Abstract
Although the pig is an accepted model species for human digestive physiology, no previous study of the pig gastric mucosa and gastric enteroendocrine cells has investigated the parallels between pig and human. In this study, we have investigated markers for each of the classes of gastric endocrine cells, gastrin, ghrelin, somatostatin, 5-hydroxytryptamine, histidine decarboxylase, and PYY cells in pig stomach. The lining of the proximal stomach consisted of a collar of stratified squamous epithelium surrounded by gastric cardiac glands in the fundus. This differs considerably from human that has only a narrow band of cardiac glands at its entrance, surrounded by a fundic mucosa consisting of oxyntic glands. However, the linings of the corpus and antrum are similar in pig and human. Likewise, the endocrine cell types are similar and similarly distributed in the two species. As in human, gastrin cells were almost exclusively in the antrum, ghrelin cells were most abundant in the oxyntic mucosa and PYY cells were rare. In the pig, 70% of enterochromaffin-like (ECL) cells in the antrum and 95% in the fundus contained 5-hydroxytryptamine (5-HT), higher proportions than in human. Unlike the enteroendocrine of the small intestine, most gastric enteroendocrine cells (EEC) did not contain colocalised hormones. This is similar to human and other species. We conclude that the pig stomach has substantial similarity to human, except that the pig has a protective lining at its entrance that may reflect the difference between a pig diet with hard abrasive components and the soft foods consumed by humans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pigs are commonly used as a translational model of gastrointestinal function, being of similar size and having a comparable gastrointestinal anatomy and physiology with humans (Gonzalez et al. 2015; Roura et al. 2016). An animal of similar size and physiology is particularly important for the investigation of devices that are being developed to treat gastric conditions, for example vagal or gastric stimulating electrodes and implanted pulse generators for electrical stimulation (Payne et al. 2018). One of the conditions that is amenable to gastric electrical stimulation in some patients is gastroparesis. Unlike most laboratory animal, the pig is a species that vomits (Szelenyi et al. 1994), which is important for testing the utility of electrical stimulation therapy for the treatment of nausea and vomiting that are common in gastroparesis. Moreover, some pathological conditions that are the targets for therapy are controlled by gastric hormones that have significant roles in regulating digestion, metabolism, appetite, and nausea. Of the major gastric hormones, gastrin and histamine are best known for regulating gastric acid secretion, ghrelin stimulates appetite and reduces nausea, and somatostatin has broad counter-regulatory effects. 5-HT may have a role in signalling the presence of toxins and initiating expulsion of potentially noxious substances, although it has several other actions (Mawe and Hoffman 2013; Diwakarla et al. 2017; Martin et al. 2017). PYY is also expressed in some cells of the gastric epithelium, but its roles in the stomach are unclear.
Gastrointestinal hormones are stored in enteroendocrine cells (EEC), which were classically defined as discrete cell types that each produces a single hormone. In the last decade, however, studies have revealed a substantial degree of overlap between EEC hormones or their genes in single EEC of the small and large intestines (Fothergill and Furness 2018). The co-expression patterns of gastric hormones were recently characterised in detail in human (Fakhry et al. 2019) and rat (Hunne et al. 2019). In contrast to the intestine, relatively few EEC in the stomach expressed more than one hormone, with the exception that 5-HT was frequently found in histamine-producing cells.
Although the pig stomach is similar to human, there are some significant differences, one being the epithelial structure of the gastric fundus. In humans, the corpus and fundus both contain oxyntic glands, which are characterised by acid-secreting parietal cells and enzyme-secreting chief cells. In contrast, the fundic mucosa in pigs is composed of cardiac glands (Meulengracht 1935). Cardiac glands can also occur in humans at the gastro-oesophageal junction; however, they are not always observed and their presence has been associated with gastro-oesophageal disease (Lenglinger et al. 2012; Chandrasoma 2013; Kim et al. 2015). The lining of the rodent fundus is different once again, being covered by non-glandular stratified epithelium. This lining in rodents resembles that of the oesophageal groove, which occurs in pigs but not humans, and is characterised by a lining composed of stratified squamous epithelium. Other aspects of the pig gastric mucosa have yet to be characterised in detail, including the distribution and co-expression patterns of gastric EEC.
We have investigated the distributions and patterns of colocalisation of gastrin, ghrelin, 5-HT, somatostatin, PYY, and histamine-producing cells. Histamine-producing cells were identified with an antibody raised against histidine decarboxylase, an enzyme involved in the synthesis of histamine. We also characterised the anatomy and mucosal structure of the pig stomach.
Methods
Tissue sources and preparation
All procedures were conducted according to the National Health and Medical Research Council of Australia guidelines and were approved by the University of Melbourne Animal Experimentation Ethics Committee. Large White/Landrace crossbred pigs (30–35 kg females) were from the University of Melbourne School of Agriculture and Food. Pigs were sedated with a xylazil and ketamine mix and euthanised by cardiac injection of pentobarbital sodium (150 mg/kg).
