori H, Yamada K, Hibi H, Ueda M, Yamamoto A (2015) Conditioned medium from the stem cells of human dental pulp improves cognitive function in a mouse model of Alzheimer’s disease. Behav Brain Res 293:189–197
Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S (2003) SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci 100:5807–5812
Morsczeck C, Götz W, Schierholz J, Zeilhofer F, Kühn U, Möhl C, Sippel C, Hoffmann K (2005) Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol 24:155–165
Mrozik K, Gronthos S, Shi S, Bartold PM (2010) A method to isolate, purify, and characterize human periodontal ligament stem cells. Oral biology, vol 666. Springer, pp 269-284
Najimi M, Berardis S, El-Kehdy H, Rosseels V, Evraerts J, Lombard C, El Taghdouini A, Henriet P, van Grunsven L, Sokal EM (2017) Human liver mesenchymal stem/progenitor cells inhibit hepatic stellate cell activation: in vitro and in vivo evaluation. Stem Cell Res Ther 8:131
Niehage C, Karbanová J, Steenblock C, Corbeil D, Hoflack B (2016) Cell surface proteome of dental pulp stem cells identified by label-free mass spectrometry. PLoS One 11:e0159824
Nosrat IV, Widenfalk J, Olson L, Nosrat CA (2001) Dental pulp cells produce neurotrophic factors, interact with trigeminal neurons in vitro, and rescue motoneurons after spinal cord injury. Dev Biol 238:120–132
Nosrat IV, Smith CA, Mullally P, Olson L, Nosrat CA (2004) Dental pulp cells provide neurotrophic support for dopaminergic neurons and differentiate into neurons in vitro; implications for tissue engineering and repair in the nervous system. Eur J Neurosci 19:2388–2398
Nuti N, Corallo C, Chan B, Ferrari M, Gerami-Naini B (2016) Multipotent differentiation of human dental pulp stem cells: a literature review. Stem Cell Rev Rep 12:511–523
Ohkoshi S, Hara H, Hirono H, Watanabe K, Hasegawa K (2017) Regenerative medicine using dental pulp stem cells for liver diseases. World J Gastrointest Pharmacol Ther 8:1
Otabe K, Muneta T, Kawashima N, Suda H, Tsuji K, Sekiya I (2012) Comparison of gingiva, dental pulp, and periodontal ligament cells from the standpoint of mesenchymal stem cell properties. Cell Med 4:13–21
Ren H, Sang Y, Zhang F, Liu Z, Qi N (2016) Chen Y (2016) Comparative analysis of human mesenchymal stem cells from umbilical cord, dental pulp, and menstrual blood as sources for cell therapy. Stem Cells Int
Rodríguez-Lozano FJ, Bueno C, Insausti CL, Meseguer L, Ramirez M, Blanquer M, Marin N, Martínez S, Moraleda JM (2011) Mesenchymal stem cells derived from dental tissues. Int Endod J 44:800–806
Roozafzoon R, Lashay A, Vasei M, Ai J, Khoshzaban A, Keshel SH, Barabadi Z, Bahrami H (2015) Dental pulp stem cells differentiation into retinal ganglion-like cells in a three dimensional network. Biochem Biophys Res Commun 457:154–160
Roubelakis MG, Pappa KI, Bitsika V, Zagoura D, Vlahou A, Papadaki HA, Antsaklis A, Anagnou NP (2007) Molecular and proteomic characterization of human mesenchymal stem cells derived from amniotic fluid: comparison to bone marrow mesenchymal stem cells. Stem Cells Dev 16:931–952
Sakai K, Yamamoto A, Matsubara K, Nakamura S, Naruse M, Yamagata M, Sakamoto K, Tauchi R, Wakao N, Imagama S (2012) Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J Clin Invest 122:80–90
Schuh EM, Friedman MS, Carrade DD, Li J, Heeke D, Oyserman SM, Galuppo LD, Lara DJ, Walker NJ, Ferraro GL (2009) Identification of variables that optimize isolation and culture of multipotent mesenchymal stem cells from equine umbilical-cord blood. Am J Vet Res 70:1526–1535
Schwab ME, Bartholdi D (1996) Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev 76:319–370
Sedgley CM, Botero TM (2012) Dental stem cells and their sources. Dent Clin 56:549–561
Shi W (2015) Lung mesenchymal stem cells. stem cells, tissue engineering and regenerative medicine. World Scientific, pp 331-336
Shimojima C, Takeuchi H, Jin S, Parajuli B, Hattori H, Suzumura A, Hibi H, Ueda M, Yamamoto A (2016) Conditioned medium from the stem cells of human exfoliated deciduous teeth ameliorates experimental autoimmune encephalomyelitis. J Immunol:1501457
Sonoda S, Tomoda E, Tanaka Y, Yamaza T (2015) Properties and possibilities of human dental pulp-derived stem cells. Arch Stem Cell Res 2:1012
Sonoyama W, Liu Y, Fang D, Yamaza T, Seo B-M, Zhang C, Liu H, Gronthos S, Wang C-Y, Shi S (2006) Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One 1:e79
Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, Huang GT-J (2008) Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod 34:166–171