Tissues for haematoxylin and eosin staining and immunohistochemistry were removed, opened along the mesenteric border, and pinned flat, mucosa up, without being stretched. Segments were washed with phosphate buffered saline (PBS; 0.15 mol.L−1 NaCl in 0.01 mol. L−1 sodium phosphate buffer, pH 7.2) and fixed at 4 °C overnight with Zamboni’s fixative (2% w/v formaldehyde and 0.2% w/v picric acid in 0.1 mol. L−1 sodium phosphate buffer, pH 7.2). Tissues were washed three times with dimethyl sulfoxide and three times with PBS, before being stored in PBS-sucrose-azide (0.1% w/v sodium azide and 30% w/v sucrose in PBS) at 4 °C.
Haematoxylin and eosin staining
Tissue was placed into histology cassettes and dehydrated through graded ethanol to histolene and embedded in paraffin. Sections (5 μm) were cut and stained with haematoxylin and eosin (H&E). Slides were coverslipped with ProLong Diamond (Thermo Fisher) mounting medium. Slides were examined and photographed using an Axioplan microscope (Zeiss, Sydney, Australia).
Immunohistochemistry
Samples for immunohistochemistry were placed in PBS-sucrose-azide and OCT compound (Tissue Tek, Elkhart, IN, USA) in a 1:1 ratio overnight before being embedded in 100% OCT and snap frozen in isopentane cooled with liquid nitrogen.
Cryosections (12 μm) were cut and air dried for 1 h on SuperFrost Plus® microscope slides (Menzel-Glaser; Thermo Fisher, Scoresby, Vic, Australia). They were then covered with normal horse serum (10% v/v with triton-X in PBS) for 30 min at room temperature and incubated with mixtures of primary antibodies (Table 1) overnight at 4 °C. The preparations were then washed three times with PBS before a 1-h incubation with mixtures of secondary antibodies (Table 1) at room temperature. Sections were washed three times with dH2O and, in some cases, incubated with Hoechst 33258 nuclear staining solution (10 μg/mL bisbenzimide-blue in dH2O; Sigma-Aldrich) for 5 min. Slides were washed three times with distilled water before being mounted under coverslips with Dako fluorescence mounting medium (Agilent, Tullamarine, Vic, Australia). Slides were examined and imaged using an Axio Imager microscope (Zeiss), or an LSM800 or LSM880 confocal microscope (Zeiss).
Immunofluorescence image quantification
Sections for cell counts were imaged as tile scans with a nominal optical thickness of 7.7 μm using a × 10 objective on the LSM800 confocal microscope (Zeiss). A 1.5-mm-wide region from each imaged section, which contained the full thickness of the mucosa, was selected for analysis in Fiji (http://imagej.nih.gov/ij/). Cells from each channel were manually circled by one investigator and verified by a second investigator, and were counted as positive if their mean pixel intensity was clearly above a threshold determined from the background fluorescence. The total mucosal area was also measured in order to determine the cell density (positive cells per mm2 of mucosa). The number of positive cells in the luminal, middle, and submucosal portions of the mucosa was also determined. Data are presented as mean ± SEM, for n = 3 animals.
Results
Anatomy of the pig stomach
The pig has a single chambered stomach similar in shape to human. The pig stomach was cut open along the greater curvature to reveal the gastric lining (Fig. 1). On gross inspection, a distinctive collar of epithelium with an irregular surface was observed around the oesophageal junction. On the lesser curvature, this extended to the boundary of the antrum as the oesophageal groove. The mucosa of the remainder of the stomach had large folds (rugae). The fundus, corpus, and antrum were distinguishable by position and colour (Fig. 1). A prominent swelling, the torus pyloricus, occurs in the stomach on the lesser curvature, adjacent to the gastro-duodenal junction, and there is a diverticulum of the fundus, on the greater curvature, adjacent to the oesophagus. Ten regions were selected for histological analysis by haematoxylin and eosin (H&E) staining (Fig. 2).
Photograph of the stomach from a 35-kg pig opened along the greater curvature to reveal the gastric lining. The regions are indicated. Es. indicates the gastric end of the oesophagus. The arrow adjacent to ‘Torus’ indicates the torus pyloricus (point of arrow is on the torus). The arrows next to ‘Antrum’ indicate the extent of the antrum along the lesser curvature. Note that the antrum extends to the collar of stratified squamous epithelium that surrounds to oesophageal entry to the stomach
Histological appearance of the pig gastric mucosa stained with haematoxylin and eosin (H&E) and diagram of the pig stomach indicating the regions sampled for histological analysis (f). The fundus (a), including the fundic diverticulum (d), was lined with cardiac glands that had prominent gastric pits lined with mucus cells (circled). A collar of thick non-keratinised stratified squamous epithelium surrounded the oesophageal entrance (asterisks mark dermal papillae) that continued as the oesophageal groove (b, c). The antrum and pylorus (h–j) were lined by an epithelium characterised by branched glands (examples circled). Note that the full thickness of the mucosa is not shown for thicker regions of mucosa such as the corpus. Scale bars are 100 μm
The epithelial lining of the peri-oesophageal collar and groove was stratified without a cornified surface, thus being similar to the lining of the oesophagus, and was around 0.5 mm thick (Fig. 2b, c). There were subepithelial papillae, similar to those seen in the skin. The muscle layer was approximately 5 mm thick near the gastro-oesophageal junction but was thinner towards the antrum.
The fundus mucosa was about 0.5 mm thick and consisted of cardiac glands (Fig. 2a). These were branched glands with mucous cells lining the parts near the gastric lumen, while the deeper branches were lined with a simple columnar epithelium. The fundic diverticulum formed a deep distendable pocket with a narrow entrance, adjacent to the esophago-gastric junction. The mucosal lining of the diverticulum was composed of cardiac glands, similar to the rest of the fundus (Fig. 2d).
The mucosa of the gastric corpus consisted of closely packed straight tubular oxyntic glands. The corpus mucosa was relatively thick, being around 1.5 mm, although the muscle layer was amongst the thinnest of the regions investigated (approximately 2 mm).
Pyloric glands in the antrum and pyloric regions of the pig stomach were convoluted, similar to the cardiac glands found in the pig fundus. The luminal ends of the glands were branched and dominated by mucus cells (Fig. 2k). Being around 1 mm thick, the antral and pyloric mucosa was thinner than the corpus mucosa but thicker than the fundic mucosa. The muscle was especially thick in these regions, reaching around 8 mm in the mid antrum. The torus pyloricus is a bulging fibro-muscular structure in the pig stomach on the lesser curvature adjacent to the gastro-duodenal junction (Fig. 1) and accordingly had the thickest wall.
Localisation and morphology of enteroendocrine cells in the pig gastric mucosa
The gastric fundus, corpus, and antrum were examined for ghrelin, somatostatin, 5-HT, PYY, HDC, and gastrin immunoreactivity (Fig. 3). Cells immunoreactivity for each marker were identified in all gastric regions examined, except that gastrin cells were extremely rare in the fundus and corpus, and PYY was uncommon in all three regions. Cell density was quantified for each of these markers (Fig. 4), and the localisation of EEC within the mucosa was determined as the proportion of EEC that was within the submucosal, middle, or luminal thirds of the mucosa (Fig. 5).
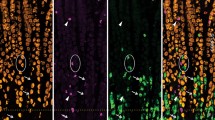
EEC immunoreactivity for ghrelin (a) and PYY (a′) in gastric fundus, 5-HT (b) and somatostatin (b′) in gastric corpus, and HDC (c) and gastrin (c′) in gastric antrum. The bases of the glands and the surface of the mucosa are marked with dotted white lines. The image is oriented with the submucosal (basal) ends of the glands at the bottom of the image. Scale bars are 200 μm
Distribution of EEC across the width of the mucosa in pig gastric fundus, corpus, and antrum. The proportion of EEC immunoreactivity for each hormone marker (indicated below each column) situated in the submucosal third of the mucosa is indicated by a striped pattern, cells in the middle third are indicated by a spotted pattern, and cells in the luminal third are indicated with no pattern. Due to their rarity, the distribution of gastrin cells in the fundus and corpus and PYY cells in all regions was not accurately determined
Ghrelin cells were the most abundant EEC type observed in the fundus and the corpus (31 ± 5 and 67 ± 4 cells/mm2 respectively) but were relatively less common in the antrum compared with most other markers investigated (16 ± 1 cells/mm2; Fig. 4). In the fundus, ghrelin cells were primarily localised in or near the submucosal third of the mucosa, whereas in the corpus and antrum, they were more evenly distributed between the middle and submucosal thirds (Fig. 5). In the corpus and fundus, ghrelin cells were round or ovoid in shape and were closed, meaning they were not in contact with the lumen (Fig. 6a). Some of these cells possessed short thin processes. In the antrum, ghrelin cells were frequently flask-shaped, although whether the apical extremities of these cells were in contact with the lumen was unclear (Fig. 6b).
EEC morphologies and relationships. Examples of cells immunoreactive for ghrelin (a, b), gastrin (c), HDC (d; a marker for histamine-producing cells), somatostatin (SST) (e, f), 5-HT (g), and PYY (h). The luminal surfaces of the epithelial cells forming the glands are marked with dotted white lines. Arrows indicate small basal processes in a, e, f, and g. Scale bars are 20 μm
Somatostatin (SST) cells were most abundant in the antrum, followed by the corpus and the fundus (76 ± 8, 34 ± 3, and 18 ± 5 cells/mm2 respectively; (Fig. 4). SST cells were fairly evenly distributed in the corpus mucosa and slightly more concentrated in the middle third of the antrum, whereas in the fundus, they were skewed towards the submucosal side (Fig. 5). In the corpus and fundus, SST cells were typically round or ovoid closed cells (Fig. 6e), whereas cells were generally flask-like or irregular in shape in the antrum and open to the lumen (Fig. 6f). Examples of small thin basal processes on some SST cells were observed in all three regions.
Gastrin cells were the most abundant type of EEC in the antrum (118 ± 15 cells/mm2), whereas they were extremely rare in the corpus and fundus (fewer than 1 cell/mm2; Fig. 4). Gastrin cells were more frequent in or near the middle third of the antrum mucosa (Fig. 5). These were generally flask-shaped open cells (Fig. 6c). Basal processes of gastrin cells were not observed.
HDC cells were most abundant in the antrum but were also common in the fundus and corpus (47 ± 7, 18 ± 3, and 16 ± 3 cells/mm2 respectively; Fig. 4). HDC cells were predominantly localised to the submucosal third of the fundus mucosa, fairly evenly distributed in the corpus mucosa, and concentrated in or near the middle third of the antrum mucosa (Fig. 5). In the corpus, HDC cells were generally round or ovoid closed cells (Fig. 6d). In contrast, cells in the fundus and antrum were a mixture of cell shapes, including round, ovoid, and flask-shaped. In the antrum, flask-shaped cells were most common.