Stenderup K, Justesen J, Clausen C, Kassem M (2003) Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone 33:919–926
Sultan N, Amin LE, Zaher AR, Scheven BA, Grawish ME (2019) Dental pulp stem cells: Novel cell-based and cell-free therapy for peripheral nerve repair. World J Stomatol 7:1–19
Taghipour Z, Karbalaie K, Kiani A, Niapour A, Bahramian H, Nasr-Esfahani MH, Baharvand H (2011) Transplantation of undifferentiated and induced human exfoliated deciduous teeth-derived stem cells promote functional recovery of rat spinal cord contusion injury model. Stem Cells Dev 21:1794–1802
Thuret S, Moon LD, Gage FH (2006) Therapeutic interventions after spinal cord injury. Nat Rev Neurosci 7:628–643
Ullah I, Park J-M, Kang Y-H, Byun J-H, Kim D-G, Kim J-H, Kang D-H, Rho G-J, Park B-W (2017) Transplantation of human dental pulp-derived stem cells or differentiated neuronal cells from human dental pulp-derived stem cells identically enhances regeneration of the injured peripheral nerve. Stem Cells Dev 26:1247–1257
Ulrich D, Muralitharan R, Gargett CE (2013) Toward the use of endometrial and menstrual blood mesenchymal stem cells for cell-based therapies. Expert Opin Biol Ther 13:1387–1400
Urraca N, Memon R, El-Iyachi I, Goorha S, Valdez C, Tran QT, Scroggs R, Miranda-Carboni GA, Donaldson M, Bridges D, Reiter LT (2015) Characterization of neurons from immortalized dental pulp stem cells for the study of neurogenetic disorders. Stem Cell Res 15:722–730
Wada N, Menicanin D, Shi S, Bartold PM, Gronthos S (2009) Immunomodulatory properties of human periodontal ligament stem cells. J Cell Physiol 219:667–676
Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ, Fu YS, Lai MC, Chen CC (2004) Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells 22:1330–1337
Wang J, Wang X, Sun Z, Wang X, Yang H, Shi S, Wang S (2010) Stem cells from human-exfoliated deciduous teeth can differentiate into dopaminergic neuron-like cells. Stem Cells Dev 19:1375–1383
Wang F, Jia Y, Liu J, Zhai J, Cao N, Yue W, He H, Pei X (2017) Dental pulp stem cells promote regeneration of damaged neuron cells on the cellular model of Alzheimer's disease. Cell Biol Int 41:639–650
Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL (2001) Physiological migration of hematopoietic stem and progenitor cells. Science 294:1933–1936
Xiao L, Nasu M (2014) From regenerative dentistry to regenerative medicine: progress, challenges, and potential applications of oral stem cells. Stem Cells Cloning: Adv Appl 7:89
Yalvac M, Ramazanoglu M, Rizvanov A, Sahin F, Bayrak O, Salli U, Palotas A, Kose G (2010) Isolation and characterization of stem cells derived from human third molar tooth germs of young adults: implications in neo-vascularization, osteo-, adipo-and neurogenesis. Pharmacogenom J 10:105
Yamamoto T, Osako Y, Ito M, Murakami M, Hayashi Y, Horibe H, Iohara K, Takeuchi N, Okui N, Hirata H (2016) Trophic effects of dental pulp stem cells on schwann cells in peripheral nerve regeneration. Cell Transplant 25:183–193
Yang C, Li X, Sun L, Guo W, Tian W (2017) Potential of human dental stem cells in repairing the complete transection of rat spinal cord. J Neural Eng 14:026005
Yao S, Pan F, Prpic V, Wise G (2008) Differentiation of stem cells in the dental follicle. J Dent Res 87:767–771
Zeichner-David M, Oishi K, Su Z, Zakartchenko V, Chen LS, Arzate H, Bringas P Jr (2003) Role of Hertwig’s epithelial root sheath cells in tooth root development. Dev Dyn 228:651–663
Zhang J, Lu X, Feng G, Gu Z, Sun Y, Bao G, Xu G, Lu Y, Chen J, Xu L (2016) Chitosan scaffolds induce human dental pulp stem cells to neural differentiation: potential roles for spinal cord injury therapy. Cell Tissue Res 366:129–142
Acknowledgments
The authors gratefully acknowledge Dr. Bruno Failla (Department of Pharmaceutical Sciences, Section of Biochemistry, University of Catania, Catania, Italy) for the recruitment of the biological material and Cristina Calì, Alfia Corsino, Maria Patrizia D’Angelo and Francesco Marino for their administrative and technical assistance.
Funding
This work was supported by a grant (CIP 2014.IT.05.SFOP.014/3/10.4/9.2.10/0008) from the European Social Fund operational programme for the Sicily region (Italy) "Development and application of biosensoristic technologies in genomics".
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bonaventura, G., Incontro, S., Iemmolo, R. et al. Dental mesenchymal stem cells and neuro-regeneration: a focus on spinal cord injury. Cell Tissue Res 379, 421–428 (2020). https://doi.org/10.1007/s00441-019-03109-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-019-03109-4