5-HT cells were most abundant in the antrum but were also found in significant numbers in the corpus and fundus (41 ± 5, 19 ± 3, and 18 ± 3 cells/mm2 respectively; Fig. 4). As with ghrelin and SST, 5-HT cells were concentrated in the basal third of the fundus mucosa. In the corpus, cells were fairly evenly distributed, whereas 5-HT cells in the antrum were predominantly localised in or near the middle third, with a tendency to be closer to the submucosal rather than luminal side (Fig. 5). Like HDC, a mixture of cell shapes was observed in the fundus and antrum, whereas cells in the corpus were generally round or ovoid. Some thin processes were evident (Fig. 6g).
PYY cells were very rare, especially in the corpus (fewer than 1 cell/mm2 in the corpus; Fig. 4). PYY cells were generally round or ovoid (Fig. 6h), although some flask-shaped cells were observed in the antrum.
Colocalisation of EEC markers
Colocalisation was assessed between all combinations of hormones, except for HDC and PYY since our only effective antibodies against these peptides were both raised in rabbit and PYY cells were rare. Furthermore, since gastrin cells were extremely rare in the corpus and fundus, we did not quantify the colocalisation of gastrin with hormones in these regions. Qualitatively, we did not observe much overlap of gastrin with other hormones in the corpus and fundus, except for rare cells containing gastrin and 5-HT or gastrin and PYY in the corpus.
Overlap between gastric hormones was generally low (Fig. 7). One significant exception to this was between 5-HT and HDC (Fig. 8). A substantial degree of colocalisation occurred in all regions investigated. For example, 83 ± 2% of 5-HT cells contained HDC in the fundus, corresponding to 94 ± 3% of HDC cells containing 5-HT.
Examples of cells showing colocalisation of HDC (a) and 5-HT (a′). Colour merge images also show Hoechst nuclear staining in blue. Most cells contain both hormones (indicated by an arrow), but two cells are immunoreactive for HDC and not 5-HT (indicated by an arrow with an asterisk). Scale bar is 20 μm
Although PYY cells were extremely rare throughout the pig stomach, a relatively high proportion of these cells, about 50%, contained other hormones, including ghrelin, somatostatin, 5-HT, and gastrin.
Localisation of parietal cells and ECL cells
Histamine is known to act in a paracrine fashion to promote acid secretion from parietal cells in the corpus (Soll and Walsh 1979). In the present study, however, HDC cells were more abundant in the antrum than the corpus (Fig. 9a, b), which led us to investigate whether HDC cells were situated near parietal cells in the antrum. However, parietal cells were extremely rare or absent in pig gastric antrum and not related to ECL cells, whereas they were abundant in the corpus (Fig. 9a′, b′). In contrast, ghrelin cells, which are abundant in the corpus, were in close proximity to parietal cells, some adjacent cells forming close associations, as seen in other species (Fakhry et al. 2019; Hunne et al. 2019).
The relationship between parietal cells and ECL cells in the gastric corpus (a), and the gastric antrum (b). ECL cells (circled in a) are stained with an anti-HDC antibody and parietal cells (circled in b′) are marked by anti-proton pump (H+/K+ ATPase). The bases of the glands and the surface of the mucosa are marked with dotted white lines. The image is oriented with the base of the glands at the bottom of the image. Scale bars are 100 μm
Discussion
The pig gastric mucosa shares many similarities with human, including similar mucosal architecture in the corpus and antrum. However, in contrast to humans, where oxyntic glands cover the mucosa of both corpus and fundus, the lining of the pig fundus consists of mucus cell-dominated cardiac glands. Furthermore, a collar, around the oesophageal entrance, and the oesophageal groove in pigs were characterised by a thick stratified squamous epithelium. Oxyntic glands were tightly packed long tubular structures, whereas the cardiac and pyloric glands were branched, convoluted, and less dense, as observed by Meulengracht (1935) in pigs. Thus, it seems that the entrance to the pig stomach is protected against abrasion by a thick epithelium and adjacent to this is an epithelium with similar appearance to the human cardiac glands that secrete watery fluid and mucus. The cardiac gland secretion can be assumed to have moistening and lubricating effects. The presence of a thick protective epithelium at the entrance to the stomach and the adjacent cardiac glands with their numerous mucus cells may be in response to the varied diets of pigs in their natural environment that can include dry, hard, and abrasive foods. This contrasts with humans, whose diets over 1000s of years have been dominated by soft processed foods (Furness et al. 2015). Once food passes this protective zone in the pig, it enters an environment very similar to the human stomach with glands of the corpus and antrum being almost indistinguishable between the two species.
Cells immunoreactive for ghrelin, somatostatin, 5-HT, PYY, HDC, and gastrin was identified in all gastric regions examined, although gastrin cells were extremely rare in the fundus and corpus, and PYY was uncommon in all three regions. These observations were on female pigs. It should be noted that EEC populations may differ between genders, for example, in the colon, 5-HT cell abundance during oestrus is 30% greater than in pro-oestrus or in males (Balasuriya et al. 2016). Unlike the small intestine where colocalisation of hormones is observed in the majority of EEC (Egerod et al. 2012; Habib et al. 2012; Sykaras et al. 2014; Cho et al. 2015; Fothergill et al. 2017), very little colocalisation was seen in pig gastric EEC. One significant exception to this is that 5-HT and HDC (a marker of histamine producing ECL cells) were generally co-expressed in all three gastric regions investigated. These results are similar to findings in human oxyntic mucosa, where the only significant overlap observed was between 5-HT and pancreastatin (an alternative marker of histamine-producing cells), although the overlap involved a significantly smaller proportion of cells in the human (Fakhry et al. 2019). In rat, 5-HT and HDC were also frequently colocalised in the antrum, but overlap was rare in the corpus (Hunne et al. 2019). Colocalisation of other hormones was observed in fewer than 5% of ghrelin, somatostatin, or gastrin cells.
Ghrelin cells
Ghrelin cells were most abundant in the oxyntic mucosa, which is consistent with findings in the rat and human (Date et al. 2000; Rindi et al. 2002; Hunne et al. 2019). In the corpus, ghrelin cells were round or ovoid closed cells, meaning they were not in contact with the lumen. This is also consistent with findings in both rat and human (Date et al. 2000; Dornonville De La Cour et al. 2001; Fakhry et al. 2019; Hunne et al. 2019). However, in the antrum, cells were frequently flask-shaped, which is often indicative that the cell is in contact with the lumen. This is contrary to the literature describing ghrelin cells. However, there were no clear examples of the apical ends of these cells reaching all the way to the lumen, so it is possible that these are closed cells, despite the flask-shaped morphology.
Gastric ghrelin has an important role in stimulating appetite, and it also increases gastric emptying in humans and laboratory animals (Levin et al. 2006; Kojima and Kangawa 2010; Avau et al. 2013). In pigs, the relationship between ghrelin and feeding behaviour is less obvious than in other mammals. Plasma ghrelin is elevated in fasting pigs and is reduced by feeding; however, administration of ghrelin did not alter food intake but did increase weight gain in weaner and grower pigs fed ad libitum (Salfen et al. 2004; Lents et al. 2016). Thus, in pigs, ghrelin has a similar distribution as in other mammals, being dominant in the stomach, with lesser amounts in the upper small intestine (Vitari et al. 2012), but seems to have a stronger effect on metabolism than on appetite.
Gastrin cells
Consistent with other species, gastrin cells were extremely rare in the corpus and fundus but were abundant in the gastric antrum. These cells were generally flask-shaped open cells, which relates to their role in sensing luminal contents (Rehfeld et al. 2007). Gastrin cells were clustered within the middle third of the mucosa in contrast to rat gastrin cells which are concentrated in a band near the base of the mucosa (Hunne et al. 2019).
Gastrin’s major role is to promote acid secretion in the stomach (Feldman et al. 1978; Eysselein et al. 1984). This is achieved by stimulating histamine secretion, which in turn promotes acid secretion from parietal cells (Friis-Hansen 2002). Furthermore, gastrin promotes the expression and activity of histidine decarboxylase, the enzyme responsible for producing histamine, and promotes the development of ECL cells and parietal cells (Sandvik et al. 1994; Friis-Hansen et al. 1998). This relationship is interesting given that histamine-producing cells were most abundant in the pig antrum, in contrast to rat and human where they are related to oxyntic glands; the antral ECL cells in pigs are well situated for interactions with gastrin cells, but not with parietal cells.
Somatostatin cells
Somatostatin cells were more abundant in the antrum than the corpus, which is also observed in the rat (Hunne et al. 2019). However, this contrasts with the human, where somatostatin cell density is higher in the corpus (Kasacka et al. 2012; Choi et al. 2014). Somatostatin cells in the rat and human frequently possessed large basal processes, which in the antrum appear to selectively connect with gastrin cells (Larsson et al. 1979; Fakhry et al. 2019; Hunne et al. 2019). This relationship is consistent with the physiological role of somatostatin to provide a negative feedback control of gastrin secretion, thereby limiting acidification of the antrum in human and in animal models (Schubert et al. 1988; Chuang et al. 1993; Vuyyuru et al. 1997; Schubert and Peura 2008), including in the pig (Holst et al. 1992). Thus, it is surprising that prominent basal processes of somatostatin cells were not observed in the pig. Although small thin processes were sometimes seen, these did not appear to extend to any particular cell type. The antral cells are generally of the open type and respond to acid in the lumen as well as neural signalling and gut hormones, including CCK, GIP, GLP-1 and secretin (Schubert et al. 1988; Gribble et al. 2018).
Somatostatin cells in the corpus were round or ovoid. This is consistent with the literature which suggests that oxyntic SST cells are typically closed type and are predominantly regulated by neural and hormonal signalling (Schubert et al. 1988; Gribble et al. 2018). Somatostatin inhibits acid production and histamine release (Schubert et al. 1988; Vuyyuru et al. 1995). SST cells associated with oxyntic glands are tonically active between meals, providing a basal inhibition of gastric acid secretion. These SST cells are temporarily inhibited following the ingestion of food, providing time for gastrin to promote gastric acid release (Li 2003; Gribble et al. 2018).
Histamine and 5-HT cells
5-HT cells were more abundant in the antrum than the corpus, consistent with human, rat, and mouse (Ito et al. 1986; Reynaud et al. 2016; Hunne et al. 2019). HDC cells were also more common in pig antrum than the corpus, which contrasts to the rat and human where they are significantly more abundant in the corpus (Choi et al. 2014; Hunne et al. 2019). Over 70% of HDC cells contained 5-HT in pig antrum, similar to rat antrum where 65% of HDC cells contained 5-HT (Choi et al. 2014; Hunne et al. 2019). In pig corpus, a high proportion of HDC cells also contained 5-HT (80%), which contrasts with both rat corpus (1%) and human corpus (11%) (Choi et al. 2014; Hunne et al. 2019). HDC cell morphology also differed between species, generally being round or ovoid in the pig corpus, whereas they are elongated, flattened cells at the bases of the epithelial cell layer in rat corpus (Håkanson et al. 1986; Hunne et al. 2019). From this, we can infer that a population of cells in which 5-HT and histamine are colocalised occurs in pig, rat, and human, but that the pig seems to lack a large population of HDC-positive 5-HT-negative cells (‘classical’ ECL cells) in the corpus. The overlap between histamine and 5-HT in all species is peculiar given that histamine promotes acid secretion whereas 5-HT inhibits acid secretion (Canfield and Spencer 1983; LePard et al. 1996).
Given histamine’s role in promoting acid secretion from parietal cells (Friis-Hansen 2002), it is peculiar that ECL cells were sparse in the corpus. On the other hand, ECL cells were common in the antrum, where histamine is unlikely to act on parietal cells, which were rare or absent in this region. The roles of histamine in the antrum are not resolved.
Concluding remarks
In many respects, the pig stomach is very similar to human. It is similar in size and shape and, like human, has prominent mucosal rugae. One difference is the thick protective layered epithelium and the mixed mucus and simple columnar (cardiac) glands at and beyond the gastric entrance. The difference here may reflect differences in the physical properties of typical pig and human food, as discussed above. Beyond the gastric fundus, the human and pig corpus and antrum are remarkably similar, suggesting similar gastric digestive physiology. In both species, ghrelin, somatostatin, 5-HT, PYY, HDC, and gastrin EEC are present with similarities in distributions and cell types, for example gastrin cells are extremely rare in the fundus and corpus, PYY cells are uncommon in all three regions, and ghrelin cells are numerous in the corpus. Some quantitative differences were noted, for example the greater proportion of somatostatin and histamine (ECL) cells in the antrum of pig, whereas they are more abundant in the corpus of human. Similar to human and rat, colocalisation of the peptide hormones was rare.
References
Avau B, Carbone F, Tack J, Depoortere I (2013) Ghrelin signaling in the gut, its physiological properties, and therapeutic potential. Neurogastroenterol Motil 25:720–732
Balasuriya GK, Hill-Yardin EL, Gershon MD, Bornstein JC (2016) A sexually dimorphic effect of cholera toxin: rapid changes in colonic motility mediated via a 5-HT3 receptor-dependent pathway in female C57Bl/6 mice. J Physiol Lond 594:4325–4338
Buchan AMJ, Sikora LKJ, Levy JG, McIntosh CHS, Dyck I, Brown JC (1985) An immunocytochemical investigation with monoclonal antibodies to somatostatin. Histochemistry 83:175–180
Canfield SP, Spencer JE (1983) The inhibitory effects of 5-hydroxytryptamine on gastric acid secretion by the rat isolated stomach. Br J Pharmacol 78:123–129
Chandrasoma PT (2013) Histologic definition of gastro-esophageal reflux disease. Curr Opin Gastroenterol 29:460–467
Cho H-J, Kosari S, Hunne B, Callaghan B, Rivera LR, Bravo DM, Furness JB (2015) Differences in hormone localisation patterns of K and L type enteroendocrine cells in the mouse and pig small intestine and colon. Cell Tissue Res 359:693–698
Choi E, Roland JT, Barlow BJ, O’Neal R, Rich AE, Nam KT, Shi C, Goldenring JR (2014) Cell lineage distribution atlas of the human stomach reveals heterogeneous gland populations in the gastric antrum. Gut 63:1711–1720
Chuang C-N, Tanner M, Lloyd KCK, Wong H, Soll AH (1993) Endogenous somatostatin inhibits histamine release from canine gastric mucosal cells in primary culture. Am J Physiol Gastrointest Liver Physiol 28:G521–G525
Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M (2000) Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 141:4255–4261
Diwakarla S, Fothergill LJ, Fakhry J, Callaghan B, Furness JB (2017) Heterogeneity of enterochromaffin cells within the gastrointestinal tract. Neurogastroenterol Motil 29:e13101
Dornonville De La Cour C, Björkqvist M, Sandvik AK, Bakke I, Zhao C-M, Chen D, Håkanson R (2001) A-like cells in the rat stomach contain ghrelin and do not operate under gastrin control. Regul Pept 99:141–150
Egerod KL, Engelstoft MS, Grunddal KV et al (2012) A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology 153:5782–5795
Eysselein VE, Maxwell V, Reedy T, Wünsch E, Walsh JH (1984) Similar acid stimulatory potencies of synthetic human big and little gastrins in man. J Clin Invest 73:1284–1290
Fakhry J, Stebbing MJ, Hunne B, Bayguinov Y, Ward SM, Sasse KC, Callaghan B, McQuade RM, Furness JB (2019) Relationships of endocrine cells to each other and to other cell types in the human gastric fundus and corpus. Cell Tissue Res 376:37–49
Feldman M, Walsh JH, Wong HC (1978) Role of gastrin heptadecapeptide in the acid secretory response to amino acids in man. J Clin Invest 61:308–313
Fothergill LJ, Furness JB (2018) Diversity of enteroendocrine cells investigated at cellular and subcellular levels: the need for a new classification scheme. Histochem Cell Biol 150:693–702
Fothergill LJ, Callaghan B, Hunne B, Bravo DM, Furness JB (2017) Costorage of enteroendocrine hormones evaluated at the cell and subcellular levels in male mice. Endocrinology 158:2113–2123
Friis-Hansen L (2002) Gastric functions in gastrin gene knock-out mice. Pharmacol Toxicol 91:363–367
Friis-Hansen L, Sundler F, Li Y, Gillespie PJ, Saunders TL, Greenson JK, Owyang C, Rehfeld JF, Samuelson LC (1998) Impaired gastric acid secretion in gastrin-deficient mice. Am J Physiol Gastrointest Liver Physiol 274:G561–G568
Furness JB, Cottrell JJ, Bravo DM (2015) Comparative physiology of digestion. J Anim Sci 93:485–491
Gonzalez LM, Moeser AJ, Blikslager AT (2015) Porcine models of digestive disease: the future of large animal translational research. Transl Res 166:12–27
Gribble FM, Reimann F, Roberts GP (2018) Gastrointestinal hormones. In: Said HM (ed) Physiology of the gastrointestinal tract, 6th edn. Academic Press, pp 31–70
Habib AM, Richards P, Cairns LS, Rogers GJ, Bannon CAM, Parker HE, Morley TCE, Yeo GSH, Reimann F, Gribble FM (2012) Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology 153:3054–3065
Håkanson R, Böttcher G, Ekblad T, Panula P, Simonsson M, Dohlsten M, Hallberg T, Sundler F (1986) Histamine in endocrine cells in the stomach. Histochemistry 86:5–17
Holst JJ, Jørgensen PN, Rasmussen TN, Schmidt P (1992) Somatostatin restraint of gastrin secretion in pigs revealed by monoclonal antibody immunoneutralization. Am J Physiol Gastrointest Liver Physiol 263:G908–G912
Hunne B, Stebbing MJ, McQuade RM, Furness JB (2019) Distributions and relationships of chemically defined enteroendocrine cells in the rat gastric mucosa. Cell Tissue Res (in press)
Ito H, Yokozaki H, Tokumo K, Nakajo S, Tahara E (1986) Serotonin-containing EC cells in normal human gastric mucosa and in gastritis. Virchows Archiv A 409:313–323
Kasacka I, Łebkowski W, Janiuk I, Łapińska J, Lewandowska A (2012) Immunohistochemical identification and localisation of gastrin and somatostatin in endocrine cells of human pyloric gastric mucosa. Folia Morphol (Warsz) 71:39–44
Kim A, Park W-Y, Shin N, Lee HJ, Kim YK, Lee SJ, Hwang C-S, Park DY, Kim GH, Lee BE, Jo H-J (2015) Cardiac mucosa at the gastroesophageal junction: an Eastern perspective. World J Gastroenterol 21:9126–9133
Kojima M, Kangawa K (2010) Ghrelin: more than endogenous growth hormone secretagogue. Ann N Y Acad Sci 1200:140–148
Kovacs TO, Lloyd KC, Lawson DC (1997) Inhibition of sham feeding-stimulated acid secretion in dogs by immunoneutralization of gastrin. Am J Physiol Gastrointest Liver Physiol 273:G399–G403
Larsson LI, Goltermann N, De Magistris L, Rehfeld JF, Schwarz TW (1979) Somatostatin cell processes as pathways for paracrine secretion. Science 205:1393–1395
Lenglinger J, See SF, Beller L, Cosentini E, Asari R, Wrba F, Riegler M, Schoppmann SF (2012) The cardia: esophageal or gastric? Critical reviewing the anatomy and histopathology of the esophagogastric junction. Acta Chir Iugosl 59:15–26
Lents CA, Brown-Brandl TM, Rohrer GA, Oliver WT, Freking BA (2016) Plasma concentrations of acyl-ghrelin are associated with average daily gain and feeding behavior in grow-finish pigs. Domest Anim Endocrinol 55:107–113
LePard KJ, Chi J, Mohammed JR, Gidener S, Stephens RL Jr (1996) Gastric antisecretory effect of serotonin: quantitation of release and site of action. Am J Physiol Endocrinol Metab 271:E669–E677
Levin F, Edholm T, Schmidt PT, Grybäck P, Jacobsson H, Degerblad M, Höybye C, Holst JJ, Rehfeld JF, Hellström PM, Näslund E (2006) Ghrelin stimulates gastric emptying and hunger in normal-weight humans. J Clin Endocrinol Metab 91:3296–3302
Li Y-Y (2003) Mechanisms for regulation of gastrin and somatostatin release from isolated rat stomach during gastric distention. World J Gastroenterol 9:129–133
Martin AM, Young RL, Leong L, Rogers GB, Spencer NJ, Jessup CF, Keating DJ (2017) The diverse metabolic roles of peripheral serotonin. Endocrinology 158:1049–1063
Mawe GM, Hoffman JM (2013) Serotonin signalling in the gut—functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol 10:473–486
Meulengracht E (1935) The glands of the stomach in relation to pernicious anaemia; with special reference to the glands in the pyloric region. Proc R Soc Med 28:841–870
Mizutani M, Atsuchi K, Asakawa A, Matsuda N, Fujimura M, Inui A, Kato I, Fujimiya M (2009) Localization of acyl ghrelin- and des-acyl ghrelin-immunoreactive cells in the rat stomach and their responses to intragastric pH. Am J Physiol Gastrointest Liver Physiol 297:G974–G980
Payne SC, Furness JB, Stebbing MJ (2018) Bioelectric neuromodulation for gastrointestinal disorders: effectiveness and mechanisms. Nat Rev Gastroenterol Hepatol 16:89–105
Rehfeld JF, Friis-Hansen L, Goetze JP, Hansen TVO (2007) The biology of cholecystokinin and gastrin peptides. Curr Top Med Chem 7:1154–1165
Reynaud Y, Fakhry J, Fothergill L, Callaghan B, Ringuet MT, Hunne B, Bravo DM, Furness JB (2016) The chemical coding of 5-hydroxytryptamine containing enteroendocrine cells in the mouse gastrointestinal tract. Cell Tissue Res 364:489–497
Rindi G, Necchi V, Savio A, Torsello A, Zoli M, Locatelli V, Raimondo F, Cocchi D, Solcia E (2002) Characterisation of gastric ghrelin cells in man and other mammals: studies in adult and fetal tissues. Histochem Cell Biol 117:511–519
Roura E, Koopmans S-J, Lallès J-P, Le Huerou-Luron I, de Jager N, Schuurman T, Val-Laillet D (2016) Critical review evaluating the pig as a model for human nutritional physiology. Nutr Res Rev 29:60–90
Salfen BE, Carroll JA, Keisler DH, Strauch TA (2004) Effects of exogenous ghrelin on feed intake, weight gain, behavior, and endocrine responses in weanling pigs. J Anim Sci 82:1957–1966
Sandvik AK, Dimaline R, Mårvik R, Brenna E, Waldum HL (1994) Gastrin regulates histidine decarboxylase activity and mRNA abundance in rat oxyntic mucosa. Am J Physiol Gastrointest Liver Physiol 267:G254–G258
Schubert ML, Peura DA (2008) Control of gastric acid secretion in health and disease. Gastroenterology 134:1842–1860
Schubert ML, Edwards NF, Makhlouf GM (1988) Regulation of gastric somatostatin secretion in the mouse by luminal acidity: a local feedback mechanism. Gastroenterology 94:317–322
Smolka AJ, Larsen KA, Hammond CE (2000) Location of a cytoplasmic epitope for monoclonal antibody HK 12.18 on H,K-ATPase α subunit. Biochem Biophys Res Commun 273:942–947
Soll AH, Walsh JH (1979) Regulation of gastric acid secretion. Annu Rev Physiol 41:35–53
Sykaras AG, Demenis C, Cheng L, Pisitkun T, Mclaughlin JT, Fenton RA, Smith CP (2014) Duodenal CCK cells from male mice express multiple hormones including ghrelin. Endocrinology 155:3339–3351
Szelenyi I, Herold H, Göthert M (1994) Emesis induced in domestic pigs: a new experimental tool for detection of antiemetic drugs and for evaluation of emetogenic potential of new anticancer agents. J Pharmacol Toxicol Methods 32:109–116
Vitari F, Di Giancamillo A, Deponti D, Carollo V, Domeneghini C (2012) Distribution of ghrelin-producing cells in the gastrointestinal tract of pigs at different ages. Vet Res Commun 36:71–80
Vuyyuru L, Schubert ML, Harrington L, Arimura A, Makhlouf GM (1995) Dual inhibitory pathways link antral somatostatin and histamine secretion in human, dog, and rat stomach. Gastroenterology 109:1566–1574
Vuyyuru L, Harrington L, Arimura A, Schubert ML (1997) Reciprocal inhibitory paracrine pathways link histamine and somatostatin secretion in the fundus of the stomach. Am J Physiol Gastrointest Liver Physiol 273:G106–G111
Acknowledgements
We thank Maree Cox and Jeremy Cottrell for assistance in tissue harvesting and Melinda Goga and Iain Burchill for assistance with preparation for histology. Confocal imaging was performed at the Biological Optical Microscopy Platform, University of Melbourne.
Funding
This work was financially supported by NIH (SPARC) grant ID # OT2OD023847 (PI Terry Powley) to JBF.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Procedures were approved by the University of Melbourne Animal Ethics Committee (ethics approval number 1714291). All applicable National and Institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fothergill, L.J., Galiazzo, G., Hunne, B. et al. Distribution and co-expression patterns of specific cell markers of enteroendocrine cells in pig gastric epithelium. Cell Tissue Res 378, 457–469 (2019). https://doi.org/10.1007/s00441-019-03065-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-019-03065-